Abstract
Electrochemical reduction of the azo dye disperse orange 1 (DO1) was performed by square wave and cyclic voltammetry using a boron-doped diamond electrode. The dissolution of DO1 was evaluated by monitoring the UV–Vis spectra of the solutions, and best results were obtained using aqueous solutions containing 5 % Fongranal FB dispersant. Three irreversible peaks were obtained for the electrochemical reduction of DO1, while the dispersant showed one irreversible peak. The overall electrodic process was mainly diffusion controlled. A mechanism is proposed for the reduction of the dye.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Synthetic dyes are widely used in the textile, pharmaceutical, food, cosmetics, and leather industries, as well as in printing and color photography [1]. The largest class of these substances is the azo category, where aromatic moieties are linked together by azo (–N=N–) chromophores, which includes reactive, disperse, and acid textile dyes [2]. The compounds are toxic, highly persistent, and are regarded as environmental pollutants that threaten aquatic life and human health [2, 3].
The disperse dyes, which include disperse orange 1 (DO1, Fig. 1), are a class of water-insoluble dyes that are applied to polyester and other fibers by hydrophobic suspension [4]. The dyeing process is generally performed in the presence of long-chain dispersing agents that help to stabilize the dye suspension and facilitate contact between the dye and the hydrophobic fiber [4–6]. Approximately 4,500 kg of dye are released into waterways annually, due to losses that occur during the process of fixing the dye to the fibers, with the disperse class contributing roughly 35 % of the total [7]. These chemicals are able to accumulate in sediments or soils, and can contaminate drinking water. Traditional wastewater treatment technologies are largely ineffective in removing these dyes, due to the chemical stability of the compounds [8].
Human exposure to azo dyes occurs mainly through ingestion of contaminated water, dermal absorption from wearing azo-dyed clothing or footwear, or inhalation in occupational settings [9]. The human intestinal microbiota plays a role in the degradation of azo dyes, with azo reduction being the most important reaction with respect to their toxicity and mutagenicity [10]. The intestinal microflora metabolizes ingested azo dyes to colorless aromatic amines, using NAD(P)H-dependent azoreductases [11–14]. Although the dyes can be reduced in the mammalian liver to form aromatic amines, the intestinal microflora is probably primarily responsible for the in vivo reduction of azo dyes [10–14]. Chequer et al. [15] demonstrated the mutagenicity of DO1 using the micronucleus assay in human lymphocytes and in the HepG2 human liver cell line. Ferraz et al. [16] used the comet assay with HepG2 cells derived from human hepatoma, together with the Ames Salmonella/microsome mutagenicity assay, and showed that DO1 is able to induce mutations in Salmonella typhimurium, cause DNA damage in human HepG2 cells, and provoke cell death by apoptosis.
DO1 (CAS 2581-69-3) possesses nitro and azo groups that can be electrochemically reduced. The reduction mechanisms of several compounds that possess the same electroactive groups have been extensively investigated and shown to be dependent on pH [17–20]. The electrochemical behavior of DO1 has been studied by oxidation of the compound at a glassy carbon electrode in a non-aqueous supporting electrolyte (0.1 mol L−1 Bu4NBF4/DMF) [21]. However, to the best of our knowledge, there are no reports in the literature concerning the electroreduction of DO1 in aqueous and non-aqueous supporting electrolytes. Owing to the low solubility of DO1 in pure water, it is necessary to add solvents or dispersing agents, and investigate the stability of the stock solutions before electrochemical investigations.
The aim of this work was to investigate the solubility and stability of DO1 in aqueous solution, and to propose a mechanism for the reduction of DO1 at a boron-doped diamond (BDD) electrode [22, 23], to suggest possible compounds that might be produced during dye degradation by the human intestinal microflora.
2 Experimental
2.1 Reagents and solutions
Monobasic sodium phosphate (NaH2PO4, 98–102 %), dibasic sodium phosphate (Na2HPO4, 99.5 %), and sulfuric acid (H2SO4, 99 %) were provided by Mallinckrodt. DO1 (96 %) was obtained from Sigma-Aldrich. The dispersants tamol (95 %, Basf), setamol (purity not declared, Basf), and Fongranal FB (purity not declared, Clariant) were kindly provided by Lupo Indústria S.A. (Araraquara, São Paulo, Brazil). The 0.1 mol L−1 sodium phosphate buffer solutions (pH 7.0) were prepared from the salts Na2HPO4 and NaH2PO4. All solutions were prepared using purified water (ρ = 18.2 MΩ cm) obtained from a Milli-Q system (Millipore). Stock solutions of 4.0 × 10−4 mol L−1 DO1 were prepared using pure water, pure solvents (acetone, dimethylformamide, acetonitrile, or ethanol), and the dispersants tamol (3.0 %, m/v), setamol (0.5 %, v/v), and Fongranal FB (5.0 %, v/v). Working solutions were prepared by dilution of the different stock solutions with deionized water, to give final concentrations of 5.0 × 10−5 mol L−1. The stabilities of the dye solutions storage at 4 °C were determined by measuring the absorption intensities at 492 nm on different days.
2.2 Apparatus
The electrochemical measurements were carried out using a PGSTAT 30 Autolab (Eco Chemie, B.V., The Netherlands) computer-controlled potentiostat. The voltammetric experiments were performed using a three-electrode arrangement fitted into a one-compartment Pyrex glass cell (20 mL). The BDD films (Adamant Technologies S.A., La Chaux-de-Fonds, Switzerland) contained 8,000 ppm of boron (working electrode area = 0.25 cm2). The reference system was an Ag|AgCl (KClsat) electrode, and the counter electrode was a 1-cm2 Pt foil. A Hewlett-Packard Model 8453 UV–Vis spectrophotometer, fitted with quartz cells (1 cm optical path length), was used to evaluate the solubility and stability of the DO1 stock solutions.
2.3 Electrochemical behavior of DO1 on the BDD electrode
The BDD electrode surface was previously activated by applying −3.0 V for 30 s in a 0.5 mol L−1 solution of H2SO4. For reactivation (which involved removal of the adsorbed species after the experiments), the electrode was first pre-treated at +3.0 V for 60 s in the H2SO4 solution, and then submitted to the activation procedure [23–25].
The electrochemical response of DO1 (at a concentration of 5.0 × 10−5 mol L−1) was analyzed by square wave voltammetry (SWV) using the BDD electrode, with a frequency (f) of 200 Hz, a pulse amplitude (a) of 60 mV, and a scan increment (ΔEs) of 2 mV. The supporting electrolyte was a pH 7.0 solution of phosphate buffer (0.1 mol L−1). The electrochemical reduction of DO1 by cyclic voltammetry (CV) was conducted using the BDD electrode in the potential range 0.0 V to −1.4 V versus Ag|AgCl (KClsat). The solutions were previously degassed by bubbling N2 for 2 min. Evaluations were made of the influences of successive scans (with adsorption of the reduction products on the electrode surface) and scan rates in the range 50–500 mV s−1. In both techniques (SWV and CV), the voltammetric response of the Fongranal FB dispersant was measured using the same supporting electrolyte and dispersant concentrations as those used in the presence of the dye.
3 Results and discussion
3.1 Evaluation of the solubility and stability of DO1 by UV–Vis spectroscopy
The electronic absorption spectrum of DO1 exhibits an intense absorption band in the range 390–570 nm, due to a π–π* electronic transition of the amino-4-nitroazobenzene chromophore [26]. This band region was used during evaluation of the solubility and stability of the solutions.
The term solubilization refers to the homogeneous mixing of an otherwise insoluble compound into a liquid medium by addition of a solubilizing agent. Successful dye preparation requires wetting of the dye particles by the liquid phase, deagglomeration of particle aggregates by mechanical destruction, and stabilization of the dispersed suspension to prevent reagglomeration [27, 28]. Special additives are required to lower the high surface tension of water and enable sufficient wetting of the pigment.
The need for an additive was first confirmed by analyzing the DO1 solution obtained by dilution of a stock solution prepared using H2O alone and heating at 100 °C for 20 min. Aqueous solutions of DO1 were then obtained by diluting the stock solutions prepared in pure organic solvents (ethanol or acetone) or in aqueous solutions containing the dispersants tamol and setamol. These solutions presented low, or zero, absorption in the visible region, since in no case was complete dissolution of the compound observed. Both tamol and setamol are anionic dispersants, which can be the reason for no dissolution of the nonionic DO1. A satisfactory aqueous solution of DO1 was obtained using the Fongranal FB dispersant, i.e., in the presence of this dispersant, the DO1 aqueous mixture changed from dispersion to an apparently clear solution. This dispersant (the composition of which is protected by a patent) is a pH-neutral oxyethylation product that is nonionic, colorless, and water soluble. It is used in the textile industry, at concentrations of between 0.5 and 2 mL L−1 (0.05–0.2 %), as an equalizing agent to insure uniformity during dyeing and printing.
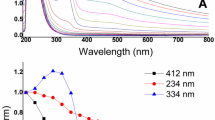
Individual aqueous solutions of DO1 (4.0 × 10−4 mol L−1) were prepared using dispersant concentrations between 0.2 and 5 %. UV–Vis spectra were obtained for 5.0 × 10−5 mol L−1 DO1 solutions, prepared by dilution of the stock solutions in pure water (Fig. 2). The molar absorptivity (ε) of the compound varies from 7,900 to 16,800 cm−1 M−1, corresponding to the cis and trans (most stable) forms, respectively [29].
The intensity of the DO1 absorption band at 492 nm increased in line with the amount of dispersant used, and a spectrum with good resolution and sensitivity was obtained for 5 % dispersant (Fig. 2). As a dispersant is added gradually to water, the surface tension falls until a certain concentration of dispersant is reached [the critical micelle concentration (CMC)]. For certain substances, the solubility can increase at dispersant concentrations higher than the CMC [30]. The increasing of the dispersant concentration caused changes in the aggregation state of dye, leading to an increase of the absorbance at 492 nm in the same DO1 concentration. Dispersant concentration has an important role in physical and chemical processes, which may cause modifications in position, intensity, and shape of absorption bands. This procedure is particularly useful for studies of the behavior of DO1 since dispersing agents are commonly used in the textile industry, while systems employing aqueous micellar media provide simple models for reactions occurring at biomembrane interfaces. These systems can provide approximations of the natural environments of organisms [31, 32]. Additional experiments were therefore performed using a Fongranal FB concentration of 5 % (which is higher than the CMC) in the dye stock solution. No significant variation (2.3 %) of the band intensity was observed during storage of the DO1 stock solution for 90 days at 4 °C, indicating good stability of the solution.
3.2 Electrochemical behavior of DO1 at the BDD electrode
Optimum activity of intestinal microflora azoreductases is achieved at pH 7.0 [13]. Since these enzymes are found in the colon region of the intestine, where the pH varies between 6.6 (transverse colon) and 7.0 (descending colon) [33], the electrochemical experiments were carried out at pH 7.0 to mimic natural conditions as best as possible.
3.2.1 SWV
Square wave voltammograms were recorded at 400 mV s−1 using a solution of 5.0 × 10−5 mol L−1 DO1 in 0.1 mol L−1 pH 7.0 phosphate buffer (Fig. 3a, voltammogram 1). Three well-defined peaks were observed, at −0.43 V (1c), −0.62 V (2c), and −0.80 V (3c), which represented processes involving the dye, while a peak at −1.02 V (4c) was related to the dispersant since the separate voltammetric response of this substance showed a single peak at −0.98 V (Fig. 3a, voltammogram 2). Previous adsorption of the dye on the electrode affected the electrochemical response for the dispersant, causing a displacement of 40 mV in the peak potential.
a Square wave voltammograms obtained in 0.1 mol L−1 phosphate buffer at pH 7.0 for 5.0 × 10−5 mol L−1 DO1 solution (full line, 1) and Fongranal FB dispersant (dashed line, 2). b Square wave voltammograms for 5.0 × 10−5 mol L−1 DO1 solution, and c for Fongranal FB dispersant: I t total current, I f forward current, and I b backward current. SWV conditions: f 200 Hz, a 60 mV, and ΔE s 2 mV
Total, forward, and backward components of the currents for the dye solution and for solution containing only the dispersant response are presented in Fig. 3b, c respectively. Irreversible electrochemical processes can be observed for peaks 1c and 2c since there were no backward current contributions. Small contributions of backward current were observed for peaks 3c and 4c in Fig. 3b. However, a very similar profile of the backward current was observed for only Fongranal FB solution in Fig. 3c, showing that the current contribution can be attributed to the dispersant process.
3.2.2 CV
The reduction behavior of the dye was studied by CV using a solution of 5.0 × 10−5 mol L−1 DO1 in 0.1 mol L−1 phosphate buffer at pH 7.0. As observed in the SWV experiments, the dye solution (Fig. 4, full line) presented peaks at −0.48 V (1c), −0.67 V (2c), −0.85 V (3c), and −1.16 V (4c). The dispersant (Fig. 4, dashed line) presented one peak at −1.07 V. Peaks 1c, 2c, and 3c represent the electrodic process involving the dye, and peak 4c represents the response from the dispersant. Peak 4c presented higher current intensity and peak potential values in the presence of DO1, compared to the values obtained using the dispersant alone (dashed line). A possible explanation for this increase of current could be that in the presence of the dye the dispersant molecules were organized around the DO1 molecules, resulting in greater exposure of the reducible groups of Fongranal FB. It is also possible that effects related to earlier adsorption of the dye on the BDD surface could have hindered reduction of the dispersant, shifting the potential to negative values. No anodic peaks were observed during the upward CV scan, indicating that the DO1 electroreduction process was irreversible.
Successive scans obtained for the same DO1 solution in 0.1 mol L−1 phosphate buffer at pH 7.0 (Fig. 5), without any intermediate cleaning of the electrode surface, showed a continuously diminishing response (voltammograms 1–5) due to the adsorption of the dye and dispersant and/or its reduction products. After reactivation of the BDD surface, a response (voltammogram 6) similar to that of the first voltammogram was obtained, indicating that the procedure used to reactivate the diamond surface was satisfactory.
Sequential cyclic voltammograms were recorded using 5.0 × 10−5 mol L−1 DO1 in pH 7.0 phosphate buffer, at different scan rates in the range 100–500 mV s−1 (Fig. 6). A clean BDD electrode surface was used for each voltammogram. No anodic peaks were observed at any scan rate, suggesting the irreversibility of the DO1 electroreduction.
The peak currents (1c, 2c, 3c, and 4c, after baseline correction) increased proportionally to the square root of the scan rate, which confirms that the processes were irreversible [34, 35]. The relationships between log I p and log ν for the different peaks could be described by the following equations:
The slopes of 0.508, 0.525, and 0.553 for peaks 1c, 3c, and 4c, respectively, were close to the theoretical value of 0.5 for a purely diffusion-controlled current, while the slope of 0.778 for peak 2c suggest a mixed diffusion–adsorption peak [36]. These findings suggest that the overall electrode process was mainly diffusion-controlled.
The peak potentials shifted to more negative values, confirming the irreversible nature of the reduction process [34], and linear relationships (E p vs. log ν) were observed in the ranges 100–500 mV s−1 (peak 1c) and 50–500 mV s−1 (all other peaks). These relationships could be described by
For irreversible electrode processes, E p is defined by the following equation [37]:
where α is the transfer coefficient, k 0 is the standard heterogeneous rate constant of the reaction, n is the number of electrons transferred, ν is the scan rate, and E 0 is the formal redox potential. Other symbols have their usual meanings. Values of αn can be calculated from the slopes of plots of E p versus log ν, and the value of E 0 can be obtained from the intercept of E p versus ν (Table 1). According to Bard and Faulkner [34], a plot of ln I p against E p − E 0, determined at different scan rates, should have a slope of –αF/RT. It was therefore possible to obtain the α values and the number of electrons transferred for each peak (Table 1).
In order to characterize each peak, successive cyclic voltammograms were recorded in the cathodic direction, from 0.0 V (start potential) to −0.60, −0.85, and −1.05 V, for peaks 1c, 2c, and 3c, respectively (Fig. 7). The anodic scans were ended at +0.2 V for all voltammograms. The cyclic voltammogram for peak 1c (from 0.0 to −0.60 V) presented a single cathodic peak at −0.48 V in the first scan, and an anodic peak at −0.01 V (1a) in the reverse sweep. No additional cathodic peak was observed during the second scan from positive to negative potentials. The same experiment carried out for peak 2c (scanning from 0.0 to −0.85 V) showed the expected two cathodic peaks, at −0.48 V (1c) and −0.67 V (2c), during the first scan. A decrease of the anodic peak at −0.01 V was observed in the reverse sweep. An additional cathodic peak was observed at −0.03 V during the second sweep from positive to negative potentials. No additional peaks were observed for the scans performed from positive potentials to the switching potential at the end of peaks 3c and 4c.
According to the literature [17, 38, 39], the electrochemical behavior of nitrobenzene in neutral aqueous buffer should reflect a four-electron reduction to produce a hydroxylamine derivative. However, the results presented here suggest that the presence of a dispersant could promote the formation of different intermediates. The reduction of DO1 generated three peaks when aqueous medium containing dispersant was used. It is proposed that the first peak at −0.48 V reflected reduction of the nitro group to the nitro radical anion (RNO −·2 ), by transfer of a single electron. Considering the reactivity of nitro radical anion [40] generated at −0.48 V, it undergoes chemical following reactions when the cathodic sweep stopped at −0.60 V. This process leads to formation of a product responsible by the anodic peak at −0.01 V showed in the reverse scan. When the switching potential is more negative, under anaerobic conditions, the nitro radical anion formed can undergo further one-electron reduction to a nitroso derivative, corresponding to the second cathodic peak (2c) at −0.67 V [41]. This intermediate could be responsible for decreasing the current of the peak at −0.01 V during the reverse scan, and could be reduced at −0.03 V during the second scan in the cathodic direction.
DO1 also contains an azo group that is liable to reduction. It is known that reduction of aromatic azo compounds, involving breakage of the N–N bond, is difficult in neutral media [42]. The cleavage of the azo group should be a four-electron process, while a reduction to a hydrazo compound should involve only two electrons. However, the presence of dispersant could alter the pathway of electron transfer, and unusual molecular surroundings could be associated with chemical steps including protonation [31]. Given these considerations, and the number of electrons transferred (~4), it is therefore reasonable to assume that the third peak was related to reduction of the N=N double bond by 2e− + 2H+, which generated the corresponding hydrazo (NH–NH) intermediate. This was then followed by cleavage of the N–N bond by 2e− + 2H+, with formation of the corresponding aromatic amines. It was not possible to propose a mechanism for the reduction of the Fongranal FB dispersant (peak 4c) since its structure was unknown. The complete proposed mechanism for DO1 reduction in the presence of the dispersant is shown in Fig. 8.
It is important to highlight some of the features of the products generated. Studies have demonstrated the tendency of the nitro radical anion to interact with certain biologically relevant species, such as glutathione and cysteamine, as well as DNA bases (adenine, thymine, and cytosine) and the RNA base (uracil) [18, 40, 43]. These interactions can induce DNA lesions and result in carcinogenesis. Nitroso aromatics can interact with polyunsaturated fatty acids, yielding radical species that can cause lipid peroxidation and cell damage [44]. The nitroso group may be mutagenic, as reported by Haack et al. [45] using Salmonella typhimurium strains TA98 and TA100. The structures of aromatic amines resulting from DO1 azo reduction contain an aniline group (C6H5–NH2) that is considered to be toxic to aquatic life, as well as possibly carcinogenic and genotoxic [46]. There is no information available concerning the toxicity of the dispersant used, which is an important omission, offering opportunities for further research. In general, the products formed from DO1 reduction can present potential hazards to human health and to the environment.
4 Conclusions
DO1 is soluble in an aqueous solution containing 5 % Fongranal FB dispersant, and is stable for at least 90 days. This aqueous micellar environment could alter the pathway for electron transfer during the electrochemical reduction of DO1. At a BDD electrode, the process showed three well-defined irreversible peaks that reflected reduction of the nitro group in two steps to form a nitro radical anion and a nitroso derivative. The process involved reduction of the N=N double bond, generating the corresponding hydrazo (NH–NH) intermediate, followed by cleavage of the N–N bond to form the corresponding toxic aromatic amines. The dispersant used in this study provides a simple microenvironment that is analogous to biological membrane systems. Electrochemical studies of dye behavior can therefore mimic the action of enzymes of the intestinal microflora, suggesting that formation of dangerous nitroso derivatives and aromatic amines might occur in the organism.
References
Ali H (2010) Water Air Soil Pollut 213:251
Carneiro PA, Umbuzeiro GA, Oliveira DP, Zanoni MVB (2010) J Hazard Mater 174:694
Hong Y, Gu J (2010) Appl Microbiol Biotechnol 88:637
Guaratini CCI, Zanoni MVB (2000) Quím Nova 23:71
Umbuzeiro GA, Freeman H, Warren SH, Kummrow F, Claxton LD (2005) Food Chem Toxicol 43:49
Osugi ME, Zanoni MVB, Chenthamarakshan CR, de Tacconi NR, Woldemariam GA, Mandal SS, Rajeshwar K (2008) J Adv Oxid Technol 11:425
Frijters CTMJ, Vos RH, Scheffer G, Mulder R (2006) Water Res 40:1249
Shaul GM, Holdsworth TJ, Dempsey CRKA (1991) Chemosphere 22:1117
U.S. Environmental Protection Agency, Dyes derived from benzidine and its congeners (2010). http://www.epa.gov/oppt/existingchemicals/pubs/actionplans/DCB%20Action%20Plan_06232010.noheader.pdf. Accessed 24 October 2011
Rafii F, Cerniglia CE (1995) Environ Health Perspect 103:17
Xu H, Heinze TM, Chen S, Cerniglia CE, Chen H (2007) Appl Environ Microbiol 73:7759
Macwana SR, Punj S, Cooper J, Schwenk E, John GH (2010) Curr Issues Mol Biol 12:43
Pricelius S, Held C, Murkovic M, Bozic M, Kokol V, Cavaco-Paulo A, Guebitz GM (2007) Appl Microbiol Biotechnol 77:321
Oliveira DP, Carneiro PA, Sakagami MK, Zanoni MVB, Umbuzeiro GA (2007) Mutat Res Genet Toxicol Environ Mutagen 626:135
Chequer FMD, Angeli JPF, Ferraza ERA, Tsuboy MS, Marcarini JC, Mantovani MS, Oliveira DP (2009) Mutat Res Genet Toxicol Environ Mutagen 676:83
Ferraz ERA, Grando MD, Oliveira DP (2011) J Hazard Mater 192:628
Baizer MM, Lund H (1983) Organic electrochemistry: an introduction and guide, 2nd edn. Marcel Dekker, New York, p 1166
Mandal PC (2004) J Electroanal Chem 570:55
Gooding JJ, Compton RG, Brennan CM, Atherton JH (1996) Electroanalysis 8:519
Ghoneim MM, El-Desoky HS, Amer SA, Rizk HF, Habazy AD (2008) Dyes Pigments 77:493
Osugi ME, Rajeshwar K, Ferraz ERA, de Oliveira DP, Araújo AR, Zanoni MVB (2009) Electrochim Acta 54:2086
Luong JHT, Male KB, Glennon JD (2009) Analyst 134:1965
Salazar-Banda GR, Andrade LS, Nascente PAP, Pizani PS, Rocha RC, Avaca LA (2006) Electrochim Acta 51:4612
Garbellini GS, Uliana CV, Yamanaka H (2011) J Braz Chem Soc 22:1241
Salazar-Banda GR, Carvalho AE, Andrade LS, Rocha RC, Avaca LA (2010) J Appl Electrochem 40:1817
Airinei A, Homocianu M, Dorohoi DO (2010) J Mol Liq 157:13
Bernhardt C (1988) Adv Colloid Interface Sci 29:79
Pegelow U, Schulte H-G, Höfer R (1999) Prog Org Coat 35:191
Hair SR, Taylor GA, Schultz LW (1990) J Chem Educ 67:709
Choudhury AKR (2006) Textile preparation and dyeing. Science Publishers, New Hampshire, p 88
Westmoreland PG, Day RA Jr, Underwood AL (1972) Anal Chem 44:737
Sivagnanam U, Palaniandavar M (1996) J Electroanal Chem 410:43
Philip AK, Philip B (2010) Oman Med J 25:70
Bard AJ, Faulkner LR (2001) Electrochemical methods: fundamentals and applications, 2nd edn. Wiley, Hoboken, p 236
Brett CMA, Brett AMO (1998) Electroanalysis. Oxford University Press, New York, pp 8–36
Gosser DK Jr (1993) Cyclic voltammetry: simulation and analysis of reaction mechanisms, 2nd edn. VCH Publishers Inc., New York, pp 27–70
Laviron E (1979) J Electroanal Chem 101:19
Karakus C, Zuman P (1995) J Electroanal Chem 396:499
Rubinstein I (1985) J Electroanal Chem 183:379
Squella JA, Bollo S, Nunez-Vergara LJ (2005) Curr Org Chem 9:565
Carbajo J, Bollo S, Núñez-Vergara LJ, Campero A, Squella JA (2002) J Electroanal Chem 531:187
Issa IM, Issa RM, Temerk YM, Mahmoud MR (1973) Electrochim Acta 18:139
Squella JA, Jimenez G, Bollo S, Núñez-Vergara LJ (1997) Electrochim Acta 42:2305
Sammartano LJ, Giganti DM (1991) Chem Biol Interact 77:63
Haack T, Erdinger L, Boche G (2001) Mutat Res 491:183
Pinheiro HM, Touraud E, Thomas O (2004) Dyes Pigments 61:121
Acknowledgments
The authors thank the Brazilian agency FAPESP (Proc. 2009/08161-8, 2008/08990-1, and 2008/10449-7), CNPq (Proc. 313307/2009-1) for scholarships and financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Uliana, C.V., Garbellini, G.S. & Yamanaka, H. Electrochemical reduction of disperse orange 1 textile dye at a boron-doped diamond electrode. J Appl Electrochem 42, 297–304 (2012). https://doi.org/10.1007/s10800-012-0403-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-012-0403-7