Abstract
Disruptions to hypothalamic-pituitary-adrenal (HPA) axis function have been associated with varying forms of psychopathology in children. Studies suggesting children with ADHD have blunted HPA function have been complicated by the prevalence of comorbid diagnoses and heterogeneity of ADHD. The goals of this research were to assess the relations between waking and stress–response salivary cortisol levels and comorbid disruptive behavior (DBD) and anxiety (AnxD) disorders and problems in boys with ADHD, and to examine whether cortisol levels varied across ADHD subtypes. One hundred seventy elementary school-age boys with ADHD provided salivary cortisol at waking and in reaction to venipuncture. Parent reports were used to assess boys’ psychiatric diagnoses and severity of behavioral problems. Boys’ comorbid AnxD and anxiety problems were associated with greater cortisol reactivity, whereas boys’ comorbid DBD and oppositional problems predicted diminished adrenocortical activity. Reactive cortisol increases were greatest in boys with ADHD and comorbid AnxD, but without DBD. ADHD subtypes were not differentially associated with waking, pre-stress baseline, or reactive cortisol levels. However, comorbid DBD predicted decreased cortisol reactivity in boys with inattentive and hyperactive subtypes of ADHD, but not in boys with combined subtype of ADHD. The results clarify previous patterns of distinct and divergent dysregulations of HPA function associated with boys’ varying kinds of psychopathology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Attention-deficit/hyperactivity disorder (ADHD) is one of the most prevalent forms of childhood psychopathology, accounting for up to 50% of referrals of children to mental health clinics (Waschbush et al. 2002). ADHD is a heterogeneous disorder, as children may present symptoms of inattentiveness, symptoms of hyperactivity-impulsivity, or a combination of both (American Psychiatric Association 2000). Further, many children with ADHD manifest comorbid disruptive behavior and anxiety problems (Connor et al. 2003; Pliszka 2000). As comorbidity exacerbates the persistence and adverse outcomes of childhood psychopathology (e.g., Capaldi 1992), further research into the processes that may contribute to comorbidity is needed (Lilienfeld 2003). Examining the etiology and nature of ADHD and its comorbid conditions, including underlying physiological processes, is necessary to improve the efficacy of treatments. Interpretation of past studies is challenging, however, as physiological correlates might vary across subtypes of ADHD or its comorbid conditions, and few samples have been large enough to support examination of phenotypic variability. This study addressed these issues by examining waking and reactive salivary cortisol levels as indicators of hypothalamic-pituitary-adrenal (HPA) axis regulation in a large sample of boys with ADHD, in order to assess potential differences in HPA function across comorbid conditions and across ADHD subtypes.
ADHD and Stress Reactivity
Poor response inhibition is a central feature of ADHD (Barkley 1997; Barkley et al. 2002), and is thought to reflect an under-active Behavioral Inhibition System (BIS; Gray 1982). The BIS, which serves to halt ongoing activities and activate withdrawal mechanisms, involves neurocircuitry incorporating the frontal cortex, septohippocampal system, locus ceruleus, raphe nucleus, hypothalamus and amygdala (Hirshfeld-Becker et al. 2004). Suboptimal BIS activity fails to support normal, adaptive arousal responses or stress reactivity, resulting in diminished responses to punishment and fear stimuli (Quay 1997). Heightened physiological activity in response to an environmental challenge is a somatic cue for the salience of the event and the need for a response (Cacioppo et al. 1993). Appropriate arousal supports behavioral responses to effectively deal with the situation, including increased vigilance and focused attention (Öhman 1993). Children who display maladaptive behavior patterns could be experiencing physiological arousal that is inappropriate or dysfunctional (Frijda 1994). Should children with ADHD manifest diminished physiological arousal or reactivity to stressors, this could indicate an under-active BIS contributing to their attentional and behavioral difficulties.
The HPA axis is one of the body’s principal stress–response and regulation systems, producing a cascade of hormones resulting in cortisol secretion from the adrenal glands. Unbound, biologically active cortisol circulates in serum, is excreted into saliva and urine, and is capable of crossing the blood-brain barrier (Lovallo and Thomas 2000). The effects of cortisol on neurophysiological activity are subject to the differential distributions of the two major types of glucocorticoid (GC) receptors. Mineralocorticoid receptors (MR) are mostly located in the hippocampus and limbic structures, and glucocorticoid receptors (GR) are located in those regions and also in the prefrontal cortex (PFC) area of the frontal lobes (Patel et al. 2000; Sanchez et al. 2000), which are part of the functional network of BIS (Hirshfeld-Becker et al. 2004). MR have higher binding affinity for cortisol than GR, and for most of the circadian cycle the majority of MRs are occupied. At peak moments of circadian levels, such as the waking period, and under conditions of acute stress and consequent reactive cortisol secretion, MR are saturated and therefore the proportion of cortisol binding to GR increases (Reul and de Kloet 1985). Under conditions of challenge or stress, cortisol binding to GC receptors in prefrontal regions supports normative and adaptive orientation and arousal. Structural and functional abnormalities in these prefrontal regions have been identified in children with ADHD and linked to their difficulties with response inhibition (Castellanos et al. 2003; Hendren et al. 2000). The distribution of GC receptors in prefrontal regions of the brain implicates HPA function in the neurophysiology of response inhibition. Thus, lowered circulating cortisol in response to stress might play a role in the maladaptive behaviours of children with ADHD.
Evidence of a possible link between HPA dysfunction and ADHD emerges from research examining whether children with ADHD manifest lower basal cortisol levels and weaker acute HPA reactivity to stressors (e.g., Hong et al. 2003). Some studies have shown such negative relations between ADHD symptoms or diagnoses and cortisol levels (e.g., Kaneko et al. 1993; Randazzo et al. 2008). However, other investigators have found positive relations between ADHD and cortisol (e.g., Sondeijker et al. 2007; White and Mulligan 2005) or comparable cortisol levels in children with and without ADHD (e.g., Snoek et al. 2004). This inconsistency across studies is further complicated by findings that the relations between ADHD and cortisol vary across sexes, age groups, and methods of inducing adrenocortical reactivity (e.g., Klimes-Dougan et al. 2001; Sondeijker et al. 2007).
ADHD, Comorbidity, and the HPA axis
The presence of comorbid problems also might obscure specific associations between inhibitory physiology and ADHD (Vaessen and Van der Meere 1990). Children with ADHD often manifest other difficulties that cut across the externalizing and internalizing spectrums (Connor et al. 2003), and which are themselves associated with disruptions in BIS and stress reactivity. One dominant model of disruptive behavior disorders (DBD), which include oppositional defiant disorder (ODD) and conduct disorder (CD), is that children with DBD have deficient or under-active BIS (Fowles 1988; Raine 1997). Youths with DBD or externalizing problems tend to experience lower physiological arousal in challenging situations (Snoek et al. 2004). Conversely, children and youths with anxiety disorders (AnxD) or internalizing problems typically have been found to show patterns of heightened arousal to challenges (Hastings et al. 2007). Their strong reactivity or inability to regulate arousal overwhelms their capacity to adapt effectively to challenging situations (Thompson and Calkins 1996). Given the connections of these multiple problems to a common underlying substrate of regulation (Hastings et al. 2007), clarifying the relations between ADHD and stress reactivity necessitates accounting for children’s profiles of comorbid diagnoses and problems.
The high comorbidity between ADHD and DBD (30–50%; Lynam 1996) has led some researchers to examine HPA function in children with varying combinations of these diagnoses (e.g., Jansen et al. 1999). Some studies have shown that children with ADHD have normative baseline or reactive cortisol levels unless they also have comorbid DBD (Snoek et al. 2004). Children with DBD have been shown to manifest lower waking or baseline cortisol levels (McBurnett et al. 2000), and smaller cortisol increases following challenges as compared to children without DBD (Snoek et al. 2004; for a recent review see van Goozen et al. 2007). According to the BIS model, threats or challenges produce weak biological arousal in children with DBD that does not evoke a fear response, and is insufficient to stop the children from engaging in dangerous, disruptive and aggressive acts. Supporting this, comorbid AnxD or sub-clinical anxiety problems in children with DBD are associated with stronger HPA reactivity to stressors (McBurnett et al. 1991; Van Goozen et al. 1998). There are inconsistencies across studies, however. Some researchers have failed to find that children with DBD or externalizing problems have lower waking, basal or reactive cortisol (Jansen et al. 1999), and some have even found positive associations (McBurnett et al. 2005).
ADHD and AnxD or anxiety problems also are often comorbid (Connor et al. 2003), but few studies of HPA function have included enough children with comorbid ADHD and AnxD to examine their combined presence in relation to cortisol (Hong et al. 2003). The physiological underpinnings of AnxD typically are portrayed as the opposite of DBD, and children with AnxD or anxiety problems tend to show exaggerated cortisol stress responses (Watamura et al. 2003). Again, though, results are not consistent across studies. Klimes-Dougan and colleagues (2001) found that young adolescents who showed decreasing cortisol levels to a parent conflict procedure had more anxiety and ADHD symptoms, whereas increasing cortisol levels to anxiety provocations were associated only with anxiety symptoms. Just as the absence versus presence of comorbid AnxD changes the association of CD with cortisol levels, so too might reactive cortisol levels differ between children with ADHD who do versus do not have comorbid AnxD.
Hyperactivity, Inattention and the HPA Axis
The heterogeneity of ADHD itself might also contribute to the complexity of findings. Treating ADHD as a global construct implicitly assumes that common processes underlie the varying subtypes. Alternatively, variations in children’s manifestations of symptoms might be related to differences in their baseline and reactive physiology. Barkley (2001) has argued that the absence of hyperactivity makes ADHD-PI (predominantly inattentive) a different disorder than ADHD-C (combined) or ADHD-HI (hyperactivity-impulsivity). Under-active BIS might only be characteristic of the latter two subtypes (Quay 1997). Analogously, attention deficits and hyperactivity-impulsivity could be two distinct disorders that are often comorbid (Hartman et al. 2004), contributing to differences in pathophysiology across the subtypes.
Few studies have compared psychophysiological functioning across ADHD subtypes. The most consistent neurophysiological evidence for decreased BIS functionality has been found for ADHD-C (Loo and Barkley 2005), or ADHD with more severe hyperactivity (Kaneko et al. 1993). However, one recent study found that children with ADHD-PI also show decreased HPA reactivity relative to comparison children (Randazzo et al. 2008). Thus, evidence is inconsistent regarding the extent to which ADHD subtypes share similar physiological correlates. Further study of HPA function across ADHD subtypes is warranted, as identifying distinct physiological substrates accompanying symptom profiles would be consistent with proposals that multiple distinct disorders are subsumed within the broader category of ADHD.
Limitations of Past Research
Most studies of ADHD and HPA function have used small samples, giving inadequate power to detect small-to-moderate effect sizes. Thus subtle but potentially important changes in cortisol levels in response to stressors, or differences across groups, might have been missed. Large samples are needed to have reasonable power to examine possible differences in HPA activity across ADHD subtypes, and across all profiles of comorbid DBD and AnxD.
Some behavioral tasks and neuropsychological tests have not been shown to be effective in eliciting strong HPA reactivity (e.g., Jansen et al. 1999; White and Mulligan 2005). It can be difficult to elicit a reliable and robust HPA response in children (Gunnar and Vazquez 2006). For this study, we took advantage of a scheduled drawing of a blood sample within a hospital’s phlebotomy clinic to examine HPA reactivity to a powerful naturalistic stressor. Fear of needles is very common (Fassler 1985), and venipuncture produces elevations in cortisol levels (Meeran et al. 1993).
Needle puncture for blood draw or inoculation evokes elevated salivary cortisol levels in young children (e.g., Braarud and Stormark 2006; Lewis and Ramsay 1997). The average latency to peak cortisol elevation after venipuncture is 20 min (Ramsay and Lewis 2003), and the magnitude of cortisol increase is associated with behavioral distress (Lewis and Thomas 1990), providing evidence for the validity of venipuncture as a stress induction task. Expectations of pain from venipuncture incorporates learning from past experiences, and studies of affective and adrenocortical reactions to venipuncture suggest that cortisol elevations are more attributable to emotional arousal than physical stress or pain (Hubert et al. 1989). Thus, a child’s visit to a hospital’s phlebotomy clinic is likely to draw upon the BIS, and could be expected to produce fear-related arousal and an elevated stress response.
Goals and Hypotheses of this Investigation
There were two primary aims of this investigation: First, to examine how comorbid disruptive behavior and anxiety problems moderated salivary cortisol levels in boys with ADHD; and second, to assess the relations between ADHD subtype and waking and stress-response salivary cortisol levels. We studied HPA function in 170 elementary school-age boys with ADHD and varying patterns of comorbid DBD and AnxD and problems by assessing salivary cortisol levels at waking, and before and after experiencing a venipuncture stressor
Based on past studies of HPA function in children with DBD and AnxD, boys with comorbid DBD were expected to show the smallest cortisol elevation in response to venipuncture, whereas boys with comorbid AnxD were expected to show the largest cortisol elevations. Boys with ADHD and no comorbid diagnoses and boys with ADHD and both comorbid DBD and comorbid AnxD were expected to show cortisol reactivity at intermediate levels. Examinations of ADHD subtype differences in HPA function were more exploratory. Based on the limited research available, we tentatively predicted that boys with ADHD-PI subtype would show higher waking and reactive cortisol levels than boys with ADHD-HI or ADHD-C subtypes. Finally, we examined whether the presence of comorbid DBD or AnxD were differentially associated with cortisol levels in boys with varying subtypes of ADHD. Relations were expected to be consistent across analyses by discrete diagnostic categories, and by continuous indices of problem severity.
Method
This research was completed in accordance with the ethical principles of the Canadian Tri-Council Ethics Guide and the American Psychological Association, and approved by the IRBs of l’Hôpital Sainte-Justine and l’Université de Montréal.
Participants
Physicians referred families to a hyperactivity clinic at a university-based pediatric hospital if children were suspected of having ADHD. The inclusionary criterion for referral was that the primary-care physician suspected a diagnosis of ADHD in the child. The exclusionary criteria for participation were child age outside targeted range of 6 to 11 years old, IQ lower than 80, premature birth, severe learning or language retardation, neurological disease or Tourette’s syndrome as a primary diagnosis. Families were retroactively excluded if the child did not meet the criteria for diagnoses of ADHD in the Diagnostic Interview Schedule for Children (DISC-IV; Shaffer et al. 2000) administered to parents, and were judged to not have clinical-level ADHD problems by clinic staff. There were 70 families referred to the clinic who declined participation, 27 children who met exclusionary criteria and were not enrolled in the study, and 15 boys excluded because the diagnosis of ADHD was not confirmed. Receipt of services (e.g., medication titration) from the clinic was not contingent upon enrolment in the study, but about half of the boys who participated also received such services. None of the boys who received services by clinic staff were re-evaluated as not having ADHD. One hundred eighty-seven boys participated in the study. Of these, 16 were excluded from the current analyses because of prescriptions for non-stimulant medication (see Results: Covariates), and one boy was excluded because he did not provide any saliva samples.
Thus, the final sample for the current analyses included 170 boys, 59 diagnosed with ADHD-PI, 25 with ADHD-HI, and 86 with ADHD-C subtype. The boys ranged in age from 6 to 11 years (M = 8.25, SD = 1.51). Families were predominantly French Canadian (88%) and Caucasian (96%), and lower-middle to upper-middle socioeconomic status, earning an average of $61000 Cdn/year (SD = 26300). Most parents were college-educated; there were 52 mothers and 68 fathers who did not proceed past secondary school education, and 14 mothers and 12 fathers who completed graduate or professional degrees.
Measures
Parents were administered the DISC-IV to assess ADHD subtype and comorbid DBD (ODD and/or CD) and AnxD (social phobia, specific phobia, separation anxiety, agoraphobia, generalized anxiety, and/or obsessive-compulsive) that reached diagnostic levels. (No boys reached diagnostic levels for major depressive disorder or dysthymia.) A French-language version of the DISC-IV was obtained through iterative translation and back-translation by bilingual health professionals in collaboration with the DISC authors. To assess the severity of boys’ problems, parents also completed the French-language version of the Conners’ Parent Rating Scales–Revised (CPRS-R; Conners 1997). The hyperactive, inattentive, oppositional and anxious-shy problem scales were used in analyses. Boys were administered the French-language version of the WISC-III (Wechsler 1991).
Procedures
All children were assessed without stimulant or other medication. Children with an existing prescription were not given any medication on the day of testing. Prior to a scheduled family visit to the ADHD clinic, parents were instructed in how to use the salivettes (Sarstedt, Inc.) with their child. A Waking sample was collected from each child on the morning of the scheduled clinic visit, within 15 min of waking and prior to eating, drinking or brushing teeth. One hundred forty-two of the 148 waking samples brought to the clinic by parents were usable (had enough saliva to perform assays of cortisol).
Each family attended a 3-h testing session at the clinic. There were 107 (63%) families seen in the morning (9 am to 11 am start-time), and 63 (37%) seen in the afternoon (1 pm to 3 pm start time). Parents completed the DISC, CPRS-R, and demographic form in one room of the clinic, while the child was assessed in another room. The WISC-III (M IQ = 100.13, SD = 12.28, range 80–137) and other procedures were administered to the child. Two hours after arriving at the clinic, a second saliva sample was collected from the child (Pre-stress). The child was then told that a nurse needed to collect a blood sample. (The broader protocol for this study included a blood draw for the purpose of genotyping, which also provided an opportunity to examine the physiology of stress reactivity.) In accord with local IRB policy, a topical analgesic was applied to the child’s arm, which the child was told would “make the needle not hurt.” The child and interviewer then went to the phlebotomy clinic, where a nurse drew the blood. The child and interviewer returned to the ADHD clinic, and exactly 20 min after the venipuncture, a third saliva sample (Post-stress) was obtained.
Two boys refused to provide pre-stress samples; thus 168 usable pre-stress saliva samples were collected. Also, 14 boys refused the blood draw procedure and therefore post-stress samples were not collected from these boys. Of 154 post-stress saliva samples collected, 153 were usable. The 16 boys who refused to provide pre- or post-stress samples did not differ from the other children in prevalence of ADHD subtype, DBD, \({{\rm{AnxD}}{\left( {{\rm{all}}\chi ^{{\rm{2}}}_{{{\left( {\rm{2}} \right)}}} {\rm{ < 1}}{\rm{.50}}} \right)}}\), inattentive, hyperactive, oppositional or anxiety problems \(\left( {{\text{all}}\left| t \right| <1.15} \right)\).
Saliva samples were stored in a −80C medical freezer, then shipped to the Pennsylvania State University Behavioral Endocrinology Laboratory (Salimetrics™) to be thawed, centrifuged, and have 50 µl of clear samples pippetted into test-wells for enzymeimmunoassay of cortisol. All samples were tested in duplicate; samples that varied by more than 5% across duplicates were re-tested; correlation across duplicates r = 0.99. Mean values of duplicates were used in analyses, in units of microgram per deciliter (µg/dL). Raw cortisol data were leptokurtic and positively skewed, therefore log-transformations were used to establish normality. These transformations corrected the skews and eliminated outliers; thus, log-transformed data were used in analyses. Untransformed data are reported in text and Tables for ease of interpretation.
Results
Examination of Possible Covariates
Child Age
Boys with ADHD-HI were younger than boys with ADHD-PI, F(2, 167) = 3.89, p < 0.05 (see Table 1). Age was not significantly correlated with any Conners’ scores or salivary cortisol levels, \({\text{all}}\left| r \right| <0.11\), and boys with and without comorbid DBD and AnxD did not differ in age, both \(\left| t \right| <0.85\). Preliminary analyses showed that controlling for age did not affect any results. Therefore, age was not controlled in final analyses.
Stimulant Medication
There were 89 boys prescribed stimulant medication (methylphenidate hydrochloride), dosage M = 22.26 mg/day, SD = 9.52, administered on average for 5.29 days/week (SD = 1.34). These boys did not differ significantly in their waking, pre-stress, or post-stress levels of salivary cortisol, or in change in cortisol from pre- to post-stress from the 81 boys not receiving ADHD medication, \({\text{all}}\left| t \right| <0.75\). Likelihood of stimulant medication did not differ across ADHD subtypes, \({\text{ $ \chi $ }}^{2}_{{{\left( 2 \right)}}} = 2.37,{\text{ }}ns\), comorbid DBD, \({\text{ $ \chi $ }}^{2}_{{{\left( 1 \right)}}} = 0.05,{\text{ }}ns\), or comorbid AnxD, \({\text{ $ \chi $ }}^{2}_{{{\left( 1 \right)}{\text{ }}}} = 2.01,{\text{ }}ns\), nor was stimulant medication associated with severity of problems reported on the Conners’ scales, \({\text{all}}\left| t \right| <1.81\). Preliminary analyses showed that controlling for stimulant medication did not affect any results. Therefore, prescription for stimulant medication was not controlled in final analyses.
Other Medications
Sixteen boys who participated in the study were excluded from current analyses because of other medications. Ten boys had been prescribed corticosteroid medication (e.g., flovent, ventolin), and six had been prescribed various other medications (e.g., risperidone, clonidine). Preliminary analyses indicated that some results varied depending on whether these 16 boys were included or excluded, and including non-stimulant medication as a covariate did not remove the differences. Thus, these boys were excluded from all final analyses.
Times of Saliva Sampling and Hospital Visit
The HPA diurnal rhythm produces morning basal cortisol levels that are higher than afternoon basal levels. Preliminary analyses examined whether time of saliva sample collection or time of hospital visit (morning versus afternoon) were associated with cortisol levels or stress responses. Correlations between time of sampling and salivary cortisol levels were non-significant for the waking and post-stress samples, rs = −0.04 and −0.06, respectively, but cortisol levels were lower for pre-stress samples collected later in the day, r = −0.18, p < 0.05. Boys tested in the afternoon had lower pre-stress cortisol levels (M = 0.09, SD = 0.06) than boys tested in the morning (M = 0.11, SD = 0.07), t(166) = 2.43, p < 0.05. Examining boys tested in the morning versus in the afternoon separately, time of pre-stress sample was non-significantly correlated with pre-stress cortisol levels, rs = 0.11 and −0.05, respectively, indicating that each testing period was an appropriately narrow window of time. As well, neither time of pre-stress sample nor time of post-stress sample was correlated with the magnitude of cortisol response to venipuncture, ΔC, whether boys were considered together or separately by time of hospital visit, \({\text{mean}}\left| r \right| = 0.09,{\text{ range}} - 0.13{\text{ to 0}}.09\).
Boys tested in the morning versus afternoon did not differ significantly on waking cortisol levels, t(140) = 0.77. A 2 × 2 (Time of Visit by Sample) mixed ANOVA, with Sample (pre- versus post-stress) treated as a repeated measure, was used to see if boys tested in the morning versus afternoon differed in cortisol levels around the stress procedure. The main effect for Sample was significant, F(1, 150) = 106.21, p < 0.001. The post-stress salivary cortisol levels were higher than the pre-stress salivary cortisol levels, showing that venipuncture elicited a robust HPA response (M ΔC = 0.13 μg/dL, SD = 0.21); increased cortisol from pre-stress to post-stress was evident in 78.9% (N = 120) of the boys. The main effect for Time of Visit approached significance, F(1, 150) = 2.81, p < 0.10, which was due to the previously described difference in morning versus afternoon pre-stress cortisol levels. The Sample Χ Time of Testing interaction was not significant, F(1, 150) = 1.48, ns. Thus, boys tested in the morning had similar increases in salivary cortisol levels (M ΔC = 0.12, SD = 0.21) in response to venipuncture as boys tested in the afternoon (M ΔC = 0.14, SD = 0.20).
Boys tested in the morning versus afternoon were similar on CPRS-R scores and prevalence of diagnoses. The two groups did not differ on prevalence of \({\text{DBD $ \chi $ }}^{2}_{{{\left( 1 \right)}}} = 2.13,{\text{ }}ns\), ratings of problems, \({\text{all}}\left| t \right| <1.65\), or distribution of ADHD subtypes, \({\text{ $ \chi $ }}^{2}_{{{\left( 2 \right)}}} = 4.16,{\text{ }}ns\). A greater proportion of boys tested in the afternoon had AnxD (29/63) than boys tested in the morning (31/107), \({\text{ $ \chi $ }}^{2}_{{{\left( 1 \right)}}} = 4.83,{\text{ }}p < 0.05\). In the fully crossed design (Time of Testing Χ DBD Χ AnxD), all but one cell had more than 10 participants; there were 9 boys with ADHD and comorbid AnxD (without DBD) who were tested in the morning.
Because boys tested in the morning versus afternoon differed in pre-stress cortisol levels and prevalence of AnxD, time needed to be accounted for in analyses of pre-stress and post-stress cortisol, and ΔC. Therefore, the exact time of pre-stress sample collection was included as a covariate in all analyses of pre-stress and post-stress cortisol, and ΔC.
Preliminary and Descriptive Analyses
Comorbid Disorders and Problem Scores
Severity of inattentive, hyperactive, oppositional and anxiety problems were assessed from parents’ responses to the CPRS-R. Parent-reports of child functioning on the DISC were used to score the presence versus absence of each diagnostic category assessed by the DISC. Frequencies of mean CPRS-R ratings and comorbid diagnoses across ADHD subtypes are presented in Table 2. More boys with ADHD-C and fewer boys with ADHD-PI were likely to have comorbid DBD than would be expected by chance, \({\text{ $ \chi $ }}^{2}_{{{\left( 2 \right)}}} = 13.15,{\text{ }}p < 0.01\). This was driven by the presence of comorbid ODD, \({\text{ $ \chi $ }}^{2}_{{{\left( 2 \right)}}} = 14.67,{\text{ }}p < 0.01\). Neither rates of comorbid \({\text{CD}},{\text{ $ \chi $ }}^{2}_{{{\left( 2 \right)}}} = 1.00,ns\), nor rates of comorbid \({\text{AnxD}},{\text{ $ \chi $ }}^{2}_{{(2)}} = 2.30,{\text{ }}ns\), varied across ADHD subtypes.
ADHD subtypes differed on Inattentive, Hyperactive, and Oppositional CPRS-R scores, all F(2, 159) > 15.00, p < 0.001, but not Anxious-Shy problems, F(2, 159) = 1.37, ns. Follow-up Student–Newman–Keuls tests showed that parents reported more hyperactive and oppositional problems in boys with ADHD-HI and ADHD-C than in boys with ADHD-PI, but fewer inattentive problems in boys with ADHD-HI than other sub-types (see Table 2).
Aim 1: Salivary Cortisol Levels in Relation to Comorbid Diagnoses and Problems
Categorical Analyses of Comorbid DBD and AnxD
There were 50 boys with no comorbid diagnoses, 60 boys with comorbid DBD only, 22 boys with comorbid AnxD only, and 38 boys with both comorbid DBD and AnxD. A 2 × 2 (DBD by AnxD) ANOVA was performed to determine whether comorbid DBD and/or AnxD were associated with Waking cortisol levels. The main effects of DBD and AnxD, and the interaction of DBD by AnxD, were non-significant, all F(1, 138) < 0.70.
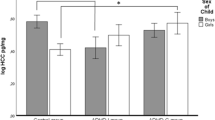
To determine whether comorbid DBD and/or AnxD were associated with HPA reactivity to venipuncture, a 2 × 2 × 2 (Sample by DBD by AnxD) mixed-design ANCOVA, with Sample treated as a repeated measure and controlling time of pre-stress sample, was performed. There were no significant main effects of comorbid diagnoses, but there were three significant interaction effects: Sample by DBD, F(1, 147) = 4.04, p < 0.05, Sample by AnxD, F(1, 147) = 4.38, p < 0.05, and Sample by DBD by AnxD, F(1, 147) = 4.59, p < 0.05 (see Fig. 1). Follow-up analyses of the significant three-way interaction revealed that, although all groups of boys showed significant elevations in cortisol from pre- to post-stress samples, all paired t > −3.80, p < 0.01, the magnitude of HPA responses (ΔC) differed, one-way F(3, 148) = 3.35, p < 0.05. Student-Newman–Keuls tests revealed that boys with only comorbid AnxD (without DBD) had significantly greater ΔC than boys in the other three groups; the latter three groups did not differ from each other significantly. (Correspondingly, the Sample by DBD interaction was driven by smaller ΔC in boys with DBD compared to boys without DBD, whereas the Sample by AnxD interaction reflected greater ΔC in boys with AnxD than in boys without AnxD).
Dimensional Analyses of Comorbid Oppositional and Anxious-Shy Problems
CPRS-R scores were examined as measures of the severity of children’s oppositional and anxious problems. Regression analyses were used to predict waking, pre-stress and reactivity cortisol levels from CPRS-R Oppositional and Anxious-Shy problem scores. Problem scores were centered prior to computing an Oppositional × Anxious-Shy interaction term, and centered scores were used in all analyses. For the prediction of waking cortisol, oppositional and anxious-shy scores were entered in Step 1, and the oppositional × anxious-shy interaction was entered in Step 2. The regression was non-significant, adj.R 2 = 0.0, F(3, 134) = 0.33, and there were no significant unique predictors, \({\text{all}}\left| \beta \right| <0.07\). Similarly, for the prediction of pre-stress cortisol, with time of pre-stress sample collection controlled in Step 1, the regression was non-significant, adj.R 2 = 0.023, F(4, 155) = 1.93, p = 0.11; only sample time predicted cortisol, β = −0.17, p < 0.05.
For the prediction of ΔC, pre-stress cortisol level and time of pre-stress sample collection were entered in Step 1, oppositional and anxious-shy scores in Step 2, and oppositional Χ anxious-shy interaction in Step 3. The regression was significant, adj.R 2 = 0.222, F(5, 140) = 9.27, p < 0.001, with three significant unique predictors: pre-stress cortisol level, β = −0.43, p < 0.001, oppositional problems, β = −0.24, p < 0.01, and anxious-shy problems, β = 0.16, p < 0.05. The severity of oppositional and anxiety problems had independent but opposite associations with the cortisol reactivity to venipuncture in boys with ADHD; boys described as more oppositional or less anxious by parents showed reduced HPA reactivity to venipuncture.
Aim 2: Salivary Cortisol Levels in Relation to ADHD Subtypes and Inattention and Hyperactivity Problems
Cortisol Levels and Reactivity across ADHD Subtypes
A one-way (ADHD Subtype) ANOVA was used to determine whether waking cortisol levels varied across ADHD subtypes (see Table 3). The effect of Subtype was not significant, F(2, 139) = 0.05, ns. A 3 × 2 (ADHD Subtype by Sample) ANCOVA was used to determine whether HPA reactivity to venipuncture varied across ADHD subtypes (see Table 3). Neither the Subtype main effect nor the Subtype by Sample interaction were significant, both F(2, 148) < 0.45. This was confirmed using a one-way (ADHD Subtype) ANCOVA on the magnitude of the stress response, ΔC. The effect of Subtype was not significant, F(2, 143) = 0.34.
Correlations between Inattentive and Hyperactive ratings and waking cortisol levels, and partial correlations controlling for time of pre-stress sample between Inattentive and Hyperactive ratings and pre-stress and post-stress cortisol levels, and ΔC, were examined to determine whether severity of ADHD was associated with salivary cortisol. The eight correlations were non-significant, \({\text{all}}\left| r \right| \leqslant 0.13\).
Exploratory Analysis: Do the Associations between Cortisol Levels and Comorbid Diagnoses and Problems differ across ADHD Subtypes?
Cortisol, ADHD Subtypes, and Comorbid Diagnoses of DBD and AnxD
Two exploratory analyses were conducted to test whether associations between comorbid DBD or AnxD and boys’ waking or reactive cortisol levels differed across ADHD Subtypes. First, waking cortisol levels were examined in a 2 × 2 × 3 (DBD by AnxD by Subtype) ANOVA. The model was limited to include only two-way interactions (DBD by AnxD, DBD by Subtype, AnxD by Subtype). All cells had at least 14 boys, with the exception of boys with ADHD-HI and AnxD (n = 6), and boys with ADHD-HI without DBD (n = 7). No effects of Subtype were significant, all F(2, 132) < 0.40.
Second, reactive cortisol levels were examined in a 2 × 2 × 2 × 3 (Sample by DBD by AnxD by Subtype) mixed-design ANCOVA. The model was limited to include only two-way interactions of the between-subjects variables (DBD by AnxD, DBD by Subtype, AnxD by Subtype). All cells had at least 15 boys, with the exception of boys with ADHD-HI and AnxD (n = 5), and boys with ADHD-HI without DBD (n = 6). Only one effect involving Subtype was significant, DBD Χ Subtype, F(2, 141) = 3.18, p < 0.05 (see Fig. 2).Footnote 1 Because this effect did not involve Sample, it suggested boys did not differ in reactivity per se, but rather in their mean pre- and post-stress samples. Higher mean cortisol levels were evident in boys with ADHD-PI alone and ADHD-HI alone, than in boys with ADHD-C alone, ADHD-PI with DBD, ADHD-HI with DBD, or ADHD-C with DBD. There were significantly higher mean cortisol levels in boys with ADHD-PI alone versus ADHD-PI with DBD, t(52) = 2.01, p = 0.05, and the comparison between boys with ADHD-HI alone versus ADHD-HI with DBD approached significance, t(19) = 1.88, p < 0.10. Conversely, mean cortisol levels were comparable in boys with ADHD-C alone versus ADHD-C with DBD, t(75) = −1.09. As well, boys with ADHD-C alone had lower mean cortisol levels than boys with ADHD-HI alone, t(31) = 2.46, p < 0.05, and boys with ADHD-PI alone, t(56) = 1.89, p < 0.10. Mean cortisol levels did not differ across ADHD subtypes in boys with comorbid DBD, \({\text{all}}\left| t \right| <1.15\).
Discussion
This investigation was conducted to determine whether comorbid disruptive behavior problems were associated with lower, and comorbid anxiety problems with higher, cortisol reactivity, and whether adrenocortical functioning differed in school-age boys with different subtypes of ADHD. Boys with comorbid AnxD or problems evidenced stronger cortisol reactivity, whereas boys with comorbid DBD or oppositional problems evidenced diminished cortisol reactivity. Boys with ADHD inattentive subtype, ADHD hyperactive-impulsive subtype, and ADHD combined subtype showed similar salivary cortisol levels at waking, prior to experiencing a stressful blood-draw procedure, and in reaction to venipuncture. There was an intriguing interaction between ADHD subtype and comorbid diagnoses, however, that indicated the presence of comorbid DBD was unrelated to cortisol levels in boys with the ADHD combined subtype. These results support developmental models of the links between dysregulated stress physiology and children’s externalizing and internalizing problems (Hastings et al. 2007), and proposals that under-active BIS functions or poor response inhibition is characteristic specifically of boys with DBD (Fowles 1988) and boys with the combined subtype of ADHD (Loo and Barkley 2005).
Our findings on the associations between boys’ comorbid conditions and cortisol levels were largely consistent with past research and serve to bolster support for the important role of HPA axis function in both DBD and AnxD, and the opposing nature of BIS dysregulation associated with these two classes of disorders. There were no robust associations between waking cortisol levels and children’s comorbid diagnoses or problems. This might reflect the fact that the relatively elevated cortisol levels observed at waking are produced by the normative diurnal cycle of the HPA axis, rather than acute responses to events (Lovallo and Thomas 2000). Compared to studies of adults, research with children and youth has not found consistent associations between waking cortisol levels and psychopathology (Klimes-Dougan et al. 2001). Future studies might benefit by undertaking a more comprehensive examination of the cortisol awakening response (CAR). In contrast to waking levels, we found that relations between cortisol reactivity and comorbid conditions were more evident. Using both diagnoses and ratings of problems, boys with ADHD who were less anxious manifested weaker adrenocortical stress responses to venipuncture than boys with ADHD who were highly anxious, provided they had no additional externalizing problems.
One question that might be posed is whether anxious boys were reacting to the fear or the pain of venipuncture. Acute pain has been found to elicit HPA arousal (e.g., Dixon et al. 2004). However, children can show cortisol elevations to invasive procedures even in the absence of observable or reported discomfort (Walco et al. 2005), and increased cortisol levels after venipuncture have been found to be more attributable to emotional than physical factors (Hubert et al. 1989). Furthermore, it is likely that the topical analgesia administered to boys in this study minimized the extent to which actual pain was experienced or contributed to stress responses. Thus, it is more probable that the cortisol elevations observed were the result of anxious boys’ anticipation and fear of potential pain. Thus, our results are consistent with developmental models identifying exaggerated arousal in response to threat as a core feature of AnxD and problems (Klimes-Dougan et al. 2001; Zahn-Waxler et al. 2000). Over-active BIS functioning is thought to contribute to fearful reactions, and greater HPA and autonomic reactivity in anxious children (Hastings et al. 2007; Hastings and Utendale 2008).
Conversely, boys with DBD and oppositional problems showed decreased adrenocortical stress responses. Therefore the results also offered support for the role of under-active BIS affecting stress physiology such that diminished reactivity in seen in children with disruptive behavior problems (van Goozen et al. 2007). Interestingly, we did not identify the “protective” role of anxiety for children with DBD that has been reported previously (e.g., McBurnett et al. 1991); the magnitudes of cortisol elevations were similar for boys with DBD alone and DBD with AnxD. This inconsistency might be due to the boys in the current sample being younger than those in most prior studies (see also Oosterlaan et al. 2005). In addition, the fact that all boys had a primary presenting condition of ADHD, and venipuncture was used as a stressor, might have affected the pattern of associations.
One strength of this study was its relatively large sample size, which allowed us to evaluate whether ADHD subtypes were associated with differences in HPA function. Arguments have been made that deficits in BIS and response inhibition might be specific to either the hyperactive-impulsive dimension of ADHD (Quay 1997), or the combined subtype (Loo and Barkley 2005) but studies are inconclusive (Randazzo et al. 2008). We found that, overall, there were not differences in adrenocortical reactivity across ADHD subtypes. However, once we took account of boys’ comorbid conditions, we found that ADHD subtypes differed with respect to how DBD were associated with daytime cortisol levels. Cortisol levels were higher in boys with ADHD-PI and ADHD-HI who did not have DBD compared to boys with these two ADHD subtypes with comorbid DBD. Conversely, the presence of comorbid DBD was not associated with cortisol reactivity for boys with ADHD-C. Cortisol levels in boys with ADHD-C alone were as low as those in boys with comorbid DBD. This could be evidence in support of the argument that ADHD-C has a distinct pathophysiology from ADHD-PI or ADHD-HI (Loo and Barkley 2005), or that the pathophysiology of ADHD-C is more closely associated with DBD. Given recent human (Blair et al. 2004) and primate research (Lyons et al. 2000) implicating adrenocortical activity in BIS function and response inhibition, it would be worthwhile to conduct further examinations of how stress-induced elevations in cortisol are related to prefrontal dopaminergic activity in children with and without ADHD and DBD.
These findings also contrast with those of earlier studies indicating that children with ADHD have normative baseline or reactive cortisol levels unless they also have comorbid DBD (e.g., Snoek et al. 2004). Despite links between both classes of disorders and BIS problems, it has been argued that ADHD and DBD have differing underlying neurobiological substrates (van Goozen et al. 2007). Studies of HPA function in children with ADHD and DBD have tended to aggregate children across ADHD subtypes into a single group, whereas our findings suggest it might be important to keep them distinct. When we considered the current sample of boys with ADHD as a single group, we also replicated the past findings: The presence of DBD and more severe oppositional problems predicted weaker adrenocortical reactivity. When subtypes were distinguished, this pattern was not evident in boys with ADHD-C. Current diagnostic systems, of course, distinguish DBD and ADHD subtypes on the basis of behavioural profiles, not pathophysiology. Given recent emphasis on the need to identify robust endophenotypes of the genetic basis of ADHD (Crosbie et al. 2008), it might be worthwhile to pursue further study of which aspects of pathophysiology are common or unique to these disorders, in order to improve etiological models and perhaps improve diagnostic procedures (Loo and Barkley 2005).
Overall, salivary cortisol concentrations in boys with ADHD followed an expected diurnal pattern: Highest at waking, and higher pre-stress cortisol levels in the morning than in the afternoon. The venipuncture challenge elicited a significant cortisol response, with mean post-stress levels more than twice as high as mean pre-stress levels, with the time of provocation being unrelated to cortisol reactivity. However, these results should not necessarily be interpreted to suggest that having ADHD is associated with typical or normative HPA activity. Lacking a comparison group of boys without ADHD, it is possible that we failed to detect deviations from non-clinical samples. We examined previously published normative data on salivary cortisol levels in school-age children without psychopathology collected at roughly comparable times (studies of community groups, or comparison groups in clinical studies; Jansen et al. 1999; Rosmalen et al. 2005; Snoek et al. 2004; Van Goozen et al. 1998). All of these studies showed higher waking, mid-morning baseline and mid-afternoon baseline cortisol levels than were evident in the boys with ADHD in this study. These studies could be taken to suggest that the boys with ADHD in our sample had low waking and pre-stress cortisol levels relative to similarly-aged peers without ADHD. This suggestion can be only tentative, of course, but further direct comparisons of HPA function in children with and without ADHD clearly are warranted.
Finally, time of day was not associated with magnitude of cortisol reactivity. Post-stress cortisol levels were more than double the pre-stress levels, and approached waking levels, whatever the time of venipuncture. Some researchers have argued it is advantageous to conduct stress-induction procedures in the afternoon, because cortisol reactions are stronger later in the day (e.g., Kirschbaum et al. 1990; Snoek et al. 2004). Perhaps such time-dependent reactivity is particular to purely psychosocial stressors. Clearly, for school-age children, the prospect of having blood drawn in a hospital clinic strongly activated the HPA stress response system in both the morning and afternoon hours. Further research on the associations between type of stressor, time of day and magnitude of cortisol response appears warranted.
Limitations
Although this study included a large and socioeconomically diverse sample, the sample was not widely varying in other demographic characteristics. Thus, the current findings may not be generalizable beyond Québec francophone families of European descent. Attention to how ADHD, comorbid conditions, and HPA regulation might vary across sociodemographic groups would be warranted in future investigations. Similarly, this study did not look at HPA function in girls with ADHD. ADHD is more common in boys than girls, and there are pronounced sex differences in typical symptom profiles (Barkley 2003), so relations between adrenocortical functioning and ADHD or its comorbid conditions may be dissimilar for boys and girls (Sondeijker et al. 2007). The current results obtained with boys with ADHD should not be generalized to girls with ADHD.
The study also lacked a normal comparison sample of boys without ADHD. Consideration of previously published data on children without diagnosed psychiatric problems was useful, but direct comparisons of results were not possible due to methodological differences across studies. Thus, although consistent with some past research (e.g., Randazzo et al. 2008), the suggestion that salivary cortisol levels might have been lower in these boys with ADHD than one would expect in a normative community sample must be treated with considerable caution. The field would benefit from more basic, descriptive studies on the development of normative HPA function (e.g., Rosmalen et al. 2005).
It should also be recognized that our results were contingent upon our methodology. We examined elementary school-age boys, assessed salivary cortisol levels at specific times on 1 day when boys were unmedicated, analyzed only the comorbid psychopathology prevalent in this sample, and relied exclusively on parent-reports of diagnoses and problems. It would be useful to examine the development of HPA function and other endocrine systems over time in children with ADHD, to evaluate whether administration of stimulant medication affects reactivity, and to utilize multiple informants on children’s psychopathology. For example, relations between youths’ anxiety-related problems and cortisol levels have been found to differ across mother-reported and self-reported symptoms (Klimes-Dougan et al. 2001). The venipuncture procedure proved to be an effective technique for eliciting HPA reactivity in most of the children, which can be challenging with behavioural stressors (Gunnar and Vasquez 2006). Given this, it could be informative to examine the relations between cortisol in response to venipuncture or similar strong challenges and children’s performance on executive function paradigms that tap cognitive regulation processes implicated in ADHD.
Additional improvements to procedures would help future studies to advance this area of work. Had children been given the opportunity to report on their experience of the venipuncture procedure, such as completing affect ratings (e.g., Hastings et al. 2007), it might have informed whether physiological and emotional arousal were associated. Collection of time-matched samples on another day (e.g., Klimes-Dougan et al. 2001) would have revealed whether pre-stress cortisol levels were elevated, perhaps due to being in the hospital setting. Finally, it would have been useful to monitor post-stress cortisol levels over a longer period of time, in order to determine whether some boys showed delayed onset of HPA arousal, or regulation problems resulting in prolonged HPA activation.
Clinical Implications
ADHD is a complex and multi-faceted diagnostic category. There has been considerable debate about whether the three subtypes should be retained under a single diagnosis. Although it is unclear whether current methods of identifying ADHD subtype are effective for informing treatment (Pelham 2001), advantages might be obtained by incorporating physiological indices into diagnostic procedures (Loo and Barkley 2005). ADHD-C might have a distinct underlying pathophysiology that could necessitate differing treatment than ADHD-PI or ADHD-HI.
Children’s anxiety problems are less obvious and troublesome than ADHD or DBD, and often go unrecognized by parents or caregivers. Undiagnosed anxiety disorders may cause over-arousal and distress to challenges in some children with ADHD, which adults may misattribute to hyperactivity or impulsivity and therefore respond to inappropriately. Accurate diagnosis of comorbid anxiety in children with ADHD could support effective parent-training interventions, and also identify children in need of therapy to improve self-regulation and coping skills.
Notes
It could be argued that dimensional scores for severity of problems, rather than categorical scores for presence of diagnoses, might be a stronger way to examine whether inattention or hyperactivity problems are differentially associated with HPA axis activity depending on the presence of comorbid difficulties. This was addressed using the scores from the Conners’ PRS-R. Regression and correlation analyses using these dimensional scores supported the categorical analyses. Specifically, the Conners’ score for Oppositional problems was negatively correlated with the magnitude of cortisol stress response for boys with ADHD-PI, r = -0.35, p = 0.012, and boys with ADHD-HI, r = -0.42, p = 0.069, but not for boys with ADHD-C, r = 0.02, ns. Considered another way, in boys without DBD, the correlation between Inattention problems and magnitude of cortisol stress response was not significant, r = 0.08, ns, but for boys with DBD, there was a significant negative correlation, r = -0.21, p = 0.046. The correlation for Hyperactive problems on the Conners’ was not significant for either group of boys, r = -0.09 and -0.12, respectively.
References
American Psychiatric Association (2000). Diagnostic and statistical manual of mental disorders (4th edn., text revision). Washington, DC: American Psychiatric Association.
Barkley, R. A. (1997). Behavioral inhibition, sustained attention, and executive function: Constructing a unifying theory of attention deficit hyperactivity disorder. Psychological Bulletin, 121, 54–94. doi:10.1037/0033-2909.121.1.65.
Barkley, R. A. (2001). The inattentive type of ADHD as a distinct disorder: what remains to be done. Clinical Psychology: Science and Practice, 8, 489–493. doi:10.1093/clipsy/8.4.489.
Barkley, R. A. (2003). Attention-deficit/hyperactivity disorder. In E. J. Mash, & R. A. Barkley (Eds.), Child psychopathology (pp. 75–143). NY: Guilford.
Barkley, R. A., et al. (2002). Consensus statement on ADHD. European Child & Adolescent Psychiatry, 11, 96–98. doi:10.1007/s007870200017.
Blair, C., Peters, R., & Granger, D. (2004). Physiological and neuropsychological correlates of approach/withdrawal tendencies in preschool: further examination of the behavioural inhibition system/behavioural activation system scales for young children. Developmental Psychobiology, 45, 113–124. doi:10.1002/dev.20022.
Braarud, H. C., & Stormark, K. M. (2006). Maternal soothing and infant stress responses: soothing, crying and adrenocortical activity during inoculation. Infant Behavior and Development, 29, 70–79. doi:10.1016/j.infbeh.2005.08.008.
Cacioppo, J. T., Klein, D. J., Berntson, G. G., & Hatfield, E. (1993). The psychophysiology of emotion. In M. Lewis, & J. M. Haviland (Eds.), Handbook of emotions (pp. 119–142). NY: Guilford.
Capaldi, D. M. (1992). Co-occurrence of conduct problems and depressive symptoms in early adolescent boys: II. A 2-year follow-up at Grade 8. Development and Psychopathology, 4, 125–144. doi:10.1017/S0954579400005605.
Castellanos, F. X., Sharp, W., Gottesman, R. F., Greenstein, D. K., Giedd, J. N., & Rapoport, J. L. (2003). Anatomic brain abnormalities in monozygotic twins discordant for attention deficit hyperactivity disorder. The American Journal of Psychiatry, 160, 1693–1696. doi:10.1176/appi.ajp.160.9.1693.
Conners, C. K. (1997). Conners’ rating scales-revised. Multi-Health Systems Publishing, North Tonawada, NY.
Connor, D. E., Edwards, G., Fletcher, K. E., Baird, J., Barkley, R. A., & Steingard, R. J. (2003). Correlates of comorbid psychopathology in children with ADHD. Journal of the American Association of Child & Adolescent Psychiatry, 42, 193–200. doi:10.1097/00004583-200302000-00013.
Crosbie, J., Pérusse, D., Barr, C. L., & Schachar, R. J. (2008). Validating psychiatric endophenotypes: inhibitory control and attention deficit hyperactivity disorder. Neuroscience and Biobehavioral Reviews, 32, 40–55. doi:10.1016/j.neubiorev.2007.05.002.
Dixon, K. E., Thorn, B. E., & Ward, C. (2004). An evaluation of sex differences in psychological and physiological responses to experimentally-induced pain: a path analytic description. Pain, 112, 188–196. doi:10.1016/j.pain.2004.08.017.
Fassler, D. (1985). The fear of needles in children. The American Journal of Orthopsychiatry, 55, 371–377.
Fowles, D. C. (1988). Psychophysiology and psychopathology: a motivational approach. Psychophysiology, 25, 373–391. doi:10.1111/j.1469-8986.1988.tb01873.x.
Frijda, N. H. (1994). Emotions are functional, most of the time. In P. Ekman, & R. J. Davidson (Eds.), The nature of emotion: Fundamental questions (pp. 112–122). NY: Oxford University Press.
Gray, J. A. (1982). The neuropsychology of anxiety: An enquiry into the functions of the septohippocampal system. Oxford: Oxford University Press.
Gunnar, M. R., & Vazquez, D. (2006). Stress neurobiology and developmental psychopathology. In D. Cicchetti, & D. Cohen (Eds.), Developmental psychopathology, Vol. 2: Developmental neuroscience (pp. 533–577). Wiley & Sons: Hoboken, NJ.
Hartman, C. A., Willcut, E. G., Rhee, S. H., & Pennington, B. F. (2004). The relation between sluggish cognitive tempo and DSM-IV ADHD. Journal of Abnormal Child Psychology, 32, 491–503. doi:10.1023/B:JACP.0000037779.85211.29.
Hastings, P. D., & Utendale, W. (2008). Il cuore del problema: la biologica dell’inibrizione comportamentale e della timidezza (English title: The heart of the matter: The biology of shyness and inhibition). In A. LoCoco, K. Rubin, & C. Zappula (Eds.), L’isolamento sociale durante l’infanzia (pp. 27–56). Milano: Edizioni Unicopli.
Hastings, P. D., Zahn-Waxler, C., & Usher, B. A. (2007). Cardiovascular and affective reactions to social stress in adolescents with internalizing and externalizing problems. International Journal of Behavioral Development, 31, 77–87. doi:10.1177/0165025407073575.
Hendren, R. L., De Backer, I., & Pandina, G. J. (2000). Review of neuroimaging studies of child and adolescent psychiatric disorders from the past 10 years. Journal of the American Academy of Child and Adolescent Psychiatry, 39, 815–828. doi:10.1097/00004583-200007000-00010.
Hirshfeld-Becker, D. R., Biederman, J., & Rosenbaum, J. F. (2004). Behavioral inhibition. In J. S. March, & T. L. Morris (Eds.), Anxiety disorders in children and adolescents (pp. 27–58, 2nd ed.). New York: Guilford.
Hong, H. J., Shin, D. W., Lee, E. H., Oh, Y. H., & Noh, K. S. (2003). Hypothalamic-pituitary-drenal reactivity in boys with attention deficit hyperactivity disorder. Yonsei Medical Journal, 44, 608–614.
Hubert, W., Moller, M., & Nieschlag, E. (1989). Stress reactions in response to the procedure of LHRH tests as measured by salivary and serum cortisol and psychological variables. Hormone Research, 32, 198–202.
Jansen, L. M. C., Gispen-de-Wied, C. C., Jansen, M. A., van-der-Gaag, R. J., Matthys, W., & van Engeland, H. (1999). Pituitary-adrenal reactivity in a child psychiatric population: salivary cortisol response to stressors. European Neuropsychopharmacology, 9, 67–75. doi:10.1016/S0924-977X(98)00003-0.
Kaneko, M., Hoshino, Y., Hashimoto, S., Okano, T., & Kumashiro, H. (1993). Hypothalamic-pituitary-adrenal axis function in children with attention-deficit hyperactivity disorder. Journal of Autism and Developmental Disorders, 23, 59–65. doi:10.1007/BF01066418.
Kirschbaum, C., Steyer, R., Eid, M., Patalla, U., Schwenzmezger, P., & Hellhammer, D. H. (1990). Cortisol and behavior: 2. Application of a latent state-trait model to salivary control. Psychoneuroendocrinology, 15, 297–307. doi:10.1016/0306-4530(90)90080-S.
Klimes-Dougan, B., Hastings, P. D., Granger, D. A., Usher, B. A., & Zahn-Waxler, C. (2001). Adrenocortisol activity in at-risk and normally developing adolescents: individual differences in salivary cortisol basal levels, diurnal variation, and responses to social challenges. Development and Psychopathology, 13, 695–719. doi:10.1017/S0954579401003157.
Lewis, M., & Ramsay, D. S. (1997). Stress reactivity and self-recognition. Child Development, 68, 621–629. doi:10.2307/1132114.
Lewis, M., & Thomas, D. (1990). Cortisol release in infants in response to inoculation. Child Development, 61, 50–59. doi:10.2307/1131046.
Lilienfeld, S. O. (2003). Comorbidity between and within childhood externalizing and internalizing disorders: reflections and directions. Journal of Abnormal Child Psychology, 31, 285–291. doi:10.1023/A:1023229529866.
Loo, S. K., & Barkley, R. A. (2005). Clinical utility of EEG in attention deficit hyperactivity disorder. Applied Neuropsychology, 12, 64–76. doi:10.1207/s15324826an1202_2.
Lovallo, W. R., & Thomas, T. L. (2000). Stress hormones in psychophysiological research. In J. T. Cacioppo, L. G. Tassinary, & G. G. Berntson (Eds.), Handbook of psychophysiology (pp. 342–367, 2nd ed.). NY: Cambridge University Press.
Lynam, D. R. (1996). Early identification of early offenders: who is the fledgling psychopath? Psychological Bulletin, 120, 209–234. doi:10.1037/0033-2909.120.2.209.
Lyons, D. M., Lopez, J. M., Yang, C., & Schatzberg, A. F. (2000). Stress-level cortisol treatment impairs inhibitory control of behavior in monkeys. The Journal of Neuroscience, 20, 7816–7821.
McBurnett, K., Lahey, B. B., Frick, P. J., Risch, C., Loeber, R., et al. (1991). Anxiety, inhibition, and conduct disorder in children: II. Relation to salivary cortisol. Journal of the American Academy of Child and Adolescent Psychiatry, 30, 192–196. doi:10.1097/00004583-199103000-00005.
McBurnett, K., Lahey, B. B., Rathouz, P. J., & Loeber, R. (2000). Low salivary cortisol and persistent aggression in boys referred for disruptive behavior. Archives of General Psychiatry, 57, 38–43. doi:10.1001/archpsyc.57.1.38.
McBurnett, K., Raine, A., Stouthamer-Loeber, M., Loeber, R., Kumar, A. M., Kumar, M., et al. (2005). Mood and hormone responses to psychological challenge in adolescent males with conduct problems. Biological Psychiatry, 57, 1109–1116. doi:10.1016/j.biopsych.2005.01.041.
Meeran, K., Hattersley, A., Mould, G., & Bloom, S. R. (1993). Venipuncture causes rapid rise in plasma ACTH. The British Journal of Clinical Practice, 47, 246–247.
Öhman, A. (1993). Fear and anxiety as emotional phenomena: Clinical phenomenology, evolutionary perspectives, and information-processing mechanisms. In M. Lewis, & J. M. Haviland (Eds.), Handbook of emotions (pp. 511–536). NY: Guilford.
Oosterlaan, J., Geurts, H. M., Knol, D. L., & Sergeant, J. A. (2005). Low basal salivary cortisol is associated with teacher-reported symptoms of conduct disorder. Psychiatry Research, 134, 1–10. doi:10.1016/j.psychres.2004.12.005.
Patel, P. D., Lopez, J. F., Lyons, D. M., Burke, S., Wallace, M., & Schatzberg, A. F. (2000). Glucocorticoid and mineralocorticoid receptor mRNA expression in squirrel monkey brain. Journal of Psychiatric Research, 34, 383–392. doi:10.1016/S0022-3956(00)00035-2.
Pelham, W. E. Jr. (2001). Are ADHD/I and ADHD/C the same or different? Does it matter?. Clinical Psychology: Science and Practice, 8, 502–506.
Pliszka, S. R. (2000). Patterns of psychiatric comorbidity with attention-deficit/hyperactivity disorder. Child and Adolescent Psychiatric Clinics of North America, 9, 525–540.
Quay, H. C. (1997). Inhibition and attention deficit hyperactivity disorder. Journal of Abnormal Child Psychology, 25, 7–13. doi:10.1023/A:1025799122529.
Raine, A. (1997). Antisocial behavior and psychophysiology: a biosocial perspective and a prefrontal dysfunction hypothesis. In D. M. Stoff, J. Breiling, & J. D. Maser (Eds.), Handbook of antisocial behavior (pp. 289–304).
Ramsay, D., & Lewis, M. (2003). Reactivity and regulation in children and behavioural responses to stress. Child Development, 74, 456–464. doi:10.1111/1467-8624.7402009.
Randazzo, W. T., Dockray, S., & Susman, E. J. (2008). The stress response in adolescents with inattentive type ADHD symptoms. Child Psychiatry and Human Development, 39, 27–38. doi:10.1007/s10578-007-0068-3.
Reul, J. M., & de Kloet, E. R. (1985). Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology, 117(6), 2505–2511.
Rosmalen, J. G. M., Oldehinkel, A. J., Ormel, J., de Winter, A. F., Buitelaar, J. K., & Verhulst, F. C. (2005). Determinants of salivary cortisol levels in 10–12 year old children; a population-based study of individual differences. Psychoneuroendocrinology, 30, 483–495. doi:10.1016/j.psyneuen.2004.12.007.
Sanchez, M. M., Young, L. J., Plotsky, P. M., & Insel, T. R. (2000). Distribution of corticosteroid receptors in the rhesus brain: relative absence of glucocorticoid receptors in the hippocampal formation. The Journal of Neuroscience, 20(12), 4657–4668.
Shaffer, D., Fisher, P., Lucas, C. P., Dulcan, M. K., & Schwab-Stone, M. E. (2000). NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry, 39, 28–38. doi:10.1097/00004583-200001000-00014.
Snoek, H., Van Goozen, S. H. M., Matthys, W., Buitelaar, J. K., & Van Engeland, H. (2004). Stress responsivity in children with externalizing behavior disorders. Development and Psychopathology, 16, 389–406. doi:10.1017/S0954579404044578.
Sondeijker, F. E. P. L., et al. (2007). Disruptive behaviors and HPA-axis activity in young adolescent boys and girls from the general population. Journal of Psychiatric Research, 41, 570–578. doi:10.1016/j.jpsychires.2006.04.002.
Thompson, R. A., & Calkins, S. D. (1996). The double-edged sword: emotional regulation for children at risk. Development and Psychopathology, 8, 163–182.
Vaessen, W., & Van der Meere, J. J. (1990). Issues in the selection of hyperactive/ADDH children for experimental clinical studies. In A. F. Kalverboer (Ed.), Developmental biopsychology: Experimental and observational studies in groups at risk. Ann Arbor: University of Michigan Press.
Van Goozen, S. H. M., Matthys, W., Cohen-Kettenis, P. T., Gispen-de Wied, C., Wiegant, V. M., & Van Engeland, H. (1998). Salivary cortisol and cardiovascular activity during stress in oppositional-defiant disorder boys and normal controls. Biological Psychiatry, 43, 531–539. doi:10.1016/S0006-3223(97)00253-9.
Van Goozen, S. H. M., Fairchild, G., Snoek, H., & Harold, G. T. (2007). The evidence for a neurobiological model of childhood antisocial behaviour. Psychological Bulletin, 133, 149–182. doi:10.1037/0033-2909.133.1.149.
Walco, G. A., Conte, P. M., Labay, L. E., Engel, R., & Zeltzer, L. K. (2005). Procedural distress in children with cancer: self-report, behavioral observations, and physiological parameters. The Clinical Journal of Pain, 21, 484–490. doi:10.1097/01.ajp.0000146166.15529.8b.
Waschbush, D. A., Pelham, W. E., Jennings, J. R., Greiner, A. R., Tarter, R. A., & Moss, H. B. (2002). Reactive aggression in boys with disruptive behavior disorders: behavior, physiology, and affect. Journal of Abnormal Child Psychology, 30, 641–656. doi:10.1023/A:1020867831811.
Watamura, S. E., Donzella, B., Alwin, J., & Gunnar, M. R. (2003). Morning-to-afternoon increases in cortisol concentrations for infants and toddlers at child care: age differences and behavioral correlates. Child Development, 74, 1006–1020. doi:10.1111/1467-8624.00583.
Wechsler, D. (1991). The Wechsler Intelligence Scale for Children-Third Edition. San Antonio, TX: Psychological Corporation.
White, B. P., & Mulligan, S. E. (2005). Behavioral and physiologic response measures of occupational task performance: a preliminary comparison between typical children and children with attention deficit. The American Journal of Occupational Therapy, 59, 426–436.
Zahn-Waxler, C., Klimes-Dougan, B., & Slattery, M. J. (2000). Internalizing problems in childhood and adolescence: prospects, pitfalls, and progress in understanding the development of anxiety and depression. Development and Psychopathology, 12, 443–466. doi:10.1017/S0954579400003102.
Acknowledgements
Fonds de Recherche en Santé du Quebec (FRSQ), Galileo Genomics, and Genizon financially supported this research, l’Hôpital Sainte-Justine provided infrastructure support, and Francois L’Heureux and Aracely Contreras assisted with data management. The participating boys and their families have our sincere gratitude.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hastings, P.D., Fortier, I., Utendale, W.T. et al. Adrenocortical Functioning in Boys with Attention-Deficit/Hyperactivity Disorder: Examining Subtypes of ADHD and Associated Comorbid Conditions. J Abnorm Child Psychol 37, 565–578 (2009). https://doi.org/10.1007/s10802-008-9292-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10802-008-9292-y