Abstract
In this work, it investigated a non-cyanide imitation gold plating system, namely a hydroxyethylidene diphosphonic acid system. The bath in this system is less toxic and less expensive, and the decorative quality of the coating can be maintained with this system. The effects of four N-based additives, namely triethanolamine (TEA), ammonium fluoride (AF), ammonia triacetic acid (NTA), and polyacrylamide (PAM), on the performance of the Cu–Zn–Sn alloy coating were studied. The results indicate that TEA can be used as an auxiliary complexing agent to promote anodic dissolution, improve the dispersibility of the bath, and control the colour and brightness of the coating. The carboxylic acid group of NTA is easily discharged in the cathode, inducing the hydrogen evolution reaction, which results in a blackened and irregular coating surface. In addition to inorganic amines, AF also contains fluoride ions, which enable the formation of uniformly sized particles, dense crystals, and a compactly arranged coating, and may promote the formation of a yellow coating. The long chain of PAM prevents the migration of Cu2+ ions in the solution, which causes a decrease in the anodic stripping peak current and thus adversely affects the electrode interface. The mechanism of the four additives in the electroplating process was studied, and the results may provide theoretical guidance for selecting additives for the Cu–Zn–Sn electroplating process.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Electroplating is one of the most commonly used methods for preparing metals and alloy coatings. Metal alloy coatings exhibit more and better properties than monometallic coatings, and they can have a wide range of compositions [1]. Currently, alloy coatings produced domestically and abroad are categorized as binary (Cu–Zn and Cu–Sn), ternary (Cu–Zn–Sn and Cu–Sn–In), and quaternary (Cu–Zn–Sn–In and Cu–Sn–In–Ni). For example, the Cu–Zn–Sn alloy coating used in the electroplating industry can be obtained by tuning the Cu content to 75% and the Zn and Sn contents to 25%. This coating exhibits the desirable flatness, brightness, smoothness, corrosion resistance, colour, and decorative effects. Furthermore, it is inexpensive, has a low porosity, and is widely used in the metal coating industry [2,3,4,5,6].
The earliest imitation gold plating process used a cyanide system because the cyanide plating solution is stable and promotes the effective deposition of the precipitated metal, which enables the evenness and thickness of imitation gold plating coatings to be substantially improved. However, the electroplating industry has recently been committed to using an environmentally friendly non-cyanide imitation gold plating solution instead of toxic cyanide electroplating solutions and has achieved satisfactory results. Recently developed non-cyanide imitation gold plating systems mainly include ethylenediaminetetraacetic acid [7], tartrate [8], pyrophosphate [9], hydroxyethylidene diphosphonic acid (HEDP) [10], d-mannitol [11], polyalcohol sorbitol [12], and citrate systems [13, 14]. In this study, the HEDP system was used for non-cyanide imitation gold plating. HEDP is a phosphate phosphonate that can form stable water-soluble complexes with metal ions and thus exhibits a stable complexing effect without introducing solubility problems. The composition of the electroplating solution is simple and stable, and the coating consists of uniform, dense crystals. HEDP can disintegrate into various products when exposed to sunlight; thus, it can be considered to be an environmentally friendly complexing agent.
The traditional electroplating process has been studied in the presence of additives to address most plating process problems. Ethylenediaminetetraacetic acid (EDTA) affects the electrodeposition of CuZn [15], FeZn [16], CuSnZn [17], and Zn [18]. EDTA not only improves the coatings but also stabilizes the electroplating solution. El-Chiekh [19] used sodium dithiolate as a surface active substance in acidic sulphate electrolytes to electrodeposit ternary Cu–Ni–Zn and Cu–Ni–Cd alloys. SAS did not affect the alloy composition significantly; however, it did affect the surface morphology of the deposited materials. Long [20] studied the electrodeposition of Zn–Ni alloy coatings on a low-carbon steel substrate using a cyanide-free alkaline bath containing tetraethylenepentamine and triethanolamine as complexing agents and found that the resulting coating was fine-grained with a smooth surface and had the highest corrosion resistance. Jung [21] fabricated a uniform Cu–Sn alloy coating in a sulphuric acid bath containing a mixture of additives such as ethylenediamine and potassium tetrathionate. Senna [22] found that a high-quality Cu–Zn alloy can be produced by adding allyl alcohol to a pyrophosphate system. Silva [23] observed that adding benzotriazole and cysteine to a Cu–Zn alloy citrate system can change the composition of the Cu–Zn alloy and improve the corrosion resistance of the coating.
Numerous types of electroplating systems and additives have been studied to date, but only their effects have been described. Their mechanisms of action have not been determined, thereby hindering advances in the use of additives in imitation gold plating. In this study, the HEDP system and four N-based additives, namely triethanolamine (TEA), ammonium fluoride (AF), ammonia triacetic acid (NTA), and polyacrylamide (PAM), were employed. AF is an inorganic amine compound with the molecular formula NH4F. NTA and TEA are small organic molecules with the molecular formulas N(CH2CH2OH)3 and N(CH2COOH)3, respectively. PAM is a high-molecular-weight organic material with the formula [CH2CHCONH2]n. The effects of these additives on the properties of the Cu–Zn–Sn alloy coatings obtained by non-cyanide imitation gold plating were investigated. The electrode reaction, the composition and surface morphology of the alloy coating, and the properties of the electroplating solution were analysed. Finally, the mechanisms of action of the additives were proposed.
2 Experiments
2.1 Electroplating solutions
The sequence in which the electroplating solution is prepared is crucial. First, 0.18 mol L−1 CuSO4·5H2O (≥ 99.0%, Tianjin Zhiyuan Chemical Reagent Co.), 0.06 mol L−1 ZnSO4·7H2O (≥ 99.5%, Tianjin Beichenfangzheng Reagent Factory), 0.05 mol L−1 Na2SnO3·3H2O (≥ 98%, Tianjin Guangfu Fine Chemical Research Institute), 22.66 g L−1 Na3C6H5O7·2H2O (≥ 99.0%, Tianjin Guangfu Technology Development Co.), and 25.0 g L−1 Na2CO3 (≥ 99.8%, Tianjin Guangfu Technology Development Co.) were dissolved in a minimal amount of water. Approximately 2.5 g L−1 NaOH (≥ 96.0%, Tianjin Beichenfangzheng Reagent Factory) was subsequently added after sodium stannate, which is insoluble in water and soluble in alkaline solutions, was completely dissolved. Then, the main complexing agent HEDP (60%, Shandong Youso Chemical Technology Co.) was added to achieve a concentration of 100.0 mL L−1. The different additives were subsequently added according to the solution requirements. The pH of the solution was adjusted to 13.0–13.5 by adding NaOH. The plating solution without additives was the blank solution (BR). Four auxiliary additives, namely TEA (≥ 99.0%, Tianjin Damao Chemical Reagent Factory), AF (≥ 96.0%, Tianjin Kaitong Chemical Reagent Co.), NTA (≥ 98.5%, Xilong Science Co.), and PAM (MW = 3,000,000, ≥ 90.0%, Tianjin Zhiyuan Chemical Reagent Co.), were selected for study. The electroplating experiments should be conducted using a fresh solution. The colour of the coating was black in the presence of the solution, which was allowed to sit for more than 48 h before the material was deposited at the bottom. Water was purified using a water purification system (PALL Cascada II I 30, USA). All reagents were analytical grade.
2.2 Electroplating experiments
The electrolysis system consisted of an electroplating bath, cathode plate, and anode plate. The effective volume of the electroplating bath was 100 mL. The cathode was a 4 mm × 7 mm × 1.0 mm stainless steel plate with a single-side effective area of 12.0 cm2. The anode consisted of a 4 mm × 7 mm × 1.0 mm Cn0.7Zn0.3 alloy with a single-side effective area of 12.0 cm2. The temperature was maintained at 298 K, and the current density and electrolysis time were 350.0 A m−2 and 60 s, respectively. The electroplating process was repeated three times to increase the thickness of the coating. An intelligent direct current constant voltage power source (WJY-30 V/10 A) was used for the electrolysis.
2.3 Electrochemical testing
Electrochemical tests were conducted on a PARSTAT PMC-1000 electrochemical workstation. A three-electrode system consisting of a mercury oxide reference electrode, stainless steel working electrode (area = 1.0 cm2; stainless steel is commonly used in electroplating experiments), and Pt counter electrode (area = 1.0 cm2) was employed. All the electrodes were finely polished and washed [24, 25]. The system was sealed by an insulating polymer to prevent fluctuations in the electrode area, except for the effective electrode area, during testing. The cyclic voltammetry (CV) scanning speed was 20 mV s−1.
2.4 Characterization
The morphologies of the coatings were studied with an optical camera (Canon A590 IS) after the cathode sheet was removed. A Hitachi SU8010 field emission environmental SEM–EDS instrument was used for surface investigations and feature detection. The images and spectra were collected at a 20 kV accelerating voltage. The crystal structure was analysed using a Bruker D2 Phaser X-ray diffractometer (XRD) (National Taiwan University of Science and Technology, China). Ultraviolet–Visible (UV–Vis) spectroscopy was performed using a TU-1901 UV–Vis spectrophotometer (Beijing Persee, China). Fourier transform infrared (FTIR) spectra were recorded by a Magna 550II FTIR spectrometer (Nicolet, USA) using the KBr pellet method.
3 Results and discussion
3.1 Effects of the additives on the colour of the coating
In Fig. 1, the appearances of the dried imitation gold coatings obtained in the presence of the four additives were observed by an optical camera. The surface of the imitation gold coating obtained from the BR without additives was smooth and purplish red. The coating appeared slightly yellow and exhibited the brightness when the concentration of NTA was 5.0 g L−1. The coating appeared scorched and blackened at the edges when the amount of NTA was 20.0 g L−1. When the amount of NTA was increased to 25.0 g L−1, most of the coating was black, and pinholes were observed in it. Therefore, an NTA dosage of 5.0 g L−1 improved the coating brightness. The analysis showed that NTA affected the grain refinement and electrolyte stability because it forms complexes with Cu, Zn, and Sn ions in the plating bath [26, 27]. Furthermore, the carboxylic acid group of NTA was easily discharged in the cathode, thereby inducing the hydrogen evolution reaction, which resulted in an increase in the number of pinholes on the surface of the coating.
The colour of the coating gradually changed from red to imitation gold when the concentration of AF was increased from 0.0 to 1.0 g L−1. The coating was yellow, and the best brightness was observed when the concentration of AF was 2.0–3.0 g L−1. However, the coating appeared nearly black when the amount exceeded 4.0 g L−1. Thus, the optimal concentration of AF was 2.0 g L−1 when cost reduction is considered. The analysis showed that AF contained fluoride ions and inorganic amines. Therefore, AF improved the flatness and brightness of the coating and enabled grain refinement.
The coating appeared purplish red when the concentration of TEA was 2.0 mL L−1. The coating changed from red to imitation gold when the concentration of TEA was increased to 5.0 mL L−1. The coating was rose gold when the concentration of TEA was further increased to 10.0–20.0 mL L−1. The coating was black when the concentration of TEA was 25.0 mL L−1. Therefore, the coating was similar to gold at the optimal TEA concentration of 5.0 mL L−1. TEA is considered an auxiliary complexing agent [20, 28] that can substitute for a fraction of the HEDP to promote anodic dissolution and improve the dispersion capability of the plating bath. TEA delays the accumulation of sodium carbonate to prevent the formation of coating burrs and other defects, thus enhancing the bright colour of the coating. It might also improve the surface roughness and reduce blackening.
The coating colour changed from pale yellow to golden yellow when the concentration of PAM increased from 3.0 to 4.0 mg L−1. The coating was nearly black when the concentration of PAM exceeded 5.0 mg L−1. The plating appeared black when the amount of PAM was 8.0 mg L−1. Therefore, the optimal concentration of PAM for electroplating was 4.0 mg L−1; at this concentration, a uniform coating with the gloss of imitation gold plating was obtained, because the organic polymer PAM plays a crucial role in reducing the grain size of the coating [29, 30].
3.2 Effects of the additives on the electrochemical reaction of the electrode
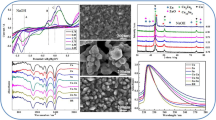
The effects of the additives on the electrode interface were analysed, as shown by the CV curves in Fig. 2. In Fig. 2a, the black line was acquired from low to high potentials in the forward scanning mode, and peak A represented the dissolution of the anode material. When the voltage exceeded 0.5 V, numerous bubbles appeared near the electrode, mainly indicating the evolution of oxygen. The red line was obtained from high to low potentials in the reversed scanning mode, and peaks B and C represented the deposition of the cathode material. When the voltage decreased below − 0.8 V, numerous hydrogen bubbles appeared near the electrode. These results showed that the electrodeposition was constantly accompanied by the hydrogen evolution reaction during the reduction of Cu–Zn–Sn. An inductive current loop was observed between − 0.8 and − 1.0 V, where the negative sweep current crossed the positive sweep current; that is, the cathode current was higher in the anode scanning direction than in the cathode scanning direction. These findings confirmed the formation and dissolution of particle nuclei during electrodeposition [31, 32].
Effects of the additives on the electrode interface determined by CV analysis CV curves of a BR, b BR and solutions of only the main salts, c BR with NTA (NTA 0, 5, 10, 15, 20, 25 g L−1.), d BR with AF (AF 0, 1, 2, 3, 4, 5, 6 g L−1.), e BR with TEA (TEA 0, 2, 5, 10, 15, 20 mL L−1.), and f BR with PAM (PAM 0, 1, 2, 3, 4, 5, 6, 7, 8, 9 mg L−1.)
The effects of the main salts on the electrode interface were determined by analysing the CV curves shown in Fig. 2b. The BR curve was the CV curve of the blank plating solution with 0.18 mol L−1 CuSO4·5H2O, 0.06 mol L−1 ZnSO4·7H2O, and 0.05 mol L−1 Na2SnO3·3H2O, and the cathodic deposition peaks B and C occurred at − 0.44 and − 0.59 V, respectively. The Cu curve was obtained using a 0.18 mol L−1 CuSO4·5H2O solution, and the cathodic deposition peaks occurred at − 0.44 and − 0.63 V. The Zn curve was obtained using a 0.06 mol L−1 ZnSO4·7H2O solution, and the cathodic deposition peaks were observed at − 0.44 and − 0.71 V. The Sn curve was obtained using a 0.05 mol L−1 Na2SnO3·3H2O solution, and the cathodic deposition peak occurred at − 0.44 V. In the positive scans of the BR and Cu curves, peak A was observed at approximately 0 V, because of the dissolution peak of the anode Cu. Moreover, of the Cu, Zn, and Sn curves, only the Zn curve exhibited a cathodic deposition peak at − 0.71 V; thus, peak D was mainly due to the precipitation of Zn or zinc compounds. Similarly, only the Cu curve exhibited a cathodic deposition peak near − 0.63 V; thus, peak C was mainly attributed to the precipitation of Cu or copper compounds. Notably, a cathodic deposition peak was observed at − 0.44 V in all three curves; thus, peak B might be due to the simultaneous precipitation of Cu, Zn, and Sn [33, 34].
The effects of the additives on the electrode interface determined by the CV analysis are shown in Fig. 2c–f. Fig. 2c is the CV curve for the BR plating solution with NTA. The peak currents of cathodic deposition peaks A, B, and C clearly decreased with increasing concentration of NTA, and peak D even disappeared. However, peak G gradually increased. The electroplating experiments showed that an increase in the concentration of NTA resulted in the blackening of the coating surface, and thus, peak G might be attributed to black oxide or sulphide formation by deposition. Therefore, the increase in peak G was unfavourable for imitation gold plating.
Figure 2d shows the CV curve for the BR plating solution with AF. The peak currents of cathodic deposition peak C decreased markedly as the concentration of AF increased, revealing that the Cu content of the coating decreased. Moreover, the obvious increases in the cathodic deposition peaks A and D indicated that AF promoted the dissolution of the anode and increased the Zn deposition at the cathode. In contrast, the considerable increase in cathodic deposition peak B suggested accelerated metal deposition. Moreover, no new peaks were observed in the CV curve, indicating that no new phase was formed. The changes in peaks B, C, and, D suggested that the amounts of Cu, Zn, and Sn deposited varied. These results showed that AF could promote the formation of the gold coating and are consistent with those of the electroplating experiments.
Figure 2e shows the CV curve for the BR plating solution with TEA. The peak currents of cathodic deposition peaks B and D clearly increased with increasing concentration of TEA, indicating accelerated metal deposition. However, the cathodic deposition peak C decreased markedly, suggesting that the Cu content of the coating decreased. Furthermore, peak A also decreased markedly with increasing concentration of TEA, indicating that the dissolution of the anode slowed down. Comparing Fig. 2d, e, that is, comparing the CV curves obtained in the presence of AF and TEA, respectively, revealed that the cathodic reduction peaks were the same, but AF and TEA exhibited opposite effects on peak A. Therefore, adding AF to the electroplating solution led to more favourable anode dissolution than adding TEA and thus eventually enabled the metal ions removed from the plating bath to be replenished.
Figure 2f shows the CV curve for the BR plating solution with PAM. The peak currents of cathodic deposition peaks B and C decreased considerably as the concentration of PAM increased, and peak D even disappeared. In contrast, peak A clearly increased, indicating that anode dissolution increased. New peaks, notably peaks H, I, and J, appeared in the CV curve. As noted previously, increasing the concentration of PAM led to the blackening of the coating surface, and peaks H, I, and J might, therefore, be attributed to black oxide formation by deposition. Thus, the increases in these peaks were not conducive to imitation gold plating. The transfer of the long polymer chain with amino groups prevented the migration of Cu2+ ions in the solution, which resulted in the observed decrease in the anode dissolution peak current and adversely affected the electrode interface. Based on this analysis, the oxidation peak current decreased with increasing concentration of PAM. The amino groups of PAM are pendant to the long chain of the polymer, and the amide groups could prevent the formation of dendrites, which would lead to a smoother coating surface. The long polymer chain would prevent the diffusion of ions at the solid–liquid interface and effectively decrease ion diffusion to the interface, thus causing a high cathode current and making the deposited gold plating appear blackened.
3.3 Effects of the additives on the surface microtopography of the coating
The effects of the additive concentrations on the coating colour and the electrochemical reaction of the imitation gold plating process were investigated to determine their optimal values. In addition, the effects of the additive structure on the morphology of the coating were also explored. The following groups of additives were selected for study: (a) electroplating solution with no additive (BR), (b) BR + 5.0 g L−1 NTA, (c) BR + 2.0 g L−1 AF, (d) BR + 5.0 mL L−1 TEA, and (e) BR + 4.0 mg L−1 PAM. The effects of these additives on the properties of the imitation gold coating, such as the surface morphology, composition, and phase, were studied.
SEM images of the cross sections and surfaces of the coatings obtained in the presence of the four additives are shown in Fig. 3. The thickness of the coating obtained from the BR without additives was approximately 1.0 µm, and its surface consisted of 0.5–1.5 µm particles (Fig. 3a). The thickness of the coating was approximately 1.5 µm when 5.0 g L−1 NTA was added to the BR, and the uneven surface was composed of approximately 1.0 µm particles (Fig. 3b). The thickness of the coating was approximately 1.2 µm when 2.0 g L−1 AF was added to the BR, and the relatively smooth surface was composed of approximately 0.2–0.4 µm particles (Fig. 3c). The gold coating thickness was nearly 1.1 µm when 5.0 mL L−1 TEA was added to the BR, and the irregular surface was composed of 0.4–1.5 µm particles and exhibited prominent dendrites (Fig. 3d). The thickness of the imitation gold coating was approximately 0.9 µm when 4 mg L−1 PAM was added to the BR, and the incomplete surface layer contained 0.5–1.0 µm particles (Fig. 3e). The size and size range of the particles in the coatings formed in the presence of the additives were smaller than those of the coating obtained from the BR, showing that grain refinement occurred. Notably, the effects of AF were the most favourable; a uniform particle size and dense crystals were obtained in the presence of this additive, and the coating had a compact arrangement and was smooth.
Effects of the additives on the SEM and EDS results a 1 , a 2 , and a 3 BR. b 1 , b 2 , and b 3 5.0 g L−1 NTA. c 1 , c 2 , and c 3 2.0 g L−1 AF. d 1 , d 2 , and d 3 5.0 mL L−1 TEA. e 1 , e 2 , and e 3 4.0 mg L−1 PAM. a 1 , b 1 , c 1 , d 1 , and e 1 show the coating cross sections. a 2 , a 3 , b 2 , b 3 , c 2 , c 3 , d 2 , d 3 , e 2 , and e 3 show the coating surfaces
EDS analysis revealed the composition of the electrodeposit formed from each bath (Table 1). Notably, the Cu, Zn, and Sn contents of the electrodeposit obtained from the BR (the 0.18 mol L−1 CuSO4·5H2O, 0.06 mol L−1 ZnSO4·7H2O, and 0.05 mol L−1 Na2SnO3·3H2O bath) were 77.200, 21.662, and 1.138 wt%, respectively. After the four N-based additives were added to the bath, the Cu content decreased, whereas the Zn content increased. However, the changes in the Sn content varied. According to the literature, pure Cu, Zn, and Sn coatings appear purple-red, silver, and silver, respectively. A gold coating can be achieved by controlling the proportions of these three elements. When AF was used, the Cu content decreased to 73.347 wt%, the Sn content was the highest at 1.212 wt%, and the Zn content was 25.441 wt%; thus, a yellow gold coating was achieved. When PAM was used, the minimum Cu and Sn contents of 73.083 and 0.769 wt%, respectively, were observed, and the highest Zn content of 26.148 wt% was obtained. The resulting coating was light yellow. The addition of TEA resulted in a high Cu content of 76.689 wt%, and the contents of Sn and Zn were 1.166 and 22.145 wt%, respectively. Thus, the coating was partly rose gold. Therefore, the EDS results explained the observed colours of the electroplated coatings.
3.4 Effects of the additives on the phase composition of the coating
Figure 4 shows the XRD patterns of the electroplated coatings obtained with different additives. The results were compared to the expected patterns provided by the Committee on Powder Diffraction Standards (JCPDS) [35]. The diffractograms of the electrodeposits obtained from the BR bath (Fig. 4) indicated the presence of CuZn (JCPDS 02–1231), Cu5Zn8 (JCPDS 25–1228), η–Cu6Sn5 (JCPDS 45–1488), CuSn (JCPDS 65-3433), and Cu (JCPDS 71–0339) phases [36,37,38,39]. The 2θ positions of the diffraction peaks were nearly constant, and only the heights of the peaks changed when 5.0 mL L−1 TEA or 2.0 g L−1 AF was added to the BR. Therefore, the alloy phases in the coating remained the same when TEA or AF was added, but the ratios of the phases varied. The 2θ diffraction peak at 73°, which was mainly attributed to the CuZn and Cu5Zn8 phases of the BR sample, disappeared when 5.0 g L−1 NTA or 4.0 mg L−1 PAM was added to the BR. Meanwhile, the Zn deposition peak at − 0.71 V disappeared when NTA or PAM was added to the BR (Fig. 2c, f). Hence, the XRD results were consistent with those of the electrochemical analysis. In all cases, a Cu–Sn–Zn ternary alloy was formed. The Cu–Sn–Zn electrodeposits produced in the BR had the same crystalline phases as those obtained in the presence of the additives TEA and AF. Therefore, TEA and AF affected the ratio of the phases in the Cu–Sn–Zn electrodeposits, and NTA and PAM influenced the phase composition.
3.5 Effects of the additives on the UV and IR spectra of the electroplating solution
To investigate the mechanisms of the four N-based additives, the UV–Vis and FTIR spectral properties of the electroplating solution were measured and compared, as shown in Fig. 5. Figure 5a shows that the absorption peak of the BR occurred at 300 nm and did not shift when TEA was added to it. The absorption peak exhibited a blue shift from 300 to 298 nm when NTA or AF was added to the BR, and the absorption peak exhibited a red shift to 301 nm when PAM was added. The maximum absorption wavelength of the –NH2 group of PAM was observed at 196 nm; however, the maximum absorption wavelength of the plating solution was unaffected by the presence of trace amounts of PAM. A comparison of the effects of the four N-based additives on the UV spectrum of the plating solution showed that their effects were nearly negligible.
The main components of the BR were 0.18 mol L−1 CuSO4·5H2O, 0.06 mol L−1 ZnSO4·7H2O, 0.05 mol L−1 Na2SnO3·3H2O, 22.66 g L−1 Na3C6H5O7·2H2O, 100.0 mL L−1 HEDP, and 25.0 g L−1 Na2CO3, and the pH ranged from 13.0 to 13.5. Figure 6a shows an absorption peak at 3450–3600 cm−1, which was attributed to the free and associated –OH stretching vibrations. The absorption peaks at 3060 and 1660 cm−1 were due to the –OH stretching and oscillating vibrations, respectively. The bands at 1575 and 1397 cm−1 were due to the asymmetric and symmetric vibrations, respectively, of the carboxylate ion [40]. The bands at 1104 and 992 cm−1 were due to the P–O stretching vibrations of HEDP, and the band at 947 cm−1 was due to the interactions between P–O and the main salt ions in the plating solution. The absorption peaks of the plating solution remained nearly unchanged after trace amounts of the four N-based additives were added (Fig. 6b).
As noted in the literature [41], the molecular formula of the main complex HEDP is C(CH3)(OH)(PO3H2)2, which is frequently expressed as H4L. HEDP mainly exists in the form of L4− when the pH is 13.0–13.5. In the strongly alkaline plating solution, Cu ions mainly form two complexes: Cu(OH)42− as the predominant species and CuL26− as the minor species. According to the literature, the electrodeposition of Cu with HEDP exhibits a two-step discharge, which is expressed as follows: CuL26− → CuL2− + L4− and CuL2− + 2e− → Cu + L4−. In the strongly alkaline plating solution, Zn ions mainly form Zn(OH)42− complexes and small amounts of (ZnLOH)3− and Zn(OH)3−. In this solution, Sn ions also form two complexes: Sn(OH)3− as the predominant species and SnL(OH)24− as the minor species. The spectroscopic analyses revealed that the UV and IR spectra of the plating solution remained nearly unchanged after the four N-based additives were added to it. These results showed that the metal ion complexes present in the electroplating baths were similar in the absence of a current. However, the influence of the additives on the electrode interface varied under an applied current; thus, differences in the morphology, composition, and crystalline structure of the deposited coatings were observed.
4 Conclusion
This study investigated the electroplating of a Cu–Zn–Sn alloy using a non-cyanide HEDP system, in which CuSO4·5H2O, ZnSO4·7H2O, and Na2SnO3·3H2O were the main salts, sodium citrate acted as an auxiliary complexing agent, and sodium hydroxide and anhydrous sodium carbonate acted as buffering agents in the electroplating solution. Four N-based additives were separately added to the basic HEDP system, and electroplating experiments and electrochemical analyses were performed. TEA, which can substitute for a fraction of the HEDP, was used as an auxiliary complexing agent to promote anodic dissolution and improve the dispersion capability of the electroplating solution. This additive favourably affected the colour and brightness of the coating, but the surface of the coating was irregular. The carboxylic acid group of NTA was easily discharged in the cathode, inducing the hydrogen evolution reaction and thus causing the formation of more pinholes and an irregular coating surface. When the concentration of NTA increased, the surface of the coating was blackened, possibly due to the formation of black oxides or sulphides by deposition. In addition to inorganic amines, AF also contains fluoride, which affected the flatness of the coating, enabled grain refinement, and improved the brightness. Furthermore, this additive could promote metal deposition and anode dissolution, enabling the formation of uniformly sized and compactly arranged particles, a smooth yellow coating, and a dense crystal structure. The long chain of the organic polymer PAM, which has amine groups, could prevent the migration of Cu2+ ions in the solution. Thus, the anodic dissolution peak current decreased, which adversely affected the electrode interface. The current of the anodic peak decreased with increasing concentration of PAM. However, the amino groups of PAM are pendant to its long chain and thus prevented dendrite formation, leading to the generation of a flat coating surface. These results might provide a theoretical basis for selecting additives for use in the electrodeposition of Cu–Zn–Sn alloys.
References
Oliveira GMD, Barbosa LL, Broggi RL, Carlos IA (2005) Voltammetric study of the influence of EDTA on the silver electrodeposition and morphological and structural characterization of silver films. J Electroanal Chem 578:151–158
Yang SC, Ho CE, Chang CW, Kao CR (2011) Strong Zn concentration effect on the soldering reactions between Sn-based solders and Cu. J Mater Res 21:2436–2439
Jung SB (2006) Interfacial reactions and shear strength on Cu and electrolytic Au/Ni metallization with Sn-Zn solder. J Mater Res 21:1590–1599
Lee JE, Kim KS, Suganuma K, Takenaka J, Hagio K (2005) Interfacial properties of Zn-Sn alloys as high temperature lead-free solder on Cu substrate. Mater Trans 46:2413–2418
Date M, Tu KN, Shoji T, Fujiyoshi M, Sato K (2004) Interfacial reactions and impact reliability of Sn Zn solder joints on Cu or electroless Au/Ni(P) bond-pads. J Mater Res 19:2887–2896
Kim YJ, Jo EJ, Kamble AS, Gang MG, Kim JH, Moon JH (2017) Improving the solar cell performance of electrodeposited Cu2ZnSn(S,Se)4 by varying the Cu/(Zn + Sn) ratio. Solar Energy 145:13–19
Oliveira GMD, Carlos IA (2009) Silver–zinc electrodeposition from a thiourea solution with added EDTA or HEDTA. Electrochim Acta 54:2155–2163
Domínguez-Ríos C, Moreno MV, Torres-Sánchez R, Antúnez W, Aguilar-Elguézabal A, González-Hernández J (2008) Effect of tartrate salt concentration on the morphological characteristics and composition of Cu-Zn electroless plating on zamak 5 zinc alloy. Surf Coat Technol 202:4848–4854
Clauwaert K, Binnemans K, Matthijs E, Fransaer J (2016) Electrochemical studies of the electrodeposition of copper-zinc-tin alloys from pyrophosphate electrolytes followed by selenization for CZTSe photovoltaic cells. Electrochim Acta 188:344–355
Pecequilo CV, Panossian Z (2010) Study of copper electrodeposition mechanism from a strike alkaline bath prepared with 1-hydroxyethane-1,1-diphosphonic acid through cyclic voltammetry technique. Electrochim Acta 55:3870–3875
Juškėnas R, Karpavičienė V, Pakštas V, Selskis A, Kapočius V (2007) Electrochemical and XRD studies of Cu–Zn coatings electrodeposited in solution with d-mannitol. J Electroanal Chem 602:237–244
Carlos IA, Almeida MRHD. (2004) Study of the influence of the polyalcohol sorbitol on the electrodeposition of copper–zinc films from a non-cyanide bath. J Electroanal Chem 562:153–159
Ibrahim MAM, Bakdash RS (2015) New non-cyanide acidic copper electroplating bath based on glutamate complexing agent. Surf Coat Technol 282:139–148
Slupska M, Ozga P (2014) Electrodeposition of Sn-Zn-Cu alloys from citrate solutions. Electrochim Acta 141:149–160
Almeida MRHD., Barbano EP, Carvalho MFD, Carlos IA, Siqueira JLP, Barbosa LL (2011) Electrodeposition of copper–zinc from an alkaline bath based on EDTA. Surf Coat Technol 206:95–102
Broggi RL, Oliveira GMD, Barbosa LL, Pallone EMJA., Carlos IA (2006) Study of an alkaline bath for tin deposition in the presence of sorbitol and physical and morphological characterization of tin film. J Appl Electrochem 36:403–409
Carvalho MFD, Barbano EP, Carlos IA (2015) Electrodeposition of copper-tin-zinc ternary alloys from disodium ethylenediaminetetraacetate bath. Surf Coat Technol 262:111–122
Carvalho MFD, Barbano EP, Carlos IA (2013) Influence of disodium ethylenediaminetetraacetate on zinc electrodeposition process and on the morphology, chemical composition and structure of the electrodeposits. Electrochim Acta 109:798–808
El-Chiekh F, El-Haty MT, Minoura H, Montaser AA (2015) Electrodeposition and characterization of Cu–Ni–Zn and Cu–Ni–Cd alloys. Electrochim Acta 50:2857–2864
Long JM, Zhang X, Pei HZ (2013) Effect of triethanolamine addition in alkaline bath on the electroplating behavior, composition and corrosion resistance of Zn-Ni alloy coatings. Adv Mater Res 738:87–91
Jung M, Lee G, Choi J (2017) Electrochemical plating of Cu-Sn alloy in non-cyanide solution to substitute for Ni undercoating layer. Electrochim Acta 241:229–236
Senna LF, Díaz SL, Sathler L (2003) Electrodeposition of copper–zinc alloys in pyrophosphate-based electrolytes. J Appl Electrochem 33:1155–1161
Silva FLG, Lago DCBD., D’Elia E, Senna LF (2010) Electrodeposition of Cu–Zn alloy coatings from citrate baths containing benzotriazole and cysteine as additives. J Appl Electrochem 40:2013–2022
Chai YC, Truscello S, Van BS, Luyten FP, Vleugels J, Schrooten J (2011) Perfusion electrodeposition of calcium phosphate on additive manufactured titanium scaffolds for bone engineering. Acta Biomater 7:2310–2319
Darban AK, Aazami M, Meléndez AM, Abdollahy M, Gonzalez I (2011) Electrochemical study of orpiment (As2S3) dissolution in a NaOH solution. Hydrometallurgy 105:296–303
Barbano EP, Carvalho MFD, Carlos IA (2016) Electrodeposition and characterization of binary Fe-Mo alloys from trisodium nitrilotriacetate bath. J Electroanal Chem 775:146–156
Carvalho MFD, Brito MMD, Carlos IA (2016) Study of the influence of the trisodium nitrilotriacetic as a complexing agent on the copper, tin and zinc co-deposition, morphology, chemical composition and structure of electrodeposits. J Electroanal Chem 763:81–89
Ramírez C, Calderón JA (2016) Study of the effect of triethanolamine as a chelating agent in the simultaneous electrodeposition of copper and zinc from non-cyanide electrolytes. J Electroanal Chem 765:132–139
Li QY, Ge W, Yang PX, Zhang JQ, An MZ (2016) Insight into the role and its mechanism of polyacrylamide as an additive in sulfate electrolytes for nanocrystalline zinc electrodeposition. J Electrochem Soc 163:D127-D132
Fabian CP, Ridd MJ, Sheehan ME (2007) Assessment of activated polyacrylamide and guar as organic additives in copper electrodeposition. Hydrometallurgy 86:44–55
Ballesteros JC, Díaz-Arista P, Meas Y, Ortega R, Trejo G (2007) Zinc electrodeposition in the presence of polyethylene glycol 20000. Electrochim Acta 52:3686–3696
Khelladi MR, Mentar L, Azizi A, Kadirgan F, Schmerber G, Nucleation AD (2012) Growth and properties of Co nanostructures electrodeposited on n-Si (1 1 1). Appl Surf Sci 258:3907–3912
Ribeaucourt L, Savidand GD. Lincot E, Chassaing (2011) Electrochemical study of one-step electrodeposition of copper–indium–gallium alloys in acidic conditions as precursor layers for Cu(In,Ga)Se2, thin film solar cells. Electrochim Acta 56:6628–6637
Wibowo RA, Hamid R, Maier T, Dimopoulos T (2015) Galvanostatically-electrodeposited Cu–Zn–Sn multilayers as precursors for crystallising kesterite Cu2ZnSnS4 Thin Films. Thin Solid Films 582:239–244
Joint Committee on Powder Diffraction Standards, JCPDS (2000), Powder Diffraction File - PDF-2, Database Sets 1–49. ICDD, Pennsylvania, (CDROM)
He X, Shen H, Wang W, Pi J, Hao Y, Shi X (2013) Synthesis of Cu2ZnSnS4 films from co-electrodeposited Cu–Zn–Sn precursors and their microstructural and optical properties. Appl Surf Sci 282:765–769
Wang H, Hreid T, Li J, Zhang Y, Spratt H (2015) Effects of metal ion concentration on electrodeposited CuZnSn film and its application in kesterite Cu2ZnSnS4 solar cells. RSC Adv 5:65114–65122
Hreid T, O’Mullane AP, Spratt HJ, Will G, Wang H (2016) Investigation of the electrochemical growth of a Cu–Zn–Sn film on a molybdenum substrate using a citrate solution. J Appl Electrochem 46:1–10
Zhang YZ, Liao C, Zong K, Wang H, Liu JB, Jiang T, Han JF, Liu GQ, Cui L, Ye QY, Yan H, Lau WM (2013) Cu2ZnSnSe4 thin film solar cells prepared by rapid thermal annealing of Co-electroplated Cu-Zn-Sn precursors. Sol Energy 94:1–7
Elbeyli İY (2015) Production of crystalline boric acid and sodium citrate from borax decahydrate. Hydrometallurgy 158:19–26
Alexandratos SD (2015) The modification of hydroxyapatite with ion-selective complexants: 1-hydroxyethane-1,1-diphosphonic acid. Ind Eng Chem Res 54:585–596
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC51604180), the Applied Basic Research Programs of Science and Technology Department of Shanxi Province (201701D221036), the start-up funds of Taiyuan Institute of Technology, and the Youth Academic Leader of Taiyuan Institute of Technology support program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ding, L., Liu, F., Cheng, J. et al. Effects of four N-based additives on imitation gold plating. J Appl Electrochem 48, 175–185 (2018). https://doi.org/10.1007/s10800-018-1148-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-018-1148-8