Abstract
TiO2 nanotube arrays sensitized by PbS nanoparticles (TiO2 NTs/PbS) with enhanced visible-light activity were synthesized by a two-step approach including an electrochemical anodization technique followed by an in situ photodeposition approach. The structural investigations indicated that PbS nanoparticles grew uniformly on the walls of the TiO2 NTs. The TiO2 NTs/PbS exhibited more excellent photoelectrochemical properties than that of the TiO2 NTs under visible-light irradiation. The enhanced photoelectrochemical activity of the TiO2 NTs/PbS could be attributed to the improvement of visible-light absorption and charge separation derived from the coupling effect of the PbS nanoparticles and TiO2 NTs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Titanium dioxide nanotube arrays (TiO2 NTs) have attracted great interest due to their unique electronic and optical properties, which can be applied in photocatalysis, biosensor, and photo-chromic devices, among other applications [1–4]. However, the large band gap of TiO2 limits its photoactivity in the UV region of the solar spectrum. A clear way to enhance the efficiency of TiO2 would be to extend its photoactivity to the visible region of the spectrum [5, 6]. The coupling of semiconductors with narrow band gap to TiO2 NTs has been proved to be an efficient method to enhance the photoresponse to the visible-light region [7]. Lead sulfide (PbS) is an attractive semiconductor for this approach because of its small band gap (0.41 eV) and large exciton Bohr radius of 20 nm, which leads to extensive quantum size effects [8]. These specific advantages give more latitude to effectively use the size quantization effect for extending the absorption into the infrared range that comprises ~40 % of the solar spectrum.
In order to improve the separation of photogenerated charge carriers, PbS nanoparticles have been often combined with the TiO2 NTs to form a heterojunction nanostructure. Craig A. Grimes and his colleagues [9] applied successive ionic layer adsorption and reaction and electrodeposition methods to synthesize PbS nanoparticles for sensitisation of the TiO2 NTs. However, the reported photoconversion efficiency was still fairly low because of the poor combination of these two materials. Recently, we have newly developed simple and versatile low-temperature photodeposition techniques for directly coupling PbS nanoparticles on the surface of TiO2 NTs by taking advantage of the photocatalysis and photoinduced surface superhydrophilicity. The application of in situ photodeposition deposition technique to sensitize TiO2 NTs allows us to obtain two unique features: one is that efficient interfacial charge transfer is inherently guaranteed because TiO2 photocatalysis is utilized, and the other is that a large amount of PbS nanoparticles can be deposited on not only the external surfaces but also the inner surfaces of the TiO2 NTs [10]. Therefore, the photodeposition sensitisation of TiO2 NTs with regulatable coverage of PbS nanoparticles is significative. In this paper, we demonstrated a photodeposition method for tethering PbS nanoparticles on the TiO2 NTs to produce nanoscale heterostructures, and the prepared TiO2 NTs/PbS exhibited an exceptionally high photoelectrochemical activity under visible-light irradiation.
2 Experimental
2.1 Preparation of PbS nanoparticles on the TiO2 nanotube arrays
The TiO2 NTs were prepared according to a two-step anodization, which was similar to our previous reports [11, 12]. PbS nanoparticles sensitized TiO2 NTs photoelectrodes were successively prepared by photodeposition technique. Briefly, ethanol solution containing 2 mM Pb(Ac)2 and 0.2 mM S was prepared under continuous stir and then the TiO2 NTs substrate was immersed in the solution. After the solution had been bubbled with argon for 0.5 h in the dark, irradiation was carried out from the direction of the TiO2 NTs with a 500 W Xe arc lamp for 30 min.
2.2 Characterization
The phase compositions of samples were determined by a Rigaku D/Max 2,400 X-ray diffractometer (XRD) equipped with graphite monochromatized Cu Kα radiation. The morphology of the prepared samples was observed using scanning electron microscopy (SEM, Quanta 200 FEG). UV–Vis diffuse reflectance spectra (DRS) of the samples were recorded on a UV-2,550 UV–Vis spectrophotometer with an integrating sphere attachment. Photoelectrochemical measurements were performed a mixture of 0.1 M Na2S and 0.1 M Na2SO3 solution. The working electrode was illuminated with a solar simulator equipped with a 500 W Xe lamp with a visible-light filter (>420 nm). The photocurrent dynamics of the working electrode were recorded according to the responses to sudden switching on and off at 0.25 V bias.
2.3 Photocatalytic activity test
The photocatalytic activity of the prepared samples was evaluated by photocatalytic decomposition of MO solution under a 500 W Xe lamp with a visible-light filter (>420 nm) irradiation. Before photodegradation, adsorption equilibrium for the dye on catalyst surface was established by mechanical stirring in the dark for 30 min. After visible-light irradiation for 1 h, the remaining dye concentration was determined with a UV 1700 UV–Vis spectrophotometer by detecting the maximum absorption wavelength for MO at 464 nm.
3 Results and discussions
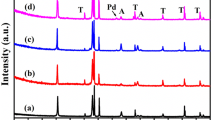
The typical XRD patterns of the TiO2 NTs and TiO2 NTs/PbS are shown in Fig. 1. The annealed TiO2 NTs (Fig. 1a) demonstrated a distinct XRD pattern that confirmed the formation of anatase TiO2 indexed to a standard JCPDS card (PDF no. 21–1272, labeled “A”). One could also observe the signals from the underlying Ti (PDF no. 44–1294, labeled “T”). The PbS sensitized TiO2 NTs had single intensities P (1 1 1), P (2 0 0), P (2 2 0), P (3 1 1), and P (2 2 2) at 2θ values: 25.96o, 30.06o, 43.03o, 50.95o, and 53.39o, respectively, which indicated a cubic structure of PbS (PDF no. 65–0692, labeled “P”), indicating that PbS nanoparticles were deposited on the TiO2 nanotubes.
Figure 2 showed the top-view and cross-sectional SEM images of the TiO2 NTs and PbS-decorated TiO2 NTs. From Fig. 2a and d, it was observed that the TiO2 NTs had a regularly arranged pore structure. The TiO2 NTs were entirely smooth with the same outer diameter of 160 nm. After photodeposition modification on the TiO2 NTs, the tube walls became decorated with aggregates of fine PbS nanoparticles partly penetrating into TiO2 NTs pores. The high-magnification SEM images (Fig. 2c, f) of the TiO2 NTs/PbS showed the uniform aggradation of PbS nanoparticles with average diameter of 50 nm.
The UV–Vis DRS of the TiO2 NTs and TiO2 NTs/PbS are presented in Fig. 3. The absorption edge of pure TiO2 NTs was observed at about 380 nm, corresponding to the band-gap energy of 3.2 eV. After photodeposition of PbS nanoparticles on the TiO2 NTs, the sample exhibited an obvious absorption in a wide wavelength range from UV to entire visible-light region. The enhanced ability of the TiO2 NTs/PbS electrode to absorb visible light made it a promising solar cell and photocatalyst for solar-driven applications.
The transient photocurrent responses and current voltage (I–V) curves of the TiO2 NTs and TiO2 NTs/PbS were measured under visible-light irradiation, respectively. From Fig. 4a, it could be seen that the average photocurrent density of the TiO2 NTs was nearly zero mA cm−2 because of none visible-light absorption, whereas that of TiO2 NTs/PbS was 9.49 mA cm−2. The photocurrent improvement of the TiO2 NTs/PbS was mainly attributed to the PbS responsive to visible light. Meanwhile, I–V curves of the samples were further measured under visible-light irradiation (Fig. 4b), which further confirmed that the photocurrent density of the TiO2 NTs/PbS was much higher than that of the plain TiO2 NTs.
To evaluate the photocatalytic degradation capability of the TiO2 NTs/PbS, we examined the decomposition of the dye MO under visible-light irradiation (Fig. 5a). The 88.7 % of MO removal was obtained after 4 h, while only 9.2 % of MO removal by TiO2 NTs was obtained in the photocatalytic process with same irradiation time. The excellent performance of the TiO2 NTs/PbS can be attributed to the interaction between PbS nanoparticles and TiO2 NTs. The detailed photocatalytic progress could be inferred as following: when illuminated, PbS nanoparticles effectively absorb visible lights and excite electrons and holes pairs. Owning to the small size effect of the PbS nanoparticles, the conduction band (CB) of the PbS could be split, and the CB of the TiO2 lays more positive than that of the PbS CB, then electron injection is expected from the photoexcited PbS nanoparticles into the TiO2 CB [13]. Thus, the photogenerated electron–hole pairs are separated effectively by the p–n junction formed on the TiO2 NTs/PbS interface, and the recombination of electron–hole pairs can be reduced. The separated electrons and holes are then free to initiate reactions with the MO adsorbed on the photocatalyst surfaces. To investigate the photocatalytic stability of the TiO2 NTs/PbS, five and ten cycles of photodegradation of MO (Fig. 5b) were conducted under visible-light irradiation. After the tenth runs, the degradation ratio of MO was well maintained, i.e., dropped only to 4.4 %, indicating that the TiO2 NTs/PbS photocatalyst could be safely reused.
4 Conclusions
In summary, we have developed a simple photodeposition method to fabricate small and uniform PbS nanoparticles on the TiO2 NTs. The sensitisation of the TiO2 NTs by PbS nanoparticles significantly enhanced visible-light response of the electrode. The TiO2 NTs/PbS showed higher photocurrents and consequently higher photocatalytic activity. The enhanced photoelectrochemical properties were attributed to the extended absorption in the entire visible-light region resulting from PbS nanoparticles, and the effective separation of photogenerated carriers driven by the photoinduced potential difference was generated at the interface of the TiO2 NTs/PbS.
References
Mirabolghasemi H, Liu N, Lee K, Schmuki P (2013) Chem Commun 49:2067–2069
Yu L, Wang ZY, Zhang L, Wu HB, Lou XW (2013) J Mater Chem A 1:122–127
Kuo YY, Li TH, Yao JN, Lin CY, Chien CH (2012) Electrochim Acta 78:236–243
Tsui LK, Homma T, Zangari G (2013) J Phys Chem C 117:6979–6989
Cai FG, Yang F, Xi JF, Jia YF, Cheng CH, Zhao Y (2013) Mater Lett 107:39–41
Wang XL, Zheng J, Sui XT, Xie H, Liu BS, Zhao XJ (2013) Dalton Trans 42:14726–14732
Li GS, Wu L, Li F, Xu PP, Zhang DQ, Li HX (2013) Nanoscale 5:2118–2125
Wang DF, Zhao HG, Wu NQ, El Khakani MA, Ma DL (2010) J Phys Chem Lett 1:1030–1035
Kang Q, Liu SH, Yang LX, Cai QY, Grimes CA (2011) ACS Appl Mater Interfaces 3:746–749
Fujii M, Nagasuna K, Fujishima M, Akita T, Tada H (2009) J Phys Chem C 113:16711–16716
Wang QY, Qiao JL, Gao SM (2014) Mater Lett 131:354–357
Wang QY, Qiao JL, Xu XH, Gao SM (2014) Mater Lett 131:135–137
Etgar L, Moehl T, Gabriel S, Hickey SG, Eychmüller A, Grätzel M (2012) ACS Nano 6:3092–3099
Acknowledgments
This work was financially supported by the Science and Technology Development Project of Jilin Province (No. 20140520161JH).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qiao, J., Wang, Q. & Xiao, Y. High-efficiency photoelectrochemical performance of PbS nanoparticles sensitized TiO2 nanotube arrays. J Appl Electrochem 44, 1007–1011 (2014). https://doi.org/10.1007/s10800-014-0725-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-014-0725-8