Abstract
The spherical LiFePO4/C and LiFe0.9Mg0.1PO4/C powders were successfully prepared from spherical FePO4 via a simple uniform-phase precipitation method at normal pressure, using FeCl3 and H3PO4 as the reactants. The FePO4, LiFePO4/C, and LiFe0.9Mg0.1PO4/C powders were characterized by scanning electron microscopies (SEM), powder X-ray diffraction (XRD), X-ray photoelectron spectrometer (XPS), and tap-density testing. The uniform spherical particles produced are amorphous, but they were crystallized to FePO4 after calcining above 400 °C. Due to the homogeneity of the basic FePO4, the final products, LiFePO4/C and LiFe0.9Mg0.1PO4/C, are also significantly uniform and the particle size is of about 1 μm in diameter. The tap-density of the spherical LiFePO4/C and LiFe0.9Mg0.1PO4/C are 1.75 and 1.77 g cm−3, respectively, which are remarkably higher than the non-spherical LiFePO4 powders (the tap-density is 1.0–1.3 g cm−3). The excellent specific capacities of 148 and 157 mAh g−1 with a rate of 0.1 C are achieved for the LiFePO4/C and LiFe0.9Mg0.1PO4/C, respectively. Comparison of the cyclic voltammograms of LiFePO4/C and LiFe0.9Mg0.1PO4/C shows enhanced redox current and reversibility for the sample substituting Mg on the Fe site. LiFe0.9Mg0.1PO4/C exhibits better high-rate and cycle performances than the un-substituted LiFePO4/C.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Phosphates LiMPO4 (M = Mn, Fe, Co, or Ni) have been investigated intensively as promising cathode materials for lithium batteries [1–9]. Among this series of materials, LiFePO4 is a low cost material and highly compatible to the environment. LiFePO4 offers several advantages compared with LiCoO2, LiMn2O4 and their derivatives. LiFePO4 offers several advantages: (i) a relatively high theoretical specific capacity of 170 mAh g−1, (ii) good reversibility of cathode reactions, (iii) high thermal and chemical stability, (iv) low material cost and toxicity, and (v) improved safety. In spite of these attractive features, LiFePO4 requires further modifications to overcome limitations of poor electronic conductivity, which leads to initial capacity loss and poor rate capability, and the low pile density, which leads to low volumetric specific capacity.
Several researchers [10–13] explained synthesis of spherical powders could be an effective way to increase the tap-density and safety of the powder. The tap-density of LiFePO4 powder is usually 1.0–1.3 g cm−3, which is much lower than the tap-density of commercially used LiCoO2 (2.3–2.5 g cm−3). The low tap-density of LiFePO4 limits the energy density of lithium ion batteries. The powders composed of spherical particles have higher density than the powders composed of irregular particles. Therefore, to obtain high tap-density of LiFePO4 powder, preparing spherical powders is expected as an effective way.
Several papers have suggested metal ion doping as a method for improving performance [6, 14–16]. Substituting Mg and other species on the Li site was reported by Chung et al. [6] to give a greatly increase in conductivity of LiFePO4 and a greatly improved electrode performance. The first reported work on substituting Mg on the Fe site (LiFe1−xMgxPO4 materials) was reported by Barker et al. [17] who successfully synthesized LiFe0.9Mg0.1PO4 via a carbothermal reaction. Later several publications confirmed the formation of this material under different synthesis conditions and reported improved capacity, conductivity, and rate capability [18–21].
In this study, spherical LiFePO4/C and LiFe0.9Mg0.1PO4/C cathode materials were synthesized by a solid-mixture of Li2CO3, spherical FePO4, and sugar. The physicochemical properties and electrochemical behaviors of the samples were characterized.
2 Experimental
Following the method reported by Wilhelmy and Matijević [22], spherical FePO4 powder was prepared by aging a solution of FeCl3 (2 × 10−3 M) and H3PO4 (3 × 10−2 M) at 40 °C. The resulting particles were thoroughly washed by distilled water and finally dried in vacuum at 60 °C for 24 h. The spherical FePO4 · xH2O powder was pre-heat treated at 550 °C for 7 h in air to obtain spherical anhydrous FePO4 powder. For preparing spherical LiFePO4/C and LiFe0.9Mg0.1PO4/C, stoichiometric amounts of LiOH, Mg(OH)2, and sugar were uniformly mixed in a molar ration of LiOH:Mg(OH)2:sugar:H2O = 1.0:0.1:0.1:4. Then, spherical FePO4 powder in a molar ratio of FePO4:LiOH = 1:1 was added into the slurry and agitated the mixture. The mixed slurry was dried and then heated at 600 °C for 5 h in Ar flow.
The sample morphology was examined by a JSM 6700 field emission scanning electron microscope (FESEM) operating at an accelerating voltage of 5 kV. The amount of carbon and Mg was determined by Oxford INCA Energy Dispersive X-ray (EDX) Spectrometer. The crystal structures of samples were characterized by X-ray diffraction (XRD) on a Bruker GADDS diffractometer using Cu Kα radiation and a graphite monochromator. The surface elements’ content of LiFePO4/C and LiFe0.9Mg0.1PO4/C powders were determined by an X-ray photoelectron spectrometer (XPS, VG ESCALAB MKII) equipped with a Mg Kα X-ray sources.
The spherical LiFePO4/C and LiFe0.9Mg0.1PO4/C were mixed with 10 wt% of carbon black and 10 wt% of poly vinylidene difluoride (PVDF) in 1-methyl-2-pyrrolidinone (NMP). The slurry was used to coat 20-μm thick aluminium disks of 13 mm diameter to a mass loading of 2 mg cm−2 after drying (at 120 °C) and compaction (at 2.0 × 106 Pa). Each coated electrode was assembled in a 2,016 coin cell using a lithium counter electrode, a microporous polypropylene separator, and an electrolyte of 1 M LiPF6 in a 50:50 (w/w) mixture of ethylene carbonate (EC) and diethyl carbonate (DEC). Cell assembly was carried out in an argon-filled glove box with less than 1 ppm each of oxygen and moisture.
The cells were discharged and charged at 25 °C on a Bitrode battery test system. Cyclic voltammetry was conducted with an EG&G model 263A potentiostat/galvonostat.
3 Results and discussion
The morphology for FePO4, LiFePO4/C, and LiFe0.9Mg0.1PO4/C powders was observed on SEM, as shown in Fig. 1. The FePO4 particles are small spherical particles that are fairly well-dispersed, and have a uniform particles size (about 1 μm) distribution (Fig. 1a). It is obviously recognized that the small spherical particles grow via the aggregation of small primary particles, similar to the case of spherical nickel [23], manganese [24], and iron [25] phosphate particles reported previously. The LiFePO4/C and LiFe0.9Mg0.1PO4/C particles are also composed of spherical particles similar to the FePO4 precursors (Fig. 1b, c), although there are small quantities of fragments. The tap-density of the spherical LiFePO4/C and LiFe0.9Mg0.1PO4/C powders are 1.75 and 1.77 g cm−3, respectively, which are remarkably higher than the non-spherical LiFePO4 powders (the tap-density is 1.0–1.3 g cm−3). For practical applications, the high tap-density of LiFePO4 cathode materials leads to the high volumetric specific capacity.
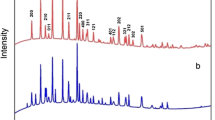
The XRD spectra of FePO4 after being treated at different temperatures are shown in Fig. 2. It can be seen obviously that the FePO4 produced are amorphous. This amorphous structure strongly supports that the uniform spherical particles are agglomerates of primary particles as same as reported for iron phosphate particles [25]. The amorphous structure is preserved up to 300 °C. After calcining the particles above 400 °C, the characteristic XRD patterns of FePO4 appear. In the present study, considerable crystallization has occurred under heating at 400 °C. Nevertheless, the crystalline peak intensities of the 400 °C samples are less than those samples prepared at higher temperatures. The crystallization temperature of LiFePO4 has been reported to be ~567 °C, based on a different thermal analysis study [26]. The spectrum of LiFe0.9Mg0.1PO4/C is almost the same as the spectrum of pure ordered orthorhombic olivine structured LiFePO4 (Fig. 3). The absence of any other signals indicates there are no unwanted impurity phases, such as Li3PO4 and Fe3+ related compounds. No evidence of diffraction peaks for crystalline carbon appeared in the diffraction patterns, which indicates that the carbon generated from sugar is amorphous carbon and its presence does not influence the structure of LiFePO4. The amounts of carbon in the LiFePO4/C and LiFe0.9Mg0.1PO4/C are about 8.15 and 8.28 wt%, respectively, through the element analysis, in other words, the molar ratios of LiFePO4:C and LiFe0.9Mg0.1PO4:C are about 1:1.17 and 1:1.19, respectively.
In order to examine the surface elements’ content of the LiFePO4/C and LiFe0.9Mg0.1PO4/C, XPS analysis was performed. As shown in Fig. 4, a sharp peak at about 283.5 eV corresponding to C 1s with a high intensity is noted. The binding energy of Fe 2p, O 1s, and P 2p are determined to be 709.4, 529.5, and 131.5 eV, respectively. As the binding energy for the Li 1s emission peak at 55.6 eV is very close to the Fe 3p peak at about 54.1 eV, accurate determination of its binding energy and estimation of the element content were precluded. Mg 2p peak at 50.0 eV is not seen clearly since it is superposed on the Fe 3p and Li 1s at about 54.1 eV. According to XPS analysis on the surface of the LiFePO4/C and LiFe0.9Mg0.1PO4/C, the C:P molar ratios are about 6:1 and 5.9:1, which indicate the surface composition should be mainly the carbon and the LiFePO4 or LiFe0.9Mg0.1PO4 particles rather perfectly coated by carbon [12]. EDX spectra of the LiFePO4/C and LiFe0.9Mg0.1PO4/C are shown in Fig. 5. The Mg:Fe molar ratio is 1:8.6 in the LiFe0.9Mg0.1PO4/C, which corresponded well with the amounts of Mg and Fe used in the starting mixture. The results of XPS and EDX analysis indicate that the Li, Fe, P, C, and Mg contents in the LiFePO4/C and LiFe0.9Mg0.1PO4/C are about the same as that in the initial mixtures.
Typical charge/discharge curves of the LiFePO4/C in the first two cycles with low current density (0.1 C) are shown in Fig. 6. The first discharge capacity is 148 mAh g−1, and then increases to 157 mAh g−1 in the second cycle, which is due to the “activation” of the first cycle reaction [27]. A flat and long voltage curve around 3.4 V indicates that the two-phase redox reaction proceeds via a first-order transition between LiFePO4 and FePO4. The large discharge capacity of the samples was due to the relatively small particle size. The electronic conductivity of LiFePO4 is very low, and diffusion of Li+ ion in the olivine structure is slow [1, 4, 28]. The smaller particle size, which is helpful for accessibility of the redox centers, is preferable to achieve larger capacity.
The results of CV experiments on LiFePO4/C and LiFe0.9Mg0.1PO4/C are presented in Fig. 7, where anodic and cathodic peaks appear at ~3.6 and 3.3 V for both materials, respectively. The main differences between LiFePO4/C and LiFe0.9Mg0.1PO4/C are in the peak shapes and heights of the voltammogram. The redox current for LiFe0.9Mg0.1PO4/C (0.85 mA) is higher than that for LiFePO4/C (0.68 mA). The larger redox current for LiFe0.9Mg0.1PO4/C results from its higher utilization due to its good electronic conductivity and lithium ion diffusivity, compared with the un-doped LiFePO4/C.
Figure 8 shows discharge curves of the LiFePO4/C and LiFe0.9Mg0.1PO4/C measured at various rates. The electrode was charged up to 4.2 V at 0.1 C prior to each discharge. The LiFePO4/C and LiFe0.9Mg0.1PO4/C showed good rate capabilities, and the discharge capacities at 1 C were 110 and 120 mAh g−1, respectively. The LiFe0.9Mg0.1PO4/C has larger capacities compared with the LiFePO4/C at all C-rates. The enhanced rate capability when the Mg-substituted for Fe in the precursor mixture is more likely to originate in the electronic conductivity. On the other hand, these good rate capabilities may attribute to the small particle size and enhanced the electronic conductivity of the LiFePO4/C and LiFe0.9Mg0.1PO4/C using carbon coating. In order to enhance the electronic conductivity of the material, extensive studies have been carried out by using some carbon coating techniques [2–7, 28]. Carbon coating on the particle is effective in decreasing the resistance of the cathode.
Figure 9 shows the specific capacity of the LiFePO4/C and LiFe0.9Mg0.1PO4/C in function of the cycle number at 1 C. The initial discharge capacities of the LiFePO4/C and LiFe0.9Mg0.1PO4/C were 110 and 120 mAh g−1, respectively. The capacity fading over 50 cycles is 12% for LiFePO4/C, but only 6% for LiFe0.9Mg0.1PO4/C. The good cycling performance of the LiFe0.9Mg0.1PO4/C is attributed to the enhancement of the electronic conductivity by the Mg substitution.
4 Conclusions
The spherical LiFePO4/C and LiFe0.9Mg0.1PO4/C powders have been synthesized via a uniform-phase precipitation method. The uniform spherical particles as prepared are amorphous, but they were crystallized to FePO4 after calcining above 400 °C. After calcinations of it with LiOH and sugar or LiOH, Mg(OH)2, and sugar, the final products, LiFePO4/C, and LiFe0.9Mg0.1PO4/C, became much denser, leading to the tap-density is as high as 1.75 g cm−3 for LiFePO4/C and 1.77 g cm−3 for LiFe0.9Mg0.1PO4/C, respectively. The excellent specific capacities of 148 and 157 mAh g−1 at 0.1 C were achieved for the LiFePO4/C and LiFe0.9Mg0.1PO4/C, respectively. Comparison of the cyclic voltammograms of LiFePO4/C and LiFe0.9Mg0.1PO4/C shows enhanced redox current and reversibility for the sample substituting Mg on the Fe site. LiFe0.9Mg0.1PO4/C exhibits good high-rate and cycle performances. This uniform-phase precipitation synthesis is an excellent powder preparation alternative method for high capacity cathode materials to be used in a Li-ion secondary battery.
References
Padhi AK, Nanjundaswamy KS, Goodenough JB (1997) J Electrochem Soc 144:1188
Yamada A, Chung SC, Hinokuma K (2001) J Electrochem Soc 148:A224
Ravet N, Chouinard Y, Magnan JF, Besner S, Gauthier M, Armand M (2001) J Power Sources 97/98:503
Huang H, Yin SC, Nazar LF (2001) Electrochem Solid-State Lett 4:A170
Chen Z, Dahn JR (2002) J Electrochem Soc 149:A1184
Hu Y, Doeff MM, Kostecki R, Fiñones R (2004) J Electrochem Soc 151:A1279
Belharouak I, Johnson C, Amine K (2005) Electrochem Commun 7:983
Chung SY, Blocking JT, Chiang YM (2002) Nat Mater 1:123
Yamada A, Yonemura M, Takei Y, Sonoyama N, Kanno R (2005) Electrochem Solid-State Lett 8:A55
Sun YK, Bae YC, Myung ST (2005) J Appl Electrochem 35:151
Ying J, Jiang C, Wan C (2004) J Power Sources 129:264
Ying J, Lei M, Jiang C, Wan C, He X, Li J, Wang L, Ren J (2006) J Power Sources 158:543
He P, Wang H, Qi L, Osaka T (2006) J Power Sources 158:529
Shi SQ, Liu LJ, Ouyang CY, Wang DS, Wang Z, Chen L, Huang X (2003) Phys Rev B 68:195108
Liu H, Cao Q, Fu LJ, Li C, Wu YP, Wu HQ (2006) Electrochem Commun 8:1553
Zhang M, Jiao LF, Yuan HT, Wang YM, Guo J, Zhao M, Wang W, Zhou XD (2006) Solid State Ionics 177:3309
Barker J, Saidi MY, Swoyer JL (2003) Electrochem Solid-State Lett 6:A53
Wang GX, Bewlay S, Yao J, Ahn JH, Dou SX, Liu HK (2004) Electrochem Solid-State Lett 7:A503
Wang D, Li H, Shi S, Huang X, Chen L (2005) Electrochim Acta 50:2955
Hong J, Wang C, Kasavajjula U (2006) J Power Sources 162:1289
Wang C, Hong J (2007) Electrochem Solid-State Lett 10:A65
Wilhelmy RB, Matijević E (1987) Colloids Surf 22:111
Springsteen LL, Matijević E (1989) Colloid Polym Sci 267:1007
Kandori K, Nakashima H, Ishikawa T (1993) J Colloid Interface Sci 160:499
Kandori K, Kuwae T, Ishikawa T (2006) J Colloid Interface Sci 300:225
Scaccia S, Carewska M, Wisniewski P, Prosini PP (2003) Mater Res Bull 38:1155
Wang YQ, Wang JL, Yang J, Nuli JN (2006) Adv Funct Mater 16:2135
Franger S, Cras FL, Bourbon C, Rouault H (2002) Electrochem Solid-State Lett 5:A231
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Z., Zhang, X. & Hong, L. Preparation and electrochemical properties of spherical LiFePO4 and LiFe0.9Mg0.1PO4 cathode materials for lithium rechargeable batteries. J Appl Electrochem 39, 2433–2438 (2009). https://doi.org/10.1007/s10800-009-9931-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-009-9931-1