Abstract
The potential of photo-catalysis as a self cleaning process is investigated for application inside refrigerator cabinets, taking advantage of the well known oxidative properties of titanium dioxide (anatase form). A low temperature in situ synthesis was adopted to obtain a catalytic layer over a polystyrene (PS) support. The synthesised material was characterised by SEM, XRD and BET analyses. Photo-catalytic activity towards organic dirt decomposition was measured through the oxidation of various organic dyes, as the perceivable decolouring of the dirt represents a clear activity indicator for users. The selected contaminants (Methylene Blue and Ortho-Cresole Red) and two refrigerator wastes (orange juice and tomato pulp) were tested at different concentrations. The results were evaluated through by colorimetric analysis. A clear photo-catalytic effect of dye discolouring was detected on the coated samples. The discolouring effect of the coated samples was comparable with that observed on the anatase powder.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Domestic appliances are a well established business, where the acquisition of as large as possible shares used to be the principal competition mandate for the main competitors. Technological quantum leaps related to primary functions have been absent from the scene for sometime, and competitiveness has mostly been related to accessory added values. This scenario has changed recently due to market globalisation: those manufacturers who are willing to maintain top positions in the market share ranking are committed to searching distinctive innovations, possibly related to the main product mission [1, 2].
Household refrigerators are a quite heterogeneous segment, and include several product categories which, in common, share the capacity of generating cold in a confined environment. The appliance geometry, and in particular the low temperature compartment (freezer) layout, define and characterise the main categories. Despite the wide range of typologies, refrigerator engineering, in particular the manufacturing process of inner surfaces, is common to all of them.
A primary area of improvement, which is directly linked to the potential benefits for customers, is product usability: internal cleaning represents a top priority area of improvement for cooling household appliances. Micro-organisms—bacteria, moulds and viruses—are always present in the household environment and are carried by several kinds of vectors such as air circulation, humans and animals, objects and foodstuff. Problems can occur when such micro-organisms multiply uncontrollably, and any situation involving moisture and nutritive substrates, such as food residuals, could lead to a potentially dangerous situation.
In the case of food storage, two situations can be distinguished:
-
Micro-organisms pre-existing on overripe foodstuff: proliferation, in this case, is usually related to erroneous storage conditions; in the case of refrigerators, high temperatures due to malfunctions of the cooling system.
-
Micro-organisms from storage environment infesting foodstuff: such a situation is usually related to an insufficient or incorrect storage space cleaning process.
The second situation is potentially the most hazardous, as it is unrelated to any known food storage methodologies: even properly stored food can become unhealthy because of external causes. Most consumers underestimate the risk of microbiologically triggered food poisoning: even in a highly sensible market, such as Germany, about 200,000 cases of gastroenteritis are reported per year, the vast majority caused by bacteria contaminated food in private homes [3].
Food cleaning is hence an essential practice. Nevertheless, it is a sporadic one for most customers, due to the relative difficulty, the internal cabinet geometry, and, above all, the reluctance to switch off the appliance, all of which lead to the deterioration of stored food. The refrigerator is in fact the only household appliance continuously in use whose switched off periods are potentially critical for its primary function.
The present work is aimed at investigating the basics of a photo-catalytic coating, to be used as, an improved automated cleaning process, which is capable of satisfying both customer needs, by significantly reducing manual operations, and manufacturers needs, by providing an increased added value to the product. This aim will be pursued by providing refrigeration liner walls with self-cleaning properties, through a photo-catalytically induced continuous degradation of accumulated soil; deodorisation of the fridge compartments and hygienisation of the coated surfaces are expected as positive side effects of the application.
1.1 Experimental details
1.1.1 TiO2 low temperature synthesis
The first step of the work was the identification of a suitable method for a potential industrial application to coat polystyrene extruded sheets. The critical point of the investigation was to identify a synthesis method that would be effective at temperatures compatible with polystyrene, i.e. within 80–100 °C: above such a temperature range (commonly used in the thermoforming process) excessive material softening would occur, potentially inducing damage or geometrical distortions. In order to achieve such a goal, an in situ TiO2 synthesis process was applied, based on the production of an amorphous type titanium peroxide gel, which was to be used as a coating [4–7].
The preparation method, described in the literature, uses, as a precursor, a titanium tetrachloride solution, which has to undergo reaction with an ammonium hydroxide solution in an acidic range of pH (from 2 to 6). As TiCl4 is a quite dangerous and difficult compound to handle, a variation of this process was conceived, using an organic titanium precursor, namely titanium isopropoxide Ti(OC3H7)4, in the same conditions.
Some 50% w/w solutions of both titanium compounds (Aldrich) were prepared, and further diluted 70 times with distilled water. All the subsequent steps were the same for both the titanium tetrachloride and the titanium isopropoxide based preparations.
A 25% w/w ammonium hydroxide NH4OH solution (Aldrich) was prepared, and further diluted 10 times with distilled water.
The titanium compound and ammonium hydroxide solutions were blended at room temperature; the pH of the obtained mixture was adjusted to a value of 6.8 (measured by a pH-meter), to allow a hydrolysis reaction of the titanium precursor to take place. After the reaction was completed, the obtained product was left to rest for 3 h: the precursor hydrolysis led to a gelification process of the mixture. The excess of liquid, which was spontaneously separated from the gel, was eliminated.
The resulting Ti(OH)4 gel (whitish in appearance) was diluted 4 times with distilled water, under continuous stirring, by a magnetic mixer (1 h), to wash away the unreacted reagent leftovers, and then left to rest (3 h). Again, the spontaneously separated liquid excess was eliminated.
A 35% aqueous hydrogen peroxide solution was prepared separately, then gradually added, under stirring, to the whitish aqueous Ti(OH)4 solution in a 1/13 volume proportion. The mixture was stirred overnight and then left to rest at room temperature for 7 days, to obtain an almost yellow transparent jelly solution.
Finally, two titania precursor preparations were available, one for each of the two titanium compounds used as a precursor. The reaction between titanium hydroxide and hydrogen peroxide, which eventually led to the titanium oxide, is here reported [8]:
Literature data [9] suggests that, on the grounds of the described preparations, an intermediate compound, namely titanium peroxide, is generated; due to its metastability, this compound easily decomposes into highly dispersed titanium dioxide, even after exposure to moderate temperatures.
1.2 Catalyst characterisation
The products obtained from titanium tetrachloride and titanium isopropoxide were characterised by SEM, XRD and BET analysis.
1.2.1 XRD measurements
A number of tests were carried out on the solution obtained after adding hydrogen peroxide to assess the actual amount of crystallised anatase available after the thermal treatments (70 °C and 250 °C) were performed at different temperatures. The yellowish powders resulting from exposure of the titanium tetrachloride and titanium isopropoxide based precursor solutions to selected temperatures for one hour were subjected to XRD analysis, performed on a PW1710 Philips diffractometer equipped with a monochromator for Cu–Kα radiation. Range 10–90, step size 2θ = 0.02, time per step 1 s, scan speed (2θ/s) = 0.020.
1.2.2 Scanning electron microscopy
The powders produced by the thermal treatment at 80 °C of the titania precursor solutions were investigated by Scanning Electron Microscope (Philips, Model 515), to analyse the microstructure of the crystal aggregates of the prepared catalysts. Moreover, the elemental composition was checked by an EDS (Energy Dispersive X-ray Spectroscopy) detector associated to the microscope.
1.2.3 Specific surface area
The BET technique (Micromeritics ASAP 2010) was used to measure the specific surface area of the catalyst powders.
1.3 Catalyst deposition on polystyrene (PS)
Small flat PS samples, previously cleaned with diluted nitric acid, ethanol and deionised water, were coated by dipping them into the titanium tetrachloride and titanium isopropoxide based titania precursor solutions that were obtained after adding hydrogen peroxide.
After dipping and removing the excess solution, the samples were placed in an oven at 80 °C for 1 h; blank reference samples were subjected to the same thermal cycle, to visually check the integrity of the PS.
As a last step, the excess coating not bonded to the surface was removed by blowing with compressed air, thus leaving the samples ready for the subsequent characterisation. The samples were investigated by SEM analysis, in order to study the achieved coating structure.
1.4 Experimental setup for photocatalytic performance tests
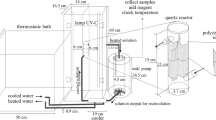
A UV test cell was used to achieve repeatable exposure conditions (Fig. 1). It was equipped with a 300 W UV Osram lamp with the following emission ranges: UVA 315–400 nm; UVB 280–315 nm, with an intensity of incident light of 19.72 W/m2. Samples were located in a fixed position (1 m from the lamp), where the differences in the irradiation of the tested samples could be considered as negligible. Forced ventilation of the samples was provided by a fan to avoid excessive substrate heating.
1.4.1 Colorimetric analysis
The progress of the photo-catalytic conversion was measured through the decolouring of various organic dyes: a colorimetric technique was applied, using RGB code values, to quantify the decolouring performances on the tested samples.
The RGB model is a typical colour encoding platform for the display of colours on a digital device, such as a television or a computer monitor. Each pixel on the screen can be represented as values of Red, Green, or Blue: a colour in the RGB model can be described by indicating how much of each of the red, green, and blue colour is included. Each can vary between the minimum (fully dark) and maximum (full intensity). If all the colours are at a minimum, the result is black. If all the colours are at a maximum, the result is white. RGB values encoded in 24 bits per pixel (bpp) are specified using three 8-bit unsigned integers (0 to 255) which represent the intensities of the red, green, and blue colour components [10].
Some sample group pictures were taken using a high resolution camera (6 MPixel) after locating the samples on a clear, non reflecting substrate, with an uncoated, non soiled nor irradiated sample, taken as a reference. Using a commercial image elaboration software, the RGB pixel values were averaged over each sample image, and normalised using the standard sample as the baseline.
Higher colorimetric indexes indicate colour whitening, hence dye decomposition, in a quantifiable and comparable way; after the three distinct R, G and B measurements, their average measurement, defined as T, was calculated. [11, 12]
1.4.2 Photo-catalytic activity tests
A set of samples was prepared with the aim of testing the photo-catalytic performance of the obtained coating. Rectangular samples, cut from a PS extruded sheet, were painted with titanium isopropoxide and hydrogen peroxide based precursor solutions in the central sector, and cured at 80 °C for 1 h.
The dyes selected for testing, prepared in different concentrations (0.4% w/w solution, further diluted by 10 or 100 times), were Methylene Blue and Ortho-Cresole Red. Moreover, photo-catalytic tests were conducted on “real” contaminants, such as orange juice and tomato pulp.
About 1 mL of soiling solution was deposited on the 10 cm2 samples and pictures were taken after increasing UV exposure time, up to 30 h, using the previously described test rig.
2 Results and discussions
As indicated in Fig. 2, an amorphous-like X-ray pattern was observed on powders treated at 70 °C, whereas anatase peaks were only observed after exposure of the powder to a temperature of 250 °C; no traces of rutile were detected. Traces of brookite were detected, indicating the correct chemical pathway had been selected. At such a temperature, it is also worth noticing the almost complete disappearing of the yellowish colour, which is likely generated by hydrogen peroxide addition. In the same way, for the case of titanium isopropoxide, weak diffraction peaks related to the anatase phase were detected, over a mostly amorphous-like spectrum for the case of thermal treatment at a low temperature (25 °C), as shown in Fig. 3.
The anatase phase was thus only partially developed, and it is possible to assume that amorphous titanium oxide was present, along with a certain part of titanium hydroxide. Moreover, the performance of the Degussa P25 commercial TiO2 photo-catalyst, consisting in a mixture of anatase and rutile in an approximate proportion of 80/20, was more active for many reactions than both the pure crystalline constituting phases [13–15], the enhanced activity arising from the increased efficiency of the electron–hole separation due to the multiphase nature of the particles [16].
The BET values of the catalyst powders are reported in Table 1. These values were quite high due to the colloidal solution constituting the intermediate step of the synthesis process, and possibly exceeded those of the powders obtained at higher temperatures.
The BET surface areas of the samples calcined at 250 °C are higher than those synthesised at room temperature or treated at 70 °C or 80 °C, probably because the precursor decomposition is not complete at a lower temperature.
The microstructure of the powders reported in Fig. 4, obtained from both titanium tetrachloride and titanium isopropoxide, were significantly different, in agreement with the differences detected in terms of specific surface area measurement (i.e. 120–220 m2 g−1 respectively). The Ti(OC3H7)4 based precursor solution gave a sponge like porous powder and at higher magnifications showed a fine structure, constituted by grains of about 0.1 μm in size. The EDS spectra highlighted, apart from Ti and O, a certain amount of C, which was probably related to some residuals of NH4OC3H7 that remained after the hydrolysis process. The TiCl4 based precursor solution instead gave a more compact-looking powder, composed of tightly bonded grains with dimensions of around 1 μm; smaller particles, similar in size to those observed for the Ti organic precursor, were evident on their surfaces. Again in this case, the EDS spectra revealed, apart from Ti and O, the presence of some NH4Cl leftovers from hydrolysis process by a clear Cl peak.
As expected, the TiO2 coated PS microstructure, appeared, quite similar to the one revealed by the free powder analysis: Fig. 5 in particular shows that the grain dimension and coating features are basically the same as the corresponding catalyst powder. For the TiCl4 based coating, the secondary structure appearing as grains of approximately 0.1 μm in size, was more frequent and evenly distributed. The finer and less coherent grains of the Ti(OC3H7)4 based coating appeared to induce a grazing effect, probably due to a lower compactness of the structure.
As previously mentioned, the colorimetric measurement method was applied to evaluate the photo-catalytic efficiency of the synthesised coatings. Reference samples, coated with commercial anatase powder (TiO2 99.8% anatase Sigma Aldrich) deposited by the solvent evaporation method (for which the photo-catalytic activity was assessed), were used as a performance benchmark. The whitening effect shown in Fig. 6 can be considered a rather quite good result.
The curves reported in Figs. 7 and 8 confirm the expected performance of the titania coating obtained through the in situ technique. The whitening effect, related to the photo-catalytic degradation of the applied dyes, was comparable to the one achieved with pure anatase, even though the coating resulted to be composed of crystalline anatase in a minimal fraction. The experimental results highlight that the most important abatement efficiency was achieved after the first exposure step, with little gain, in terms of whitening, over the following exposure periods.
Test runs were carried out using “real” contaminants, such as orange juice and tomato pulp, deposited on PS covered with titania. The photocatalytic process took place with evident favourable results, in terms of abatement, for the orange juice. Conversely, in the case of tomato juice, the obtained results appeared inadequate (see Figs. 9, 10).
Tomato pulp very likely has more fibres and for this reason it is difficult to spread on the catalytic material. On the other hand, fibres are not easily attackable by the photo-catalytic reactions.
This result highlights the importance of the self cleaning of the photocatalytic layer by the contaminating substance.
3 Conclusions
Refrigerator cabinet liner cleaning is a major issue for household users, particularly due to the need for continuous functionality. The exploitation of photo-catalytic effects to provide internal fridge surfaces, made up of thermoformed polystyrene PS extruded sheets, with self-cleaning capabilities, was explored as a possible solution to such issues and to provide significant added value to target products.
A technology combining the in situ synthesis of TiO2 and its deposition on a PS substrate has been developed. The possibility of obtaining an effective anatase based photo-catalytic layer on PS substrate was tested.
A comparison was carried out, in terms of photo-catalytic effect, with coatings obtained through the deposition of commercial powder on PS substrates. The photo-catalysis process exhibited a clear whitening effect on colorants for both the synthesised and commercial TiO2. The adhesion of the titania based coating most probably suffers from a lack of compatibility with the polyolefin based substrate, due to poor wettability; the extreme smoothness of the PS surface also prevents mechanical grip of the coating. For future experiments, considering the aqueous precursor solution and the nature of the precursors themselves, a surface treatment aimed at producing polar-philic groups (e.g. flame or plasma etching) on the surface could be beneficial for coating adhesion.
The test results have shown the feasibility of self-cleaning surfaces, but an optimization of the coating procedure and of the compatibility of the catalytic layer characteristics (wear/scratch resistance, adhesion, crystallinity) with identified industrial applications appears to be necessary. Moreover, the photo-catalytic coating could be used for sterilization purposes.
However, the in situ synthesis on a PS sheet surface of a titania based coating with photo-catalytic properties, still needs to be improved. A suitable precursor solution spreading technique, that is compatible with industrial high-throughput production, should be identified, while at the same time tuning the solution properties to have a correct rheology. Evenness, aesthetic appearance and fair wear resistance of a coating are mandatory for a successful application. Precursor spreading and its decomposition to anatase through thermal treatment should also avoid deformation of the PS sheet.
However, the effectiveness achieved with real soiling substances was limited; this was presumably due to an insufficiently close contact with the catalyst, and to severe hindering of the catalyst to UV radiation. Increasing the porosity to improve the available surface would most probably lead to the soaking of the structure by soiling agents, making them almost impossible to remove.
The photo-catalytic coating activity will not completely eliminate the need for manual cleansing operations, in particular during massive soiling events. However, the well known capability of pathogenic organism removal (including bacteria, viruses and fungi [17]) of TiO2 photo-catalytic layers from surfaces prone to contact with food and biological hosts will remain available.
References
Yearly Global Appliances Market Survey 2006—GfK Marketing Services Italia (2006)
55th Annual Report: Ten-Year Review of Appliance Industry—Appliance Magazine, May 2008—Canon Communications LLC
Heinzel M (2000) Veranderung der Hygienerisiken in Deutschen Haushalten. SÖFW J 126/10:38
Ogata S, Matsui Y. US patent application 2001\0019776A1. http://www.patft.uspto.gov/9
Ogata S, Matsui Y. US patent 6107241A1. http://www.patft.uspto.gov/9
Deorsola FA, Vallauri D (2008) J Mater Sci 43:3274
Shankar MV, Kako T, Wang D, Ye J (2009) J Collid Interf Sci 331:132
Yin S, Hasegawa H, Maeda D, Ishitsuka M (2004) J Photochem Photobiol A: Chem 163:1
Ogata S, Matsui Y. Coating method of amorphous type titanium peroxide. US Patent 6379811. http://www.patentstorm.us/patents/6379811/fulltext.html
Hunt RWG (2004) The reproduction of colour, 6th edn. Wiley-IS&T Series in Imaging Science and Technology, Chichester
de Souza LKC, Zamian JR, da Rocha Filho GN, Soledade LEB, dos Santos IMG, Souza AG, Scheller T, Angélica RS, da Costa CEF (2009) Dyes Pigments 81:187
Kuo CY (2009) J Hazard Mater 163:239
Bickley RI, Gonzales T, Lees JL, Palmisano L, Tilley RJD (1991) J Solid State Chem 92:178
Ohno T, Sarukawa K, Tokieda K, Matsumura M (2001) J Catal 203:82
Zhang Q, Gao L, Guo J (2000) Appl Catal B: Environ 26:207
Gerischer H, Heller A (1992) J Electrochem Soc 139:113
Carp O, Huisman CL, Reller A (2004) Prog Solid State Ch 32:33
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Di Giulio, S., Faraldi, P., Russo, N. et al. Photo-catalytic coating of polystyrene for household cooling appliances with self cleaning surfaces. J Appl Electrochem 39, 2265–2273 (2009). https://doi.org/10.1007/s10800-009-9858-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-009-9858-6