Abstract
Attention-Deficit/Hyperactivity Disorder (ADHD) is a prime candidate for exploration of gene-by-environment interaction (i.e., G x E), particularly in relation to dopamine system genes, due to strong evidence that dopamine systems are dysregulated in the disorder. Using a G x E design, we examined whether the DRD4 promoter 120-bp tandem repeat polymorphism, previously associated with ADHD, moderated the effects of inconsistent parenting and marital conflict on ADHD or Oppositional-Defiant Disorder (ODD). Participants were 548 children with ADHD and non-ADHD comparison children and their parents. Homozygosity for the DRD4 promoter 120-bp tandem repeat insertion allele increased vulnerability for ADHD and ODD only in the presence of inconsistent parenting and appeared to increase susceptibility to the influence of increased child self-blame for marital conflict on ADHD inattention. DRD4 genotypes may interact with these proximal family environmental risk factors by increasing the individual’s responsivity to environmental contingencies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An extremely common, costly, and impairing disorder, Attention-Deficit/Hyperactivity Disorder (ADHD) is highly heritable (Faraone et al. 2005; Waldman and Gizer 2006) with heritability estimates exceeding 0.8 in some reports. Replicated molecular genetic findings from candidate gene studies have highlighted associations within the monoaminergic neurotransmission system (see reviews by Faraone et al. 2005; Gizer et al. 2009; Waldman and Gizer 2006). Despite these associations, genome wide association (GWA) studies have so far failed to find genetic markers of major effect, although small effects have emerged (Kuntsi et al. 2006). One possibility is that G x E effects may be contributing to the high heritability term, as has been demonstrated mathematically (Purcell 2002) and highlighted as a possibility for ADHD (Nigg et al. 2010).
Indeed, limitations of gene main-effect findings, as well as growing appreciation of epigenetics and gene-by-environment interplay (Goldsmith et al. 1997; Purcell 2002), have re-awakened interest in environmental influences on ADHD (Banerjee et al. 2007; Sonuga-Barke 2010) and consequently in G x E effects. A small literature on G x E in relation to ADHD has begun to be established (for reviews, see Ficks and Waldman 2009; Nigg et al. 2010). Nigg et al. (2010) concluded that psychosocial variables had an aggregate interaction with gene markers and warranted more investigation. A key finding was a twin study by Pennington et al. (2009) showing that a psychosocial variable (in this example, parental education) moderated latent genetic influences on ADHD, such that the overall contribution of genetic factors to the disorder varied across different levels of parental education. Nonetheless, it is probable that more proximal family process-related variables contribute to that effect. Yet, interplay of identified candidate genes with promising contextual or family environmental effects remains markedly under-investigated.
The dopamine system is particularly interesting as a possible potentiator of environmental inputs in ADHD due to its known role in modulating perceptual sensitivity. We theorized that genes affecting dopaminergic neurotransmission were a logical starting point for examining G x E in relation to psychosocial environments for several reasons. Alterations in brain dopamine functioning have known effects on cognition (Diamond 2007). Dopaminergic receptor genes are closely involved in brain regulatory systems in ascending limbic-frontal circuits as well as descending and reciprocal striatal-thalamo-cortical circuits (Nigg and Casey 2005), areas which may be especially sensitive to alterations in the environment (Arnsten and Goldman-Rakic 1998). In healthy adults, dopamine genes moderate a number of perceptual experiences, including sensitivity to pain (Treister et al. 2009) and responsivity to acute psychosocial stressors (White et al. 2009). Specific to ADHD, one possibility is that decreased dopaminergic neurotransmission influences an individual’s sensitivity to environmental contingencies by enhancing saliency of highly affective stimuli with immediate, rapidly changing, or unpredictable consequences (Holroyd and Coles 2002; Sagvolden et al. 2005). We reasoned that dopamine genes in particular may be involved in G x E processes via altering an individual’s sensitivity to various environmental exposures at the neurobiological level. This, in turn may influence how behavior is modified and encoded in response to those events (Belsky et al. 2009).
We selected one of the most widely-studied genes in the ADHD literature: the dopamine D4 receptor gene (DRD4), located on the short arm of chromosome 11 (11p15.5). DRD4 is expressed in brain regions often implicated in ADHD, most notably in prefrontal cortical regions believed to play a role in executive control (Swanson et al. 2000). Polymorphisms within DRD4 have shown reliable, meta-analytic association with ADHD (Gizer et al. 2009). Although several markers have been studied as potential functional variants, the current study focuses on the tandem repeat polymorphism within the promoter region of DRD4 (with the 240 bp “long” allele associated with ADHD), referred to hereafter as the promoter polymorphism. We selected this promoter polymorphism because it is located in a region important for the binding of transcription factors, which may be relevant for subsequent DRD4 protein expression levels (Kereszturi et al. 2007; McCracken et al. 2000; Seaman et al. 1999). Note that this polymorphism has shown inconsistent associations with ADHD (Faraone et al. 2005; Gizer et al. 2009), suggesting the possibility of G x E.
Research on the interplay between DRD4 and the environment is limited, particularly in regard to examination of psychosocial risk, even though psychosocial risk has shown the largest G x E interaction effects to date (Nigg et al. 2010). Thus, in the current study, we chose to move toward the relatively less examined domain of psychosocial risk factors, partly on the strength of Nigg et al. (2010), Pennington et al. (2009), Lasky-Su et al. (2007), and Sonuga-Barke et al. (2009). Family factors have recently emerged as an environmental potentiator of externalizing behaviors in young children (Bakermans-Kranenburg et al. 2008; Burt et al. 2005a; Propper et al. 2007), and family processes seem to influence the development of self regulation and ADHD via socialization (Cummings et al. 2002). Thus, family environment factors are a set of viable, but understudied, environmental factors that may interact with relevant genetic polymorphisms to increase risk for ADHD (Nigg et al. 2006).
Among the numerous variables related to the family environment, there is mounting evidence suggesting that parenting behavior is part of the maintaining causal structure of ADHD from preschool into middle childhood (Campbell et al. 2000). Indeed, modification of parenting techniques are a common and empirically-supported intervention for reducing impairment associated with ADHD and other disruptive behavior disorders (Barkley 2006). Although effects are likely reciprocal in nature, inconsistent parenting style in particular has been robustly associated with childhood ADHD, even after controlling for oppositional and conduct problems (Ellis and Nigg 2009).
In addition, the role of conflict in the family environment has been shown to be important in the development of behavioral regulation, deficiencies which likely underlie ADHD symptomatology (Nigg et al. 2006). Marital conflict in particular has been shown to be a robust predictor of child behavioral and adjustment problems (Fosco and Grych 2008; Grych et al. 2003; Grych et al. 2000) and ADHD specifically (Wymbs et al. 2008). Child appraisals of marital conflict, particularly cognitive appraisals of self-blame and threat, have been argued to be the central mechanism by which exposure to conflict influences child behavior problems (Grych and Fincham 1990). In line with this, a recent meta-analysis indicated robust associations between children’s appraisals of self-blame and threat with their later internalizing and externalizing problems (Rhoades 2008). Self-blame in particular has shown specific association with ADHD symptom dimensions over and above other psychosocial risk factors (Counts et al. 2005), indicating that these appraisals may have a particularly important role in the etiology of ADHD. Further, recent work indicates that youth appraisals of marital conflict interact with 5HTTLPR genotype in ADHD (Nikolas et al. 2010).
Thus, we theorize that psychosocial variables directly pertinent to the child’s self regulation capacities, including both parenting style and child cognitions about family relations (in this case, interparental conflict) interact with child genotype in ADHD. These specific suppositions find some support in prior G x E studies (e.g., Bakermans-Kranenburg et al. 2008; Propper et al. 2007; Waldman 2007) that have established clear linkages between psychosocial variables such as parenting, marital stability, and genes affecting dopaminergic neurotransmission. With this thinking in mind, we pursued two family-based measures that we theorized are related to regulatory development due to their interpersonal salience and significance to the child, that prior data indicated are likely associated with ADHD, and that enable us to consider both behavioral style and child cognitions about family process in children of school age (during the period when maintaining causes are thought to be operative for the disorder). We evaluated their interplay with a promising but under-studied marker in DRD4, due to the gene’s expression in prefrontal cortex and potential importance to regulatory ability. The main study hypothesis was that children who are homozygous for the “long” allele (240 bp allele) of the DRD4 promoter polymorphism are more susceptible to the influence of inconsistent and negative processes in the socialization environment, operationalized via inconsistent parenting and child-perceived blame for interparental conflict. The long allele has previously been suggested as the risk allele at this marker (Gizer et al. 2009). This basic prediction is in line with a diathesis-stress or multiplicative model of ADHD as suggested by Pennington et al. (2009).
The relative specificity of effects on ADHD versus other externalizing behavior problems is an important question that is often neglected in genetic or G x E studies of psychopathology. This question was explored in the current study in secondary analyses of the association of DRD4 with symptoms of Oppositional Defiant Disorder—the most common comorbid disorder with ADHD. Twin and family studies have suggested that while there is some overlap in the genetic and environmental etiologies of ADHD and disruptive behavior disorders, unique genetic and environmental influences also remain (Burt et al. 2005b; Coolidge et al. 2000; Jester et al. 2005; Tuvblad et al. 2009; Waldman et al. 2001). However, few unique specific gene effects have been identified for most psychopathology, including ADHD and overlapping disruptive behavior problems. Therefore, we set out to examine whether G x E effects influence ADHD and comorbid disruptive behavior disorders generally, or if these variables interact to exert specific effects on ADHD symptoms.
Method
Participants
Overview
Participants were 548 children (59% male) between the ages of six and 18 years. Children were included in two groups: those who met DSM-IV criteria for ADHD (n = 302) and non-ADHD comparison youth (“controls,” n = 199). Forty-seven additional children who were classified as having situational or sub-threshold ADHD (did not meet criteria for either ADHD or control group) were included to provide more complete coverage of the dimensional trait space for ADHD (Levy et al. 1997). The ADHD group included 110 ADHD-Predominantly Inattentive type (ADHD-PI; i.e., met criteria for six or more inattentive symptoms, plus impairment, onset, and duration, and never in the past met criteria for combined type) and 192 ADHD-Combined type (ADHD-C; i.e., met criteria for six or more inattentive symptoms and six or more hyperactive-impulsive symptoms, plus impairment, onset, and duration). One hundred sixty-one children (29%) met DSM-IV criteria for Oppositional-Defiant Disorder (ODD), and 19 (3.4%) met criteria for Conduct Disorder (CD). Children came from 468 families; 388 families had one child in the study, and 80 families had two children in the study.
Recruitment and Identification
In order to avoid the potential inferential bias that can result from identifying cases only through clinic referred cases, a broad community-based recruitment strategy was used, with mass mailings to parents in local school districts and public advertisements, as well as fliers at local clinics. Most cases were ascertained through community sources, including many clear cases that were untreated (percentages below). Families who initially volunteered were then passed through a standard multi-gate screening process to establish diagnostic groupings. At Stage 1, all families were screened by phone to rule out youth prescribed long-acting psychotropic medication for ADHD or other conditions (e.g., antidepressants, Strattera, Atomoxetine), neurological impairments, seizure history, head injury with loss of consciousness, other major medical conditions, or a prior diagnosis of mental retardation or autistic disorder.
At Stage 2, parents and teachers of remaining eligible youth completed standardized rating scales, and parents completed a structured clinical interview to ascertain symptom presence, duration, and impairment. Children completed an IQ screen (a three- or four-subtest short form of the WISC-III or WISC-IV, Wechsler 1991, 2003) and achievement testing. They had to have estimated Full Scale IQ of greater than or equal to 75 for inclusion.
The diagnostic interview used depended on the year of data collection. For participants who participated between 1997 and 2001 (N = 218), the Diagnostic Interview Schedule for Children (DISC-IV; Shaffer et al. 2000) was completed with the parent by telephone or during on-campus visits with a trained interviewer. For participants who participated from 2002 to 2008, youth and their primary caregiver completed the Kiddie Schedule for Affective Disorders and Schizophrenia (KSADS-E; Puig-Antich and Ryan 1986), and the data from the interviews and parent and teacher rating scales were presented to a clinical diagnostic team consisting of a board certified child psychiatrist and licensed clinical child psychologist. Pooling the data across families that received the KSADS and the DISC was justified based on our analysis of agreement between the two methods in 430 youth (including some who were screened out of the study for other reasons) for whom a parent completed both a KSADS and a DISC-IV ADHD module. The two interviews agreed adequately for total number of symptoms (inattention, ICC = 0.88; hyperactivity, ICC = 0.86), presence of six or more symptoms of ADHD (kappa = 0.79), presence of impairment (kappa = 0.64), and presence of ADHD (defined as six or more symptoms + cross situational impairment; kappa = 0.79). Thus, it seemed that similar cases of ADHD were being identified using both methods so they could be pooled.
ADHD Symptoms
Inattentive, hyperactive-impulsive, and total ADHD symptom counts were obtained via separate parent and teacher report on the ADHD Rating Scale (DuPaul et al. 1998). Symptoms were rated on a scale ranging from zero to three. Teacher-rated symptoms were utilized as the primary dependent variable in the current study to eliminate the potential confound of shared method variance between ADHD symptoms and parent-rated parenting style.
Comorbid Child Diagnoses and Symptoms
The structured diagnostic interview was used for establishing the presence of ODD and CD based on DSM-IV criteria. Symptom counts for ODD were obtained via teacher report on the Swanson, Nolan, & Pelham Rating Scale-Fourth Edition (SNAP-IV; Swanson 1992).
Measures
Parenting
Parents completed the Alabama Parenting Questionnaire, a 42-item self-report questionnaire on parenting practices. Each item was rated on a Likert scale from one (never) to five (always). The scale assessed six domains: involvement, positive parenting, poor monitoring/supervision, inconsistent discipline, corporal punishment, and other discipline practices. These subscales exhibited satisfactory reliability (Essau et al. 2006). The questionnaire also demonstrates satisfactory convergent validity, exhibiting significant correlations with conduct problems and interviews about parenting (Essau et al. 2006), as well as with ADHD and ODD symptoms (Ellis and Nigg 2009). Internal consistency in the current study was 0.74 for the six-item inconsistent parenting scale (e.g., “you let your child out of a punishment early;” “you threaten to punish your child and then do not do it”). This scale was emphasized in the current study based on our prior finding (Ellis and Nigg 2009), indicating it was the scale most specific to ADHD when comorbid symptoms were held constant statistically.
Child Perception of Inter-parental Conflict
Children completed the Children’s Perception of Interparental Conflict (CPIC; Grych et al. 1992) with a staff person while the parent was absent. They were instructed to complete the measure in regard to the most familiar current relationship between parents or parental figures. Thus, the child could rate two parents living in the home, whether both biological parents (n = 347) or a biological parent and a partner (n = 73), or co-parents who were living apart if the child often observed their interactions (n = 114). If two parental-type figures that interacted with each other in the child’s presence could not be identified by the child, then the CPIC was not completed (n = 14). Although 72 of the ADHD families were separated, divorced, or single parents (compared to 32 of the non-ADHD families), in 48 of these instances the mother had a boyfriend or partner who was frequently in the home and was perceived by the child as a potential parental figure. The CPIC consists of 48 items rated on a three-point scale. Recent factor analytic empirical validation of the scale items in the current sample and a cross-validation sample indicated that the instrument’s variance is best summarized in samples of this type by four latent factors: threat to self (6 items), conflict properties (11 items), triangulation/stability (13 items), and self-blame (9 items; Nigg et al. 2009). In the current study, these were treated as manifest variables by summing the items to create each scale (after appropriate reversal of negatively-worded items). Child self-blame for marital conflict was emphasized in the current study based on results by Counts et al. (2005) which found that children’s self blame (in that study, using the closely related original self blame scale suggested by Grych and Fincham 1990) was independently associated with inattentive and hyperactive symptoms, after controlling for other associated risk factors, as well as due to theoretical considerations that a child’s perception of conflict may be more important than the objective measurement of conflict. This scale demonstrated adequate internal reliability (α = 0.81) in the current data set.
Genotyping
Buccal and salivary DNA samples were obtained from participating children. DNA samples were purified using a method by Meulenbelt et al. (1995). The DRD4 120-bp tandem repeat polymorphism was assayed according to the method of McCracken et al. (2000) with minor modifications to the amplification parameters. Genomic DNA (40 to 60 ng) was amplified using 0.5U of Taq polymerase (Invitrogen Corp., Carlsbad, CA) in standard PCR buffer consisting of 20 mM Tris HCl and 50 mM KCl, 1.5 mM MgCl2, 0.2 mM dNTPs, and 1 μM of primer (5′-GTTGTCTGTCTTTTCTCATTGTTTCCATTG-3′ and 5′-GAAGGAGCAGGCACCGTGAGC-3′). Reaction conditions consisted of an initial denaturing step at 94°C for 3 min, followed by 35 cycles at 94°C for 30 s, 61°C annealing temperature for 30 s and an extension at 72°C for 1 min, followed by a final extension step for 7 min at 72°C. Expected products sizes of 429 bp for the “short” allele and 549 bp for the “long” allele were analyzed on a 1.5% agarose gel.
Genotype frequencies were consistent with previous reports (see McCracken et al. 2000). When Mendelian errors were examined in 467 trios, only four errors (0.009%) were encountered. Within the control group, no significant deviations from the Hardy-Weinberg Equilibrium test were detected (p = 0.94). DRD4 genotype was coded into two groups. Children with the short/short and long/short genotypes were coded as “low-risk.” Children homozygous for the long allele were coded as “high risk” based on prior reports of over-transmission of this genotype to ADHD (McCracken et al. 2000), as well as current study findings that children homozygous for the long allele had marginally more ADHD symptoms (M = 20.03) than did children with the short/short (M = 18.67) and long/short genotypes (M = 16.41; F[2] = 1.97, p = 0.14).
Data Analysis
Data analysis was conducted using Mplus software package 5.1 (Muthen and Muthen 1998–2008). Robust maximum likelihood estimation (i.e., the MLR estimator) was utilized to address non-normality and outliers (Curran et al. 1996). To address the minimal missingness on the independent variables (< 5%), full information maximum likelihood estimation (i.e., FIML), a method of directly fitting models to raw data without imputing values (McCartney et al. 2006), was utilized. The presence of siblings, resulting in non-independence of data points, was addressed using the clustering feature of Mplus. It takes into account the non-independence of the data when computing test statistics and significance tests (Muthen and Muthen 1998).
To assess G x E interaction, multivariate regressions analyses were conducted in MPlus with main effects entered at step 1, and the interaction term entered at step 2. This strategy controls for gene-environment correlations by controlling for main effects and collinearity between variables (Cohen et al. 2003). To correct for multiple testing (two outcome variables per model), a modified Bonferroni correction was applied requiring p < 0.025 (p < 0.05/2 for the two ADHD symptom domains) to attain statistical significance. Because we had specific, distinct hypotheses about both risk variables, we did not correct for the two risk variables tested.
Results
Preliminary Data Review
Descriptive statistics on the sample are shown in Table 1. Compared to typically developing children, children with ADHD were significantly more likely to be male, were younger, and had lower mean family income (all p < 0.05). Children with ADHD experienced higher levels of inconsistent parenting and more frequently blamed themselves for marital conflict (p < 0.01), confirming that these were viable environmental risk factors for our models based on Moffitt et al. (2005) criteria. Although children with ADHD were slightly more likely to carry the “long” allele of the DRD4 120-bp tandem repeat polymorphism than typically developing children, this difference was not significant in the current sample (p = 0.24). Although ethnicity was significantly related to DRD4 genotype (Χ 2[8] = 25.62, p < 0.01), there were no significant ethnic differences in ADHD symptoms (inattentive or hyperactive-impulsive) or ODD symptoms (F[3,415] = 1.61, p > 0.05). Although the latter finding suggested population stratification was not a major concern (per criteria of Hutchison et al. 2004), these group differences suggested that child ethnicity, age, and sex should be covaried in all analyses. There were no significant differences in inconsistent parenting or child self-blame for marital conflict, based on DRD4 risk status (all p > 0.18). Thus, gene-by-environment correlation was not considered a major confound to the planned G x E analysis. Note however that, as mentioned earlier, it was nonetheless statistically controlled.
Correlations between teacher-rated ADHD symptoms, DRD4 genotype, and environmental risk (i.e., parenting and marital conflict) are shown in Table 2 and confirm prior results. Inconsistent discipline and child self-blame for marital conflict were associated with teacher-rated ADHD symptoms, in the expected direction (all p < 0.01). DRD4 did not exhibit significant associations with teacher-rated ADHD symptoms (p = 0.21) in this data set.
Question 1: Do DRD4 and Inconsistent Parenting Interact?
In multivariate regression analyses that controlled for main effects and for the correlation between genotype and family risk, the interaction between DRD4 genotype and inconsistent parenting was significant in predicting inattentive ADHD symptoms (standardized estimate = 0.32, t = 2.26; p < 0.025; R 2 = 0.04), but not hyperactive-impulsive ADHD symptoms (estimate = 0.26, t = 1.84; p = 0.07; R 2 = 0.03). The interaction was also significant for total ADHD symptoms (estimate = 0.32, t = 2.34; p < 0.025; R 2 = 0.04) and ODD symptoms (estimate = 0.31, t = 2.46; p < 0.025; R 2 = 0.02). The relation between inconsistent parenting and inattention, total ADHD symptoms, and ODD symptoms was somewhat stronger for those homozygous for the long allele of DRD4 versus the other genotypes (as shown in Fig. 1).
The interactive effect for inattentive and oppositional-defiant symptoms did not hold when controlling for the alternate symptom domain, suggesting that the effect generalized across behavior problems, rather than being specific to a particular symptom domain. Although the interaction in predicting total ADHD symptoms remained significant controlling for child age, sex, and ethnicity (p = 0.04), the interaction for inattentive symptoms dropped to nonsignificant (p = 0.08).
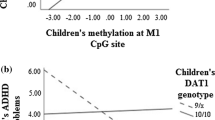
Question 2: Do DRD4 and Child Self-Blame for Marital Conflict Interact?
Using the same modeling logic detailed above, we examined the interaction between DRD4 and child self-blame for marital conflict. The interaction was statistically significant in predicting inattentive ADHD symptoms (estimate = 0.47, t = 2.82; p < 0.01; R 2 = 0.07), but not hyperactive-impulsive ADHD symptoms (estimate = 0.07, t = 0.40; p = 0.69; R 2 = 0.04), total ADHD symptoms (estimate = 0.31, t = 1.76; p = 0.08; R 2 = 0.05), or ODD symptoms (estimate = 0.31, t = 1.66; p = 0.10; R 2 = 0.03). The interactive effect for inattentive symptoms remained significant when controlling for hyperactive-impulsive and oppositional-defiant symptoms (p < 0.01), as well as child age, sex, and ethnicity (p < 0.05).
As shown in Fig. 2, the relation between child self-blame and inattentive ADHD symptoms appears to vary based on children’s DRD4 genotype. Homozygosity for the long allele of the DRD4 genotype appears to be associated with more symptoms of inattention in the presence of high child self-blame for marital conflict, but with relatively fewer symptoms of inattention in the presence of lower levels of child self-blame for marital conflict.
Discussion
Using a strategic approach to examining G x E interactions related to ADHD in children, it was hypothesized that interactions between DRD4 and family processes would be involved in ADHD, due to the importance of dopaminergic neurotransmission in the encoding of environmental contingencies related to inconsistent parenting and child perception of marital conflict. The main finding was that DRD4 “risk” status (e.g., those homozygous for the long allele of the promoter polymorphism) made children differentially susceptible to self blame related to marital conflict. That is, for children with two long alleles, more child self-blame was associated with more inattentive symptoms and less self-blame was associated with relatively fewer inattentive symptoms, an effect that was specific to ADHD inattention versus overlapping externalizing symptoms. A secondary finding was that DRD4 genotype was associated with more symptoms of ADHD and ODD only in the presence of inconsistent parenting, an effect that generalized across most disruptive behavior domains. Thus, the current study provides novel findings of G x E interaction in ADHD. The results are consistent with a theory of regulation breakdown via the interaction between genes influencing brain neuromodulatory systems in prefrontal cortex (where DRD4 is widely expressed) and socially-mediated family processes on which the development of self regulation is presumed to depend. Since the DRD4 120-bp repeat insertion allele appears to play a role in transcriptional regulation of the DRD4 gene (Kereszturi et al. 2007), it is an attractive candidate for G x E effects between genes related to dopaminergic function and broad environmental factors such as parenting (Swanson et al. 2000).
Recently, there has been renewed interest in environmental risk factors for ADHD due to the possibility of the influence of G x E interactions in this highly heritable disorder (Banerjee et al. 2007; Faraone et al. 2005; Nigg 2006; Purcell 2002). Empirical work has highlighted the importance of family characteristics and process (Campbell et al. 2000; Counts et al. 2005; Ellis and Nigg 2009). In that self-regulation develops through extensive interchange between child and family from the earliest years and ADHD emerges developmentally in early childhood (Task Force on Research Diagnostic Criteria: Infancy and Preschool 2003), the family likely serves as an important proximal environment through which genetic risk is realized.
Genetic liability can increase vulnerability to environmental threats or increase susceptibility to the environment more generally, magnifying both positive and negative influences (Belsky et al. 2009). Some evidence for both kinds of effects emerged here. The DRD4 marker appeared to exhibit somewhat different kinds of interactive effects based on the specific type of family-level environmental influence. Homozygosity for the DRD4 120-bp tandem repeat insertion allele appeared to increase vulnerability for ADHD and ODD in the presence of inconsistent parenting, but appeared to make children differentially susceptible to the effects of self-blame for marital conflict on inattentive ADHD symptoms. The importance of this distinction should be further explored as the graphical depiction of the interactions in the current study appeared somewhat similar to one another. Specificity of effects of G x E on externalizing symptoms domains were illustrative. In line with previous work establishing inconsistent parenting as a general risk factor for childhood disruptive behaviors (Burt et al. 2005a; Pfiffner et al. 2005), G x E interactions involving inconsistent parenting exhibited general effects across all disruptive behavior domains, including ADHD and ODD. However, G x E involving child self blame for marital conflict exhibited more specific effects on inattentive ADHD symptoms, suggesting that dopaminergic genetic risk may increase risk more specifically for inattention and, more speculatively, associated internalizing problems (Milich et al. 2001) via exposure to prominent psychosocial stressors like marital conflict. It is important to note that both inconsistent parenting and children’s appraisals of self-blame are likely complex proximal processes that are themselves influenced by both genetic/temperament factors as well as by the environment. Empirical work has shown modest genetic influences on reports of self-blame and parenting style (Nikolas et al. under review; Towers et al. 2001), and recent reviews have indicated that many “environmental” risk factors associated with psychopathology are influenced, in part, by genetic factors (Kendler and Baker 2007). However, this does not contradict the possibility that inconsistent parenting and self-blame may be important psychosocial exposures that increase risk for ADHD via main effects or via moderation of genetic influences on ADHD.
Genes underlying dopaminergic function serve as good candidates for modulators of family processes due to their importance in the brain’s higher level regulatory networks. Dopamine enhances responsivity to personally significant signals from the environment (Holroyd and Coles 2002). As a result, a change in dopamine neurotransmitter levels would be likely to indirectly influence a child’s response to personally important environmental factors like parenting or interparental conflict. Thus, DRD4 may interact with marital conflict or inconsistent parenting to increase susceptibility to ADHD by influencing sensitivity to broad-based environmental context and an individual’s vulnerability to maladjustment in the face of uncertainty about environmental contingencies (Mill and Petronis 2008).
Although G x E can appear artifactually in the presence of gene-environment correlations, the present findings were not explained by gene-environment correlations involving DRD4 and our family environment variables. We cannot ensure, however, that other unmeasured genetic markers would show relations with inconsistent parenting, self-blame, and ADHD. The current study results should be considered in light of other limitations. A candidate gene approach was employed based on our theoretical approach, but other genes not studied may carry larger or more important effects, and epigenetic effects are an important direction for future work. The same holds for the environmental (family) measures, which were narrowly construed and might be more powerful with the consideration of additional measures. Parenting and interparental conflict were measured using questionnaires; other measurement approaches might be superior. It is unclear whether child self blame for interparental conflict should be considered a family effect or a child personality effect. Initial behavioral genetic work examining self-blame specifically showed that non-shared environmental factors accounted for the majority of the variance (i.e., 72%) in self-blame appraisals (Nikolas et al. under review). Yet, further work examining the child-specific and family-wide effects of these appraisals is needed. Additionally, the sample covered a large age range. G x E effects may emerge as more pronounced depending on developmental stage, a possibility that would be important to examine in a larger sample or with a more narrowly restricted age range. False positive findings are always a concern in genetic studies; although replication is needed, the present results provide an important next step in evaluating specific family measures in relation to G x E. They are also consistent with prior G x E studies of ADHD examining other psychosocial measures (e.g., Pennington et al. 2009). Finally, causality or direction of effects cannot be assessed in a cross-sectional study.
In conclusion, homozygosity for the DRD4 120-bp tandem repeat insertion allele interacted with child self-blame for marital conflict and inconsistent parenting to increase susceptibility to inattention and ADHD. DRD4 exerted more potent effects on ADHD symptoms in the presence of environmental risk factors. Results are consistent with the supposition that DRD4 and family environmental processes interact to shape the development of self-regulation abilities related to ADHD. Inconsistent parenting and child attributions about interparental conflict are important environmental variables to consider in future work examining G x E interactions in ADHD.
References
Arnsten, A. F., & Goldman-Rakic, P. S. (1998). Nose impairs prefrontal cognitive function in monkeys: Evidence for a hyperdopaminergic mechanism. Archives of General Psychiatry, 55, 362–368.
Bakermans-Kranenburg, M. J., van IJzendoorn, M. H., Pijlman, F. T. A., Mesman, J., & Juffer, F. (2008). Experimental evidence for differential susceptibility: dopamine D4 receptor polymorphism (DRD4 VNTR) moderates intervention effects on toddlers’ externalizing behavior in a randomized controlled trial. Developmental Psychology, 44(1), 293–300.
Banerjee, T. D., Middleton, F., & Faraone, S. V. (2007). Environmental risk factors for attention-deficit hyperactivity disorder. Acta Paediatrica, 96, 1269–1274.
Barkley, R. A. (2006). Attention-deficit hyperactivity disorder: A handbook for diagnosis and treatment (3rd Ed.). New York: Guilford Press.
Belsky, J., Jonassaint, C., Pluess, M., Stanton, M., Brummett, B., & Williams, R. (2009). Vulnerability genes or plasticity genes. Molecular Psychiatry, 1–9.
Burt, S. A., McGue, M., Krueger, R. F., & Iacono, W. G. (2005a). How are parent-child conflict and childhood externalizing symptoms related over time? Results from a genetically informative cross-lagged study. Development and Psychopathology, 17, 145–165.
Burt, S. A., McGue, M., Krueger, R. F., & Iacono, W. G. (2005b). Sources of covariation among the child externalizing disorders: informant effects and the shared environment. Psychological Medicine, 35, 1133–1144.
Campbell, S. B., Shaw, D. S., & Gilliom, M. (2000). Early externalizing behavior problems: toddlers and preschoolers at risk for later maladjustment. Development and Psychopathology, 12, 467–488.
Cohen, J., Cohen, P., West, S. G., & Aiken, L. S. (2003). Applied multiple regression/correlation analysis for the behavioral sciences (3rd ed.). Mahwah: Lawrence Erlbaum Associates.
Coolidge, F. L., Thede, L. L., & Young, S. E. (2000). Heritability and the comorbidity of attention deficit hyperactivity disorder with behavioral disorders and executive function deficits: a preliminary investigation. Developmental Neuropsychology, 17(3), 273–287.
Counts, C. A., Nigg, J. T., Stawicki, J. A., Rappley, M. D., & von Eye, A. (2005). Family adversity in DSM-IV ADHD combined and inattentive subtypes and associated disruptive behavior problems. Journal of the American Academy of Child and Adolescent Psychiatry, 44(7), 690–698.
Cummings, E. M., Davies, P. T., & Campbell, S. B. (2002). Developmental psychopathology and family process: Theory, research, and clinical implications. New York: Guilford.
Curran, S. G., West, S. G., & Finch, J. F. (1996). The robustness of test statistics to nonnormality and specification error in confirmatory factor analysis. Psychological Methods, 1(1), 16–29.
Diamond, A. (2007). Consequences of variations in genes that affect dopamine in the prefrontal cortex. Cerebral Cortex, 17, 1161–i170.
DuPaul, G. J., Power, T. J., Anastopolous, A. D., & Reid, R. (1998). ADHD rating scale—IV: Checklists, norms, & clinical interpretation.
Ellis, B., & Nigg, J. (2009). Parenting practices and attention-deficit/hyperactivity disorder: new findings suggest partial specificity of effects. Journal of the American Academy of Child & Adolescent Psychiatry, 48(2), 1–9.
Essau, C. A., Sasagawa, S., & Frick, P. J. (2006). Psychometric properties of the Alabama Parenting Questionnaire. Journal of Child and Family Studies, 15(5), 597–616.
Faraone, S. V., Perlis, R. H., Doyle, A. E., Smoller, J. W., Goralnick, J. J., Holmgren, M. A., et al. (2005). Molecular genetics of attention-deficit/hyperactivity disorder. Biological Psychiatry, 57(11), 1313–1323.
Ficks, C. A., & Waldman, I. D. (2009). Gene-environment interactions in attention-deficit/hyperactivity disorder. Current Psychiatry Reports, 11(5), 387–392.
Fosco, G. M., & Grych, J. H. (2008). Emotional, cognitive, and family systems mediators of children’s adjustment to interparental conflict. Journal of Family Psychology, 22(6), 843–854.
Gizer, I. R., Ficks, C., & Waldman, I. D. (2009). Candidate gene studies of ADHD: a meta-analytic review. Human Genetics, 126, 51–90.
Goldsmith, H. H., Gottesman, I. I., & Lemery, K. S. (1997). Epigenetic approaches to developmental psychopathology. Development and Psychopathology, 9, 365–387.
Grych, J. H., & Fincham, F. D. (1990). Marital conflict and children’s adjustment: a cognitive-contextual framework. Psychological Bulletin, 108(2), 267–290.
Grych, J. H., Seid, M., & Fincham, F. D. (1992). Assessing marital conflict from the child’s perspective: the children’s perception of interparental conflict scale. Child Development, 63, 558–572.
Grych, J. H., Fincham, F. D., Jouriles, E. N., & McDonald, R. N. (2000). Interparental conflict and child adjustment: testing the mediational role of appraisals in the cognitive-contextual framework. Child Development, 71, 1648–1661.
Grych, J. H., Harold, G., & Miles, C. (2003). A prospective investigation of appraisals as mediators of the link between interparental conflict and child adjustment. Child Development, 74, 1176–1193.
Holroyd, C. B., & Coles, M. G. H. (2002). The neural basis of human error processing: reinforcement learning, dopamine, and error-related negativity. Psychological Review, 109(4), 679–709.
Hutchison, K. E., Stallings, M., McGeary, J., & Bryan, A. (2004). Population stratification in the candidate gene study: fatal flaw or red herring? Psychological Bulletin, 130(1), 66–79.
Jester, J. M., Nigg, J. T., Adams, K., Fitzgerald, H. E., Puttler, L. I., Wong, M. M., et al. (2005). Inattention/hyperactivity and aggression from early childhood to adolescence: heterogeneity of trajectories and differential influence of family environment characteristics. Development and Psychopathology, 17, 99–125.
Kendler, K. S., & Baker, J. H. (2007). Genetic influences on measures of the environment: a systematic review. Psychological Medicine, 37, 615–626.
Kereszturi, E., Kiraly, O., Csapo, Z., Tarnok, Z., Gadoros, J., Sasvari-Szekely, M., et al. (2007). Association between the 120-bp duplication of the dopamine D4 receptor gene and attetnion deficit hyperactivity disorder: genetic and molecular analyses. American Journal of Medical Genetics Part B (Neuropsychiatric Genetics), 144B, 231–236.
Kuntsi, J., Neale, B. M., Chen, W., Faraone, S. V., & Asherson, P. (2006). The IMAGE project: methodological issues for the molecular genetic analysis of ADHD. Behavioral and Brain Functions, 2, 1–13.
Lasky-Su, J., Faraone, S. V., Lange, C., Tsuang, M. T., Doyle, A. E., Smoller, J. W., et al. (2007). A study of how socioeconomic status moderates the relationship bewteen SNPs encompassing BDNF and ADHD symptom counts in ADHD families. Behavior Genetics, 37, 487–497.
Levy, F., Hay, D. A., McStephen, M., Wood, C. H., & Waldman, I. (1997). Attention-deficit/hyperactivity disorder: a category or a continuum? Genetic analysis of a large-scale twin study. American Academy of Child & Adolescent Psychiatry, 36(6), 737–744.
McCartney, K., Burchinal, M. R., & Bub, K. L. (2006). Best practices in quantitative methods for developmentalists. Monographs of the Society for Research in Child Development, 71(3).
McCracken, J. T., Smalley, S. L., McGough, J. J., Crawford, L., Del’Homme, M., Cantor, R. M., et al. (2000). Evidence for linkage of a tandem duplication polymorphism upstream of the dopamine D4 receptor gene (DRD4) with attention deficit hyperactivity disorder (ADHD). Molecular Psychiatry, 5(5), 531–536.
Meulenbelt, I., Droog, S., Trommelen, G. J., Boomsa, D. I., & Slagboom, P. (1995). High-yield non-invasive human genomic DNA isolation method for genetic studies in geographically dispersed families and populations. American Journal of Human Genetics, 57, 1252–1254.
Milich, R., Balentine, A. C., & Lynam, D. R. (2001). ADHD combined type and ADHD predominantly inattentive type are distinct and unrelated disorders. Clinical Psychology: Science and Practice, 8(4), 463–488.
Mill, J., & Petronis, A. (2008). Pre- and peri-natal environmental risks for attention-deficit hyperactivity disorder (ADHD): the potential role for epigenetic processes in mediating susceptibility. Journal of Child Psychology and Psychiatry, 49(10), 1020–1030.
Moffitt, T. E., Caspi, A., & Rutter, M. (2005). Strategy for investigating interactions between measured genes and measured environments. Archives of General Psychiatry, 62, 473–481.
Muthen, L. K., & Muthen, B. O. (1998-2008). Mplus user’s guide (5th ed.). Los Angeles: Muthen & Muthen.
Nigg, J. T. (2006). What causes ADHD? Understanding what goes wrong and why. New York: Guilford.
Nigg, J. T., & Casey, B. J. (2005). An integrative theory of attention-deficit/hyperactivity disorder based on the cognitive and affective neurosciences. Development & Psychopathology, 17(3), 785–806.
Nigg, J. T., Hinshaw, S. P., & Huang-Pollock, C. (2006). Disorders of attention and impulse regulation. In D. Cicchetti & D. J. Cohen (Eds.), Developmental psychopathology, vol.3: Risk, disorder, and adaptation (2nd ed., pp. 358–403). Hoboken: John Wiley and Sons.
Nigg, J. T., Nikolas, M., Miller, T., Burt, S. A., Klump, K. L., & von Eye, A. (2009). Factor structure of the child perception of marital conflict scale for studies of youth with externalizing behavior problems. Psychological Assessment, 21(3), 450–456.
Nigg, J. T., Nikolas, M., & Burt, S. A. (2010). Measured gene by environment interaction in relation to Attention-Deficit/Hyperactivity Disorder (ADHD). Journal of the American Academy of Child and Adolescent Psychiatry.
Nikolas, M., Friderici, K., Waldman, I., Jernigan, K., & Nigg, J. T. (2010). Gene x environment interactions for ADHD: synergistic effect of 5HTTLPR genotype and youth appraisals of inter- parental conflict. Behavioral and Brain Functions, 6, 23–37.
Pennington, B. F., McGrath, L. M., Rosenberg, J., Barnard, H., Smith, S. D., Willcutt, E. G., et al. (2009). Gene X environment interactions in reading disability and attention-deficit/hyperactivity disorder. Developmental Psychology, 45(1), 77–89.
Pfiffner, L. J., McBurnett, K., Rathouz, P. J., & Judice, S. (2005). Family correlates of oppositional and conduct disorders in children with attention deficit/hyperactivity disorder. Journal of Abnormal Child Psychology, 33(5), 551–563.
Propper, C., Willoughby, M., Halpern, C. T., Carbone, M. A., & Cox, M. (2007). Parenting quality, DRD4, and the prediction of externalizing and internalizing behaviors in early childhood. Developmental Psychobiology, 49, 619–632.
Puig-Antich, J., & Ryan, N. (1986). Kiddie schedule for affective disorders and schizophrenia. Pittsburgh: Western Psychiatric Institute.
Purcell, S. (2002). Variance components models for gene-environment interaction in twin analysis. Twin Research, 5(6), 554–571.
Rhoades, K. A. (2008). Children’s responses to interparental conflict: a meta-analysis of their associations with child adjustment. Child Development, 79, 1942–1956.
Sagvolden, T., Aase, H., Johansen, E. B., & Russell, V. A. (2005). A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behavioral and Brain Sciences, 28(3), 397–468.
Seaman, M. I., Fisher, J. B., Chang, F., & Kidd, K. D. (1999). Tandem duplication polymorphism upstream of the dopamine D4 receptor gene (DRD4). American Journal of Medical Genetics, 88, 705–709.
Shaffer, D., Fisher, P., Lucas, C., Dulcan, M. K., & Schwab-Stone, M. (2000). NIMH diagnostic interview schedule for children, version IV (NIMH DISC-IV): description, differences from previous versions and reliability of some common diagnoses. Journal of American Academy of Child and Adolescent Psychiatry, 39, 28–38.
Sonuga-Barke, E. J. S. (2010). ‘It’s the environment stupid!’ On epigenetics, programming, and plasticity in child mental health. Journal of Child Psychology and Psychiatry, 51(2), 113–115.
Sonuga-Barke, E. J. S., Oades, R. D., Psychogiou, L., Chen, W., Franke, B., Buitelaar, J. et al. (2009). Dopamine and serotonin transporter genotypes moderate sensitivity to maternal expressed emotion: The case of conduct and emotional problems in attention deficit/hyperactivity disorder. Journal of Child Psychology and Psychiatry.
Swanson, J. M. (1992). School-based assessments and interventions for ADD students. Irvine: KC.
Swanson, J. M., Flodman, P., Kennedy, J., Spence, M. A., Moyzis, R., Schuck, S., et al. (2000). Dopamine genes and ADHD. Neuroscience and Biobehavioral Reviews, 24, 21–25.
Task Force on Research Diagnostic Criteria: Infancy and Preschool. (2003). Research diagnostic criteria for infants and preschool children: the process and empirical support. Journal of the American Academy of Child and Adolescent Psychiatry, 42(12), 1504–1512.
Towers, H., Spotts, E. L., & Neiderhiser, J. (2001). Genetic and environmental influences on parenting and marital relationships: Current findings and future directions. In K. Deater-Deckard & S. Petrill (Eds.), Gene-environment processes in social behaviors and relationships (pp. 11–29). London: Haworth.
Treister, R., Pud, D., Ebstein, R. P., Laiba, E., Gershon, E., Haddad, M., et al. (2009). Assocations between polymorphisms in dopamine neurotransmitter pathway genes and pain response in healthy humans. Pain, 147, 187–193.
Tuvblad, C., Zheng, M., Raine, A., & Baker, L. A. (2009). A common genetic factor explains the covariation among ADHD, ODD, and CD symptoms in 9–10 year old boys and girls. Journal of Abnormal Child Psychology, 37, 153–167.
Waldman, I. D. (2007). Gene-environment interactions reexamined: does mother’s marital stability interact with the dopamine receptor D2 gene in the etiology of childhood attention-deficit/hyperactivity disorder? Development and Psychopathology, 19, 1117–1128.
Waldman, I. D., & Gizer, I. R. (2006). The genetics of attention deficit hyperactivity disorder. Clinical Psychology Review, 26, 396–432.
Waldman, I. D., Rhee, S. H., Levy, F., & Hay, D. A. (2001). Genetic and environmental influences on the covariation among symptoms of attention deficit hyperactivity disorder, oppositional defiant disorder, and conduct disorder. In D. A. Hay & F. Levy (Eds.), Attention, genes and ADHD. East Sussex: Brunner-Routledge.
Wechsler, D. (1991). Wechsler intelligence scale for children, third edition: Administration and scoring manual. New York: Psychological Corporation.
Wechsler, D. (2003). Wechsler intelligence scale for children, fourth edition: Administration and scoring manual. San Antonio: Psychological Corporation.
White, M. J., Lawford, B. R., Morris, C. P., & Young, R. M. (2009). Interaction between DRD2 C957T polymorphism and an acute psychosocial stressor on reward-related behavioral impulsivity. Behavior Genetics, 39, 285–295.
Wymbs, B. T., Pelham, W. E., Molina, B. S. G., & Gnagy, E. M. (2008). Mother and adolescent reports of interparental discord among parents of adolescents with and without attention-deficit/hyperactivity disorder. Journal of Emotional and Behavioral Disorders, 16, 29–41.
Acknowledgements
This research was supported by NIH National Institute of Mental Health Grants R01-MH63146, MH59105, and MH70542. We are indebted to the families and staff who made this study possible.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martel, M.M., Nikolas, M., Jernigan, K. et al. The Dopamine Receptor D4 Gene (DRD4) Moderates Family Environmental Effects on ADHD. J Abnorm Child Psychol 39, 1–10 (2011). https://doi.org/10.1007/s10802-010-9439-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10802-010-9439-5