Abstract
DSM-IV distinguishes two symptom domains of attention deficit hyperactivity disorder (ADHD): inattentiveness and hyperactivity-impulsivity. The present study examines the aetiologies and developmental relations underlying the associations between inattentiveness and hyperactivity-impulsivity over time, based on a representative population sample from the United Kingdom of approximately 7,000 twin pairs. ADHD symptoms were assessed as continuous dimensions using the DSM-IV items from the Conners’ Parent Rating Scale at two ages: middle childhood (age 1) and early adolescence (age 2). Quantitative genetic cross-lagged analyses showed that the association of the ADHD dimensions over time is influenced by stable as well as newly developing genetic factors. Moreover the longitudinal relationship between the ADHD dimensions appears to be unidirectional, with hyperactivity-impulsivity in middle childhood predicting the presence of inattentiveness in early adolescence, but not vice versa. Thus, hyperactivity-impulsivity may serve to exacerbate inattentiveness over time. Findings are discussed in the context of developmental changes in ADHD symptoms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Attention deficit hyperactivity disorder (ADHD) is a highly prevalent childhood psychiatric disorder (Polanczyk et al. 2007). DSM-IV recognizes two ADHD symptom domains: inattentiveness and hyperactivity-impulsivity (American Psychiatric Association 1994). The relative balance in the number of symptoms in the two domains leads to the subtypes of ADHD: the combined, predominantly inattentive and predominantly hyperactive-impulsive subtypes. Twin studies find that both inattentiveness and hyperactivity-impulsivity are highly heritable (Nikolas and Burt 2010); and analysis of cross-sectional data suggests that their association is largely attributable to shared genetic influences, although there are additional genetic influences that are unique to each of the two symptom dimensions (Greven et al. 2011; McLoughlin et al. 2007).
However, little attention has been paid to the influences that link these two groups of symptoms over time. For example, it is plausible that early problems with hyperactivity-impulsivity may increase the risk for developing inattentiveness later in development, or vice versa. Understanding such longitudinal relationships during development may help clarify the primary processes that influence developmental outcomes for children with ADHD. One such outcome concerns the development of the DSM-IV ADHD subtypes. The stability of the subtypes has been noted to be poor during development, during the transition from infancy through childhood (Lahey et al. 2005), as well as childhood through adolescence (Todd et al. 2008). Overall, there appears to be a trend for some children to shift from the hyperactive-impulsive subtype to the combined subtype, and from the combined to the inattentive subtype (Larsson et al. in press). Studying the longitudinal relationships between inattentiveness and hyperactivity-impulsivity may help shed light on the processes underlying developmental shifts in ADHD subtypes.
A previous population-based study examined the relationship of inattentiveness and hyperactivity-impulsivity (and oppositional defiant disorder) using a cross-lagged panel design (Burns and Walsh 2002). This 2-year longitudinal study beginning during the pre-school years found that inattentiveness and hyperactivity-impulsivity did not predict each other after accounting for the ability of each variable to predict itself. To date, no comparable study has investigated the relationship of the two symptom dimensions during the transition from childhood to early adolescence. The present study addresses their developmental relationships using a longitudinal cross-lagged design applied to data from approximately 7,000 population-representative twin pairs from the United Kingdom. Inattentiveness and hyperactivity-impulsivity were assessed as continuous dimensions at two ages, middle childhood and early adolescence.
This is an interesting period for studying developmental processes underlying the ADHD dimensions, as hyperactivity is still an important feature of ADHD during early adolescence; yet previous studies find that hyperactivity-impulsivity declines relative to inattentiveness during the childhood to adolescent transition in both population (Larsson et al. 2006) and clinical samples (Biederman et al. 2000; Hart et al. 1995). Whether this represents a true decline in hyperactive-impulsive symptoms continues to be debated, since the observed decline may be attributable to insensitivity of current diagnostic criteria to detect age-appropriate manifestations of ADHD symptoms. For example hyperactivity may manifest itself as internal restlessness as children grow older (Weyandt et al. 2003) and externalising symptoms may ‘internalize’ as processes that regulate behavioural control mature during the adolescent years.
Since the present study is based on a twin sample, it was also possible to study the genetic and environmental aetiologies underlying the longitudinal relations between the ADHD dimensions. Previous twin studies have shown that genetic influences on composite measures of ADHD symptoms demonstrate substantial stability, despite significant age-specific effects (e.g. Kuntsi et al. 2005; Larsson et al. 2004; Price et al. 2005). The stability of genetic and environmental contributions to each ADHD symptom dimension has faced less empirical scrutiny (Ebejer et al. 2010; Hay et al. 2004; see also trajectory analyses by Larsson et al. in press), and only two twin studies have focused on the genetic and environmental aetiologies of the co-variance between inattentiveness and hyperactivity-impulsivity over time. The first was based on a United States sample of 1,097 8–16 year old twin pairs assessed at two ages 19 months apart (Nadder et al. 2002). The study found that the longitudinal relations between inattentiveness, hyperactivity and impulsivity were largely influenced by shared genetic influences, with some dimension-specific genetic effects. However, the wide age range of the twins and the short follow-up period presented limitations to the study of developmental trends. The second study was based on a Swedish sample of 1,480 twin pairs assessed at ages 8–9, 13–14 and 16–17 years (Larsson et al. 2006). Consistent with the earlier report, this study found a combination of shared genetic influences on inattentiveness and hyperactivity-impulsivity across ages, with some additional age and symptom dimension specific effects. The present study helps consolidate these previous findings in a substantially larger sample. Furthermore, the present study extends these reports, by examining aetiological stability of the ADHD dimensions in a cross-lagged causal design.
The aims of this study were addressed in two sets of related questions. The first aim was to examine the phenotypic relationships between the two dimensions of ADHD as they develop over time. A particular focus was on the cross-lagged (i.e. across-age across-dimension) relations from middle childhood to early adolescence, which delineate the direction of effects underlying the ADHD dimensions. Since there are several potential developmental relationships that can be postulated we did not have a strong a priori hypothesis, but rather aimed to generate an empirical evaluation of their developmental relationship during the transition from childhood to adolescence. The ADHD dimensions have been shown to load onto separate but highly correlated factors (e.g., Burns et al. 1997; DuPaul et al. 1997). Because of these high correlations, we might expect that the ADHD dimensions do not predict each other over time after accounting for pre-existing associations; and findings from the previous cross-lagged study of the ADHD dimensions in young children are consistent with this hypothesis (Burns and Walsh 2002). On the other hand, the trend towards a shift from the hyperactive-impulsive to the combined, and from the combined to the inattentive subtype, raises the possibility that childhood hyperactivity-impulsivity influences levels of adolescent inattentiveness.
The second aim of this study was to examine the influences of genes and environments on the associations between the ADHD dimensions. Although the overlap in genetic influences on the ADHD dimensions has been shown to be of similar magnitude at different ages (see Greven et al. 2011), much less is known about whether the same genes are involved in genetic overlap at different developmental stages. The genetic and environmental aetiologies underlying the longitudinal associations between the ADHD dimensions were therefore of particular interest to this study, as they facilitated insights into the stability of genetic and environmental effects on the association between the ADHD dimensions across middle childhood to early adolescence. Based on previous research we expected to find stable genetic effects on the ADHD dimensions over time, in addition to age-specific and dimension-specific genetic effects.
Methods
Participants
Participants came from the Twins Early Development Study, a United Kingdom population-representative sample of twins born in England and Wales between 1994 and 1996 (Oliver and Plomin 2007). Zygosity of twins was initially determined using physical similarity ratings and was later confirmed for same-sex pairs using DNA markers (Freeman et al. 1997; Oliver and Plomin 2007). Twins were excluded from the present study following severe pregnancy and perinatal complications (e.g., low birth weight, gestation less than 27 weeks) or if one or both twins had a severe medical condition (e.g., Down’s syndrome, cerebral palsy, autism). Unknown sex or zygosity and missing recruitment data were also grounds for excluding twin pairs. After exclusions, the total number of twin pairs included in the model fitting analyses was 7,234 twin pairs: 1,186 monozygotic (MZ) male, 1,149 dizygotic (DZ) male, 1,355 MZ female, 1,208 DZ female and 2,336 DZ opposite-sex pairs.
Conners’ Parent Rating Scale – Revised
Inattentiveness and hyperactivity-impulsivity were assessed in middle childhood (mean age = 7.87 years, SD = 0.51) and early adolescence (mean age = 11.34 years, SD = 0.65). For brevity, we will refer to middle childhood as age 1, and early adolescence as age 2 throughout the methods and results. Assessments were made by parents using the DSM-IV-based ADHD subscales from the Conners’ Parent Rating Scale—Revised (CPRS-R; Conners et al. 1998), which was sent to families via postal questionnaire. Informed parental consent was obtained at each assessment. The Conners’ inattentiveness subscale includes nine items, such as “avoids, expresses reluctance about, or has difficulties engaging in tasks that require sustained mental effort”, and “easily distracted by extraneous stimuli”. The Conners’ hyperactivity-impulsivity subscale includes nine items, such as “is always ‘on the go’ or acts as if driven by a motor”, “talks excessively”, and “blurts out answers to questions before the questions have been completed”. Parents rated each child’s behaviour on a 4-point Likert scale from 0 ‘not true at all’ to 3 ‘very much true’. Cronbach’s alpha internal consistencies were high: 0.89/0.90 for inattentiveness and 0.84/0.83 for hyperactivity-impulsivity at ages 1 and 2, respectively.
ADHD Symptoms in the Present Sample
At ages 1 and 2, boys demonstrated significantly greater variability and higher levels of inattentive and hyperactive symptoms than girls (see Greven et al. 2011; McLoughlin et al. 2007). Attrition analyses on the present data have previously been conducted (Greven et al. 2011), and revealed modest albeit significant attrition linked to the presence of higher ADHD symptoms. However, families who did and did not return the questionnaire at age 2 were largely comparable on a number of background variables (e.g., ethnicity, sex of the child, employment status of father). Moreover, the age 2 sample has been shown to be largely representative of the UK population based on comparisons with UK Census data for families with children (Greven et al. 2011). Based on the Conners’ manual, children who score 3 on six or more of the nine items on either one or both Conners’ subscales are considered suspected ADHD cases, who have a ‘pronounced chance’ for meeting criteria for a DSM-IV diagnosis (Ronald et al. 2008). 2% and 1.5% of the sample could be classified as suspected ADHD cases at ages 1 and 2, respectively. However, it is important to note that the present study examined the full range of the normal distribution of ADHD symptoms across a population that includes a representative range of largely typically developing children, as well as a small percentage of children who were suspected ADHD cases. ADHD medication status was not assessed.
Analyses
This study was based on standard assumptions of the twin method (Plomin et al. 2008). The twin method capitalizes on the fact that similarities between children in the same family can be attributed to shared genes or shared environments. MZ (identical) twins share all of their genetic variation, whereas DZ (fraternal) twins share on average half of their genetic variation, just like non-twin siblings. Shared environments, which make children in the same family more alike, are assumed to be comparable for both types of twins. In contrast, differences between children in the same family can be attributed to genetic differences, or to environmental differences, so called non-shared environments, which have a differential impact upon siblings. Based on the twin method it is possible to construct structural equation models of similarities and differences between and within MZ and DZ twin pairs to estimate genetic (also heritability A), shared environmental (C) and non-shared environmental (E) contributions to individual differences in inattentiveness and hyperactivity-impulsivity and to the covariation between them.
Following standard procedures (McGue and Bouchard 1984), Conners’ raw scores were regressed for sex and age using assessment-appropriate age variables, and residual scores were created. Conners’ scores were positively skewed and transformed using the optimized minimal skew command ‘lnskew0’ in STATA. A quantitative genetic cross-lagged model, first described in a study by Burt and colleagues (Burt et al. 2005), was then fitted to the data in Mx (Neale et al. 2006). Full information maximum likelihood estimation was used, which allows for the inclusion of missing data with minimised bias (Finkbeiner 1979). Cross-sectional analyses on the same data (Greven et al. 2011; McLoughlin et al. 2007) had previously revealed no evidence for qualitative or quantitative sex differences, and favoured a model incorporating shared environment over one incorporating genetic dominance (i.e. the interaction between alleles at the same locus). However, based on these previous analyses variances were modelled separately for each gender. Descriptive statistics, and twin and cross-twin cross-trait correlations are available from these previous publications.
The Cross-Lagged Model
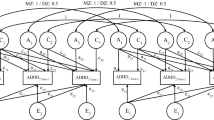
The quantitative genetic cross-lagged model of the ADHD dimensions is presented in Fig. 1. Inattentiveness and hyperactivity-impulsivity are modelled at ages 1 and 2, and the age 2 measures are regressed on the age 1 measures. At the heart of the model are the stability paths (b11, b22) which connect the same measures across ages, and the cross-lagged paths (b12, b21) which connect different measures across ages. The cross-lagged paths are of central interest to the present analyses. The stability and cross-lag paths take the form of partial regression coefficients. That is, they take into account the pre-existing relation between inattentiveness and hyperactivity-impulsivity at age 1, as well as controlling for stability or cross-lagged effects. Figure 1 also depicts genetic (a1–a4), shared environmental (c1–c4) and non-shared environmental (e1–e4) influences on the ADHD dimensions. Non-shared environmental influences also include measurement error. Lastly, Fig. 1 presents genetic and environmental correlations (rA1, rA2; rC1, rC2; rE1, rE2), which can range from −1 to 1, and which represent the extent to which genetic and environmental influences are shared at the two ages.
Quantitative genetic cross-lagged model. Rectangles refer to observed variance in inattentiveness (INN) and hyperactivity-impulsivity (HYP-IMP) in middle childhood (age 1) and early adolescence (age 2). Circles represent latent genetic (A), shared environmental (C) and non-shared environmental (E) factors. Single-headed arrows from the latent A, C, E factors to the observed variables (a1–a4, c1–c4, e1–e4) represent genetic and environmental influences on inattentiveness and hyperactivity-impulsivity. Curved double-headed arrows connecting the latent A, C, E factors represent genetic and environmental correlations (rA1, rA2, rC1, rC2, rE1, rE2). Stability paths connect the same observed variables across ages (b11, b22). Cross-lagged paths connect different variables across ages (b12, b21)
Results
Phenotypic Correlations
Phenotypic within-age across-dimension correlations (Table 1) were high (0.60, and 0.53). Phenotypic ‘cross-lagged’ (i.e. across-age across-dimension) correlations were moderate, and similar in magnitude between age 1 inattentiveness and age 2 hyperactivity-impulsivity (0.40), and age 1 hyperactivity-impulsivity and age 2 inattentiveness (0.43). Across-age within-dimension correlations were also high (0.62 and 0.65), suggesting that each dimension demonstrated high stability across ages.
Model Fit
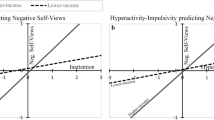
Cross-lagged model results are presented in Fig. 2. The fit of the cross-lagged model was evaluated against a baseline saturated model which estimates the maximum number of parameters (means, variances and covariances) without decomposing variance into ACE components. Model fit was good based on fit indices that heavily reward parsimony, as indicated by negative values on the Bayesian Information Criterion (BIC = −528.86) and Deviance Information Criterion (DIC = −357.93). However, according to the significant χ 2 test (χ 2 (186) = 595.18, p < 0.001) and positive Akaike’s Information Criterion (AIC = 223.18) the model fitted the data significantly worse than the saturated model. However, it should be noted that inadequate fit is the rule rather than the exception in multivariate models (Barrett 2007).
Quantitative genetic cross-lagged model results. For presentational purposes, genetic and environmental estimates at age 2 refer to genetic and environmental contributions to total variance in inattentiveness (INN) and hyperactivity-impulsivity (HYP-IMP), rather than contributions to residual variance specific to age 2. All estimates are standardized. 95% confidence intervals presented in parentheses. Dashed lines represent non-significant paths
Phenotypic Cross-Lagged Model Results
The cross-lagged model revealed notable stabilities for inattentiveness and hyperactivity-impulsivity over time (standardized stability paths b11 = 0.58 and b22 = 0.64; Fig. 2). Moreover, mean comparisons revealed that inattentiveness increased from age 1 (M = 1.99) to age 2 (M = 2.12) as indicated by the decrease in cross-lagged model fit when means were equated across ages, χ 2(1) = 579.474, p < 0.001. In contrast, hyperactivity-impulsivity demonstrated a decrease from age 1 (M = 2.09) to age 2 (M = 1.72), χ 2(1) = 2996.963, p < 0.001.
The most notable finding was that age 1 hyperactivity-impulsivity contributed significantly to age 2 inattentiveness (standardized cross-lagged path b21 = 0.08; Fig. 2). This contribution was significant as the confidence interval did not include zero, and as fixing b21 to zero resulted in a significant deterioration in fit, χ 2(1) = 54.76, p < 0.001. In contrast, age 1 inattentiveness did not significantly contribute to age 2 hyperactivity-impulsivity (standardized cross-lagged path b12 = 0.02), as the confidence interval included zero, and as fixing b12 to zero did not result in a significant deterioration in fit, χ 2(1) = 2.77, p = 0.10. Moreover the cross-lagged path from hyperactivity-impulsivity to inattentiveness was significantly larger than the reverse path, as confidence intervals did not overlap, and as equating b12 and b21 led to a significant decrease in fit, χ 2(1) = 13.50, p < 0.001.
Genetic and Environmental Cross-Lagged Model Results
Squaring the standardized paths from the latent A, C, E factors to the observed variables \( \left( {{\text{a}}_1^2 - {\text{a}}_4^2,\;{\text{c}}_1^2 - {\text{c}}_4^2,\;{\text{e}}_1^2 - {\text{e}}_4^2} \right) \) in Fig. 1, estimates univariate genetic (heritability), shared (C) and non-shared (E) environmental contributions. In line with previous cross-sectional analyses of the same data at age 1 (McLoughlin et al. 2007) and age 2 (Greven et al. 2011), each ADHD dimension was substantially influenced by genetic influences, with heritabilities ranging from 0.63 to 0.72. Shared and non-shared environmental influences were modest, ranging from 0.11 to 0.26. More than half of genetic influences were shared between inattentiveness and hyperactivity-impulsivity, as indicated by the genetic correlations of 0.59 at age 1, and 0.52 at age 2 (Fig. 2). The proportions of the phenotypic within-age across-dimension correlations due to genes were calculated as \( \left[ {\left( {{{\text{a}}_{{1}}} * {\text{r}}{{\text{A}}_{{1}}} * {{\text{a}}_{{2}}}} \right)/{\text{phenotypic}}\;{\text{correlation}}\;{\text{at}}\;{\text{age}}\;{1}} \right] \), and \( \left[ {\left( {{{\text{a}}_{{3}}} * {\text{r}}{{\text{A}}_{{2}}} * {{\text{a}}_{{4}}}} \right)/{\text{phenotypic}}\;{\text{correlation}}\;{\text{at}}\;{\text{age}}\;{2}} \right] \). Due to the high heritabilities and high genetic correlations these proportions were substantial: 0.67 (95% confidence interval: 0.62–0.73) at age 1, and 0.63 (95% confidence interval: 0.58–0.68) at age 2. The proportions of the phenotypic within-age across-dimension correlations attributable to shared environments were 0.23 (95% confidence interval: 0.18–0.29) at age 1, and 0.27 (95% confidence interval: 0.23–0.31) at age 2. For non-shared environments, the proportions were 0.09 (95% confidence interval: 0.08–0.11) at age 1 and 0.10 (95% confidence interval: 0.09–0.11) at age 2.
Moreover, although residual variance specific to age 2 is not presented in Fig. 2, it was possible to estimate the proportion of the age 2 within-age across-dimension correlation due to genes specific to age 2, which can be obtained by calculating \( \left[ {\left( {{{\text{a}}_{{{3}\;{\text{residual}}}}} * {\text{r}}{{\text{A}}_{{{2}\;{\text{residual}}}}} * {{\text{a}}_{{{4}\;{\text{residual}}}}}} \right)\left. {/{\text{phenotypic}}\;{\text{correlation}}\;{\text{at}}\;{\text{age}}\;{2}} \right)} \right] = \left[ {\left( {\surd 0.{37} * 0.{4}0 * \surd 0.{36}} \right)/0.{53}} \right] \approx 0.{27}\left( {{95}\% \;{\text{confidence}}\;{\text{interval}}:0.{22} - 0.{32}} \right) \). Thus, almost half (27% out of 63%) of the age 2 within-age across-dimension correlation attributable to genes was due to age-specific genetic effects. The proportions of the age 2 within-age across-dimension correlation attributable to shared environmental influences and non-shared environmental influences specific to age 2 were 0.15 (95% confidence interval: 0.12–.0.19) and 0.05 (95% confidence interval: 0.04–0.06), respectively. Thus, new environmental influences were also involved in the covariation of the ADHD dimensions at age 2.
The cross-lagged model also allows the estimation of the extent to which genetic and environmental influences at age 2 are specific to age 2 or transmitted from age 1. Effects can be transmitted via three possible routes. First via the stability paths; for example, heritable variance in age 2 inattentiveness due to the stability path is calculated as \( {\text{b}}_{{{11}}}^2 * {\text{a}}_1^2 \). Second, via the cross-lagged paths; for example, heritable variance in age 2 inattentiveness due to age 1 hyperactivity-impulsivity is calculated as \( {\text{b}}_{{21}}^2 * {\text{a}}_2^2 \). Third, via the co-variation of the ADHD dimensions at age 1; for example, variance in age 2 inattentiveness due to the covariation of the ADHD dimensions at age 1 is calculated as \( {2} * \left( {{{\text{b}}_{{{21}}}} * {{\text{a}}_{{2}}} * {\text{r}}{{\text{A}}_{{1}}} * {{\text{a}}_{{1}}} * {{\text{b}}_{{{11}}}}} \right) \). Results are summarized in Table 2 and show that less than half (38%–48%) of genetic and shared environmental influences on the dimensions at age 2 were transmitted from age 1, whereas more than half (52%–62%) of genetic and shared environmental influences were specific to age 2. Moreover only around a third (30%–31%) of non-shared environmental influences on the ADHD dimensions at age 2 were transmitted from age 1, whereas two thirds (69%–70%) were specific to age 2.
Discussion
We found that hyperactivity-impulsivity in middle childhood contributed significantly to the presence of inattentiveness in early adolescence, whereas the converse effect was non-significant. Thus, we show the novel finding that cross-lagged relations between the ADHD dimensions during the transition from childhood to early adolescence are driven by hyperactivity-impulsivity.
In addition, we found that the ADHD dimensions were highly heritable in middle childhood and early adolescence. Genetic and shared environmental influences on each ADHD dimension across this period were moderately stable, although more than half of the familial (genetic and shared environmental) influences on the ADHD dimensions in early adolescence were new, age-specific effects. In contrast, non-shared environments demonstrated little stability over time, as more than two thirds of non-shared environmental influences on the ADHD dimensions in early adolescence were age-specific. Moreover, the ADHD dimensions shared more than half of their genetic influences. Some of the genetic influences shared between the two dimensions in early adolescence were new genetic effects that did not play a role in the co-variation of the symptom dimensions during middle childhood; while some shared genetic influences were transmitted from middle childhood. This implicates the role of novel and enduring genetic influences in the association of the ADHD dimensions across the two developmental periods in this study.
Strengths and Limitations
One possible weakness of this study is its use of continuous ratings of ADHD symptoms in a population-based sample rather than focusing on the clinical phenotype. However, this is also a strength for three main reasons. First, the use of an unselected population sample facilitated the collection of data from a large number of twin pairs, thereby increasing power for quantitative genetic analyses. Second, it helped to avoid some of the biases associated with referral patterns and ascertainment of clinical samples. Finally, the focus on the whole distribution of ADHD symptoms follows from the strong previous evidence that complex disorders like ADHD represent the extreme and impairing tail of continuously distributed traits (Chen et al. 2008; Plomin et al. 2009). Indeed nearly all twin studies of ADHD reported to date have adopted a quantitative approach to the phenotype.
An additional strength is the use of a cross-lagged model based on prospective ratings of ADHD symptoms. This enabled a strong design for studying temporal, and potentially causal, relationships because cross-lagged effects are independent of pre-existing associations and stability of the ADHD dimensions. This is also why it is possible to find significant differences in cross-lagged partial regression coefficients, even when the phenotypic across-age across-dimension ‘cross-lagged’ correlations do not differ—in fact this is the point of cross-lagged analysis.
Because families were not asked to provide information on ADHD medication status, it was not possible to assess whether medication effects may have biased some parents’ ratings of ADHD symptoms. However, as this was a population-based study, such effects would only have affected a very small subset of children. Moreover findings from the present sample map nicely onto the symptom representation in clinical samples; for example we demonstrate an age-dependent decline of hyperactivity and a gender bias in the frequency of ADHD symptoms. This adds validity to the examination of ADHD symptoms in the present sample.
Moreover this study was subject to methodological overlap since inattentiveness and hyperactivity-impulsivity were rated at the same time by the same informant (parents). On the other hand, using the same rater avoided between-rater ‘noise’, as agreement on ADHD symptoms is only modest among different informants, such as parents and teachers (Wolraich et al. 2004). In addition, rating inattentiveness and hyperactivity-impulsivity at the same time was also a strength because synchronicity of ratings bolsters the case for causal inference in cross-lagged designs (Kenny 1975). Methodological overlap and measurement ‘noise’ may also help explain why our cross-lagged findings are inconsistent with a previous study, which found no evidence for significant cross-lagged effects between the ADHD dimensions (Burns and Walsh 2002). In the previous study, different teachers rated ADHD symptoms on different ADHD measures at different ages, decreasing shared method variance, but introducing measurement ‘noise’. In contrast, in the present study the same informant (parent) rated their children on the same DSM-IV based ADHD measure at both ages, increasing methodological overlap, but decreasing measurement ‘noise’.
Although following children over a 4-year period from middle childhood to early adolescence created a strong longitudinal design, the present study does not tell us about the onset of the direction of effects. The possibility of developmental changes in the trajectories of cross-lagged effects may also help explain inconsistencies with the previous cross-lagged study of ADHD symptoms (Burns and Walsh 2002), as the previous study followed children of pre-school age across a 2-year period, whereas the present study followed children over a 4-year period spanning middle childhood to early adolescence. This is particularly relevant given the developmental changes in inattentive and hyperactive-impulsive ADHD symptoms throughout childhood and adolescent development. The aetiological influences and the neurobiological and social processes that mediate such developmental effects on ADHD will need to be studied closely.
The present study gave us no indication of the extent to which the cross-lagged effect from hyperactivity-impulsivity to inattentiveness was attributable to genes or environments. This is because cross-lagged paths in the quantitative genetic cross-lagged model are merely weighted by the univariate genetic and environmental influences (Luo et al. 2010). For example the cross-lagged path from hyperactivity-impulsivity to inattentiveness is attributable to genes and environments to exactly the same extent that hyperactivity-impulsivity in middle childhood is attributable to genes and environments. Lastly, possible weaknesses of this study also include standard limitations of the twin method (Plomin et al. 2008).
Implications
The cross-lagged results suggest that hyperactivity-impulsivity may serve to exacerbate inattentiveness during development from middle childhood to adolescence. The mechanisms underlying this effect remain unclear, and could involve behavioural, cognitive or neurobiological pathways, in addition to changing social influences. One potential mechanism may be linked to developmental changes in the manifestation of ADHD symptoms, as hyperactivity may manifest itself as internal restlessness as children get older (Weyandt et al. 2003). We hypothesize that the increasing internalization of hyperactivity throughout childhood, perhaps by contributing to mental restlessness and distractibility, may lead to the development of behavioural inattentiveness in adolescence. A prediction from this hypothesis is that the predictive relationship from hyperactivity-impulsivity to inattentiveness will be mediated by internal restlessness. This is an interesting area for future research. A related point is that if hyperactivity-impulsivity was to contribute to the development of inattentiveness, as suggested by our hypothesis, this would help explain, at least in part, the observation of a trend for children to shift from the hyperactive-impulsive to the combined, and from the combined to the inattentive ADHD subtype. For example, in our recent study of children with combined type ADHD, we found that more than 50% fulfilled criteria for the inattentive subtype at follow-up in adolescence (Asherson et al., unpublished data); and this is consistent with other studies.
It is important to highlight, however, that the cross-lagged path from hyperactivity-impulsivity to inattentiveness, although statistically significant, was of small effect. Thus, the clinical significance of this effect may be limited. On the other hand, small cross-lagged effects are the rule rather than exception in cross-lagged analyses (Burt et al. 2005; Gregory et al. 2009; Hallett et al. 2010; Larsson et al. 2008), and may reflect the fact that cross-lagged effects are conservative, as they are independent of pre-existing relations and stability effects. The full predictive relations between inattentiveness and hyperactivity-impulsivity are of course stronger and are captured by the across-age across-dimension ‘cross-lagged’ phenotypic correlations, which were moderate. Moreover small cross-lagged effects may reflect the fact that complex traits, such as inattentiveness and hyperactivity-impulsivity, are likely to involve multiple aetiologies which may be genetic or environmental in origin (Pennington 2006), and which at the level of specific risk factors involve small effects (Donnelly 2008; Turkheimer and Waldron 2000). The present study suggests that one such aetiological factor in the development of adolescent inattentiveness may be childhood hyperactivity-impulsivity.
The other key observation from this study was that both stable and newly developing genetic influences were involved in the ADHD dimensions and their associations at the two developmental stages included in this study. This may be a critically important observation in our understanding of the development of ADHD throughout the lifespan, because it suggests that both enduring and novel molecular and hence neurobiological processes might play an important role in developmental changes seen in ADHD. This might be reflected in the developmental hypothesis by Halperin and colleagues (Halperin and Schulz 2006; Halperin et al. 2008) who postulate the role of both early enduring neurobiological processes involved in the aetiology of ADHD, and later developing moderating neurobiological processes involved in the persistence or desistance of ADHD during adolescence. It may also be reflected in the identification of two distinct familial cognitive factors, which largely account for familial (thought to be genetic) influences on ADHD, and which may map onto the neurocognitive processes proposed by Halperin and colleagues (Kunsti et al. 2010).
A final implication concerns molecular genetic studies. We found that the ADHD dimensions are highly heritable and share many of the same genes at each age. However, because genetic stability was only moderate, many genes involved in heritability and genetic overlap were not shared across ages. Sampling strategies in molecular genetic studies should therefore differ depending on whether the goal is to identify age-specific or age-persistent genes.
Final Conclusions
The present study highlighted factors underlying the relationship between inattentiveness and hyperactivity-impulsivity from middle childhood to early adolescence, based on data from a large population-based sample. Overall, the present data suggest that several factors act in concert to explain the associations between the ADHD dimensions across this period. These factors include the substantial genetic overlap between the ADHD dimensions at both ages, which involved both stable and newly developing genetic influences. Moreover, hyperactivity-impulsivity significantly predicted later inattentiveness, whereas the converse did not apply. This finding suggests that hyperactivity-impulsivity may serve to exacerbate inattentiveness during development from middle childhood to adolescence.
References
American Psychiatric Association. (1994). Diagnostic and statistical manual of mental disorders (4th ed.). Washington: Author.
Barrett, P. (2007). Structural equation modelling: adjudging model fit. Personality and Individual Differences, 42, 815–824.
Biederman, J., Mick, E., & Faraone, S. V. (2000). Age-dependent decline of symptoms of attention deficit hyperactivity disorder: impact of remission definition and symptom type. The American Journal of Psychiatry, 157, 816–818.
Burns, G. L., & Walsh, J. A. (2002). The influence of ADHD-hyperactivity/impulsivity symptoms on the development of oppositional defiant disorder symptoms in a 2-year longitudinal study. Journal of Abnormal Child Psychology, 30, 245–256.
Burns, G. L., Walsh, J. A., Patterson, D. R., Holte, C. S., Sommers-Flanagan, R., & Parker, C. M. (1997). Internal validity of the disruptive behavior disorder symptoms: implications from parent ratings for a dimensional approach to symptom validity. Journal of Abnormal Child Psychology, 25, 307–319.
Burt, S. A., McGue, M., Krueger, R. F., & Iacono, W. G. (2005). How are parent-child conflict and childhood externalizing symptoms related over time? Results from a genetically informative cross-lagged study. Development and Psychopathology, 17, 145–165.
Chen, W., Zhou, K., Sham, P., Franke, B., Kuntsi, J., Campbell, D., et al. (2008). DSM-IV combined type ADHD shows familial association with sibling trait scores: a sampling strategy for QTL linkage. American Journal of Medical Genetics. Part B: Neuropsychiatric Genetics, 147B, 1450–1460.
Conners, C. K., Sitarenios, G., Parker, J. D., & Epstein, J. N. (1998). The revised Conners’ Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. Journal of Abnormal Child Psychology, 26, 257–268.
Donnelly, P. (2008). Progress and challenges in genome-wide association studies in humans. Nature, 456, 728–731.
DuPaul, G. J., Power, T. J., Anastopoulos, A. D., Reid, R., McGoey, K. E., & Ikeda, M. J. (1997). Teacher ratings of attention deficit hyperactivity disorder symptoms: factor structure and normative data. Psychological Assessment, 9, 436–444.
Ebejer, J. L., Coventry, W. L., Byrne, B., Willcutt, E. G., Olson, R. K., Corley, R., et al. (2010). Genetic and environmental influences on inattention, hyperactivity-impulsivity, and reading: kindergarten to grade 2. Scientific Studies of Reading, 14, 293–316.
Finkbeiner, C. (1979). Estimation for the multiple factor model when data are missing. Psychometrika, 44, 409–420.
Freeman, B., Powell, J., Ball, D. M., Hill, L., Craig, I. W., & Plomin, R. (1997). DNA by mail: an inexpensive and noninvasive method for collecting DNA samples from widely dispersed populations. Behavior Genetics, 27, 251–257.
Gregory, A. M., Rijsdijk, F. V., Lau, J. Y., Dahl, R. E., & Eley, T. C. (2009). The direction of longitudinal associations between sleep problems and depression symptoms: a study of twins aged 8 and 10 years. Sleep, 32, 189–199.
Greven, C. U., Rijsdijk, F. V., & Plomin, R. (2011). A twin study of ADHD symptoms in early adolescence: hyperactivity-impulsivity and inattentiveness show substantial genetic overlap but also genetic specificity. Journal of Abnormal Child Psychology, 39, 265–275.
Hallett, V., Ronald, A., Rijsdijk, F., & Happe, F. (2010). Association of autistic-like and internalizing traits during childhood: a longitudinal twin study. The American Journal of Psychiatry, 167, 809–817.
Halperin, J. M., & Schulz, K. P. (2006). Revisiting the role of the prefrontal cortex in the pathophysiology of attention-deficit/hyperactivity disorder. Psychological Bulletin, 132, 560–581.
Halperin, J. M., Trampush, J. W., Miller, C. J., Marks, D. J., & Newcorn, J. H. (2008). Neuropsychological outcome in adolescents/young adults with childhood ADHD: profiles of persisters, remitters and controls. Journal of Child Psycholology and Psychiatry, 49, 958–966.
Hart, E. L., Lahey, B. B., Loeber, R., Applegate, B., & Frick, P. J. (1995). Developmental change in attention-deficit hyperactivity disorder in boys: a four-year longitudinal study. Journal of Abnormal Child Psychology, 23, 729–749.
Hay, D. A., Bennett, K. S., McStephen, M., Rooney, R., & Levy, F. (2004). Attention deficit - hyperactivity disorder in twins: a developmental genetic analysis. Australian Journal of Psychology, 56, 99–107.
Kenny, D. A. (1975). Cross-lagged panel correlation: a test for spuriousness. Psychological Bulletin, 82, 887–903.
Kunsti, J., Wood, A. C., Rijsdijk, F., Johnson, K. A., Andreou, P., Albrecht, B., et al. (2010). Separation of cognitive impairments in attention deficit/hyperactivity disorder into 2 familial factors. Archives of General Psychiatry, 67, 1159–1167.
Kuntsi, J., Rijsdijk, F., Ronald, A., Asherson, P., & Plomin, R. (2005). Genetic influences on the stability of attention-deficit/hyperactivity disorder symptoms from early to middle childhood. Biological Psychiatry, 57, 647–654.
Lahey, B. B., Pelham, W. E., Loney, J., Lee, S. S., & Willcutt, E. (2005). Instability of the DSM-IV subtypes of ADHD from preschool through elementary school. Archives of General Psychiatry, 62, 869–902.
Larsson, J., Larsson, H., & Lichtenstein, P. (2004). Genetic and environmental contributions to stability and change of ADHD symptoms between 8 and 13 year of age: a longitudinal twin study. Journal of the American Academy of Child and Adolescent Psychiatry, 43, 1267–1275.
Larsson, H., Lichtenstein, P., & Larsson, J. (2006). Genetic contributions to the development of ADHD subtypes from childhood to adolescence. Journal of the American Academy of Child and Adolescent Psychiatry, 45, 973–981.
Larsson, H., Viding, E., Rijsdijk, F. V., & Plomin, R. (2008). Relationships between parental negativity and childhood antisocial behavior over time: a bidirectional effects model in a longitudinal genetically informative design. Journal of Abnormal Child Psycholology, 36, 633–645.
Larsson, H., Dilshad, R., Lichtenstein, P., & Barker, E. D. (in press). Developmental trajectories of DSM-IV symptoms of attention-deficit/hyperactivity disorder: genetic effects, family risk and associated psychopathology. Journal of Child Psychology and Psychiatry. doi:10.1111/j.1469-7610.2011.02379.x
Luo, Y. L., Haworth, C. M., & Plomin, R. (2010). A novel approach to genetic and environmental analysis of cross-lagged associations over time: the cross-lagged relationship between self-perceived abilities and school achievement is mediated by genes as well as the environment. Twin Research and Human Genetics, 13, 426–436.
McGue, M., & Bouchard, T. J. (1984). Adjustment of twin data for the effects of age and sex. Behavior Genetics, 14, 325–343.
McLoughlin, G., Ronald, A., Kuntsi, J., Asherson, P., & Plomin, R. (2007). Genetic support for the dual nature of attention deficit hyperactivity disorder: substantial genetic overlap between the inattentive and hyperactive-impulsive components. Journal of Abnormal Child Psychology, 35, 999–1008.
Nadder, T. S., Rutter, M., Silberg, J. L., Maes, H. H., & Eaves, L. J. (2002). Genetic effects on the variation and covariation of attention deficit-hyperactivity disorder (ADHD) and oppositional-defiant disorder/conduct disorder (Odd/CD) symptomatologies across informant and occasion of measurement. Psychological Medicine, 32, 39–53.
Neale, M. C., Boker, S. M., Xie, G., & Maes, H. (2006). Mx: Statistical modeling (7th ed.). Richmond: Department of Psychiatry, Medical College of Virginia.
Nikolas, M. A., & Burt, S. A. (2010). Genetic and environmental influences on ADHD symptom dimensions of inattention and hyperactivity: a meta-analysis. Journal of Abnormal Psychology, 119, 1–17.
Oliver, B., & Plomin, R. (2007). Twins Early Development Study (TEDS): a multivariate, longitudinal genetic investigation of language, cognition and behaviour problems from childhood through adolescence. Twin Research and Human Genetics, 10, 96–105.
Pennington, B. F. (2006). From single to multiple deficit models of developmental disorders. Cognition, 101, 385–413.
Plomin, R., DeFries, J. C., McClearn, G. E., & McGuffin, P. (2008). Behavioral genetics (5th ed.). New York: Worth Publishers.
Plomin, R., Haworth, C. M., & Davis, O. S. (2009). Common disorders are quantitative traits. Nature Reviews. Genetics, 10, 872–878.
Polanczyk, G., de Lima, M. S., Horta, B. L., Biederman, J., & Rohde, L. A. (2007). The worldwide prevalence of ADHD: a systematic review and metaregression analysis. The American Journal of Psychiatry, 164, 942–948.
Price, T. S., Simonoff, E., Asherson, P., Curran, S., Kuntsi, J., Waldman, I., et al. (2005). Continuity and change in preschool ADHD symptoms: longitudinal genetic analysis with contrast effects. Behavior Genetics, 35, 121–132.
Ronald, A., Simonoff, E., Kunsti, J., Asherson, P., & Plomin, R. (2008). Evidence for overlapping genetic influences on autistic and ADHD behaviours in a community twin sample. Journal of Child Psychology and Psychiatry, 49, 535–542.
Todd, R. D., Huang, H., Todorov, A. A., Neuman, R. J., Reiersen, A. M., Henderson, C. A., et al. (2008). Predictors of stability of attention-deficit/hyperactivity disorder subtypes from childhood to young adulthood. Journal of the American Academy of Child and Adolescent Psychiatry, 47, 76–85.
Turkheimer, E., & Waldron, M. (2000). Nonshared environment: a theoretical, methodological, and quantitative review. Psychological Bulletin, 126, 78–108.
Weyandt, L. L., Iwaszuk, W., Fulton, K., Ollerton, M., Beatty, N., Fouts, H., et al. (2003). The internal restlessness scale: performance of college students with and without ADHD. Journal of Learning Disabilities, 36, 382–389.
Wolraich, M. L., Lambert, E. W., Bickman, L., Simmons, T., Doffing, M. A., & Worley, K. A. (2004). Assessing the impact of parent and teacher agreement on diagnosing attention-deficit hyperactivity disorder. Journal of Developmental and Behavioral Pediatrics, 25, 41–47.
Acknowledgements
Study supported by the United Kingdom Medical Research Council (G0500079). We are grateful to Twins Early Development Study families.
Conflict of Interest
Asherson has acted in an advisory capacity to Shire, Janssen-Cilag, Eli-Lilly and Flynn Pharma. He is the holder of an educational grant from Janssen-Cilag and a research grant from Shire. He has given talks at meetings or educational program sponsored by to Shire, Janssen-Cilag, Eli-Lilly and Flynn Pharma. The other authors have no competing interests to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Greven, C.U., Asherson, P., Rijsdijk, F.V. et al. A Longitudinal Twin Study on the Association Between Inattentive and Hyperactive-Impulsive ADHD Symptoms. J Abnorm Child Psychol 39, 623–632 (2011). https://doi.org/10.1007/s10802-011-9513-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10802-011-9513-7