Abstract
The cathodic reaction kinetics and anodic behavior of Al alloy 3003 in aerated ethylene glycol–water solution, under well-controlled hydrodynamic conditions, were investigated by various measurements using a rotating disk electrode (RDE). The transport and electrochemical parameters for cathodic oxygen reduction were fitted and determined. The results demonstrate that the cathodic reaction is a purely diffusion-controlled process within a certain potential region. The experimentally fitted value of diffusion coefficient of oxygen is 3.0 × 10−8 cm2 s−1. The dependence of cathodic current on rotation speed was in quantitative agreement with Levich equation. At potentials more positive than the diffusion controlled region, the cathodic process was controlled by both diffusion and electrochemical kinetics. The electrochemical reaction rate constant, k 0, was determined to be 1.1 × 10−9 cm s−1. There is little effect of electrode rotation on anodic behavior of Al alloy during stable pitting. However, fluid hydrodynamics play a significant role in formation of the oxide film and the Al alloy passivity. An enhanced electrode rotation would increase the mass-transfer rate of solution, and thus the oxygen diffusion towards the electrode surface for reduction reaction. The generated hydroxide ions are favorable to the formation of Al oxide film on electrode surface.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
It has been acknowledged [1, 2] that corrosion resistance of aluminum (Al) alloy is dependent on the formation of a layer of oxide film on its surface. Al alloys, due to their good formability and excellent heat efficiency, have been used widely in various industrial applications [3]. However, the environmental factors including an elevated temperature, a strong alkalinity, the presence of halide ions and a high fluid flow velocity [4, 5] could damage, thinner or evenly complete remove the film and enhance corrosion of Al alloy significantly. In automobile cooling system, Al alloy heat exchanger pipe has encountered flow-assisted corrosion (FAC) due to the presence of chloride ions in the flowing coolant (mainly ethylene glycol) [3].
Corrosion of Al alloy has been studied extensively in various aqueous solutions, and theories and models have been developed to illustrate corrosion, especially pitting corrosion, of Al [1]. However, to date, there has been few work performed in ethylene glycol–water solution to investigate corrosion of Al alloy in automobile cooling system. In the previous work, Niu and Cheng [6] studied the electrochemical corrosion behavior of 3003 Al alloy in ethylene glycol–water solution, and concluded that, in the presence of oxygen, a layer of oxide film forms on Al electrode surface to protect the substrate from corrosion. In the absence of oxygen, the film formed is mainly aluminum alcohol film that is less compact and less resistant to corrosion. Furthermore, they used rotating disk electrode (RDE) and rotating cylinder electrode (RCE) to study the effects of fluid hydrodynamics on Al corrosion [7]. It was found that the Al corrosion is mixed-controlled by both activation and diffusion steps at low and intermediate electrode rotating speeds. When the rotating speed is sufficiently high, there is a synergistic effect of fluid hydrodynamics and sand particles on Al erosion–corrosion (E–C) when the solution contains solid sands.
Cathodic process, which is an essential part of corrosion reaction, is usually ignored in investigation of metallic corrosion. During corrosion of Al alloy, cathodic reaction, i.e., reduction of the dissolved oxygen, is expected to play a critical role in corrosion of Al alloy. In this work, cathodic reaction kinetics and anodic behavior of Al alloy in ethylene glycol–water solution were studied by RDE technique. It is anticipated that this research provides an essential insight into the mechanism of Al alloy corrosion in automotive coolant.
2 Experimental
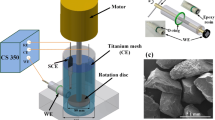
Rotating disk electrodes of 6 mm in diameter were made of 3003 Al alloy, supplied by DANA Canada Corporation, with a chemical composition (wt.%): Cu 0.20, Fe, 0.70, Si 0.60, Mn 1.50, Mg 0.05, Cr 0.05, Zn 0.10, Ti 0.05, and Al balance. The unexposed faces and edges of RDE were sealed with Teflon epoxy. Before tests, the working surface of the electrode was ground with 800 and 1200 grit silicon carbide papers, polished with a 1 micron alumina paste, and then cleaned by deionized water and acetone.
The test solution was a 50% ethylene glycol + 50% deionized water + 100 ppm Cl− solution to simulate the automotive coolant. All solutions were made from analytic grade reagents and ultra-pure deionized water (18 MΩ cm). All the tests were conducted at the ambient temperature (20 ± 0.2 °C).
Electrochemical measurements were performed using a three-electrode cell, with Al RDE as working electrode, a saturated calomel electrode (SCE) as reference electrode and a platinum wire as counter electrode. Polarization curves were measured through a PAR Model 263A Potentilstat/Galvanostat. The electrode rotation was controlled by a Pine AFM-SRX rotator system. Linear sweep voltammetry tests were performed at a potential sweep rate of 10 mV/s. In potentiostatic current transient tests, the Al RDE was potentiostatically controlled and the current density was recorded as a function of electrode rotating speed that was stepped up with time.
3 Results
3.1 Cathodic voltammograms of Al RDE
Figure 1 shows the cathodic voltammograms of Al RDE at the various rotating speeds. It is seen that, under the static condition, the reductive current density decreased with the positive shift of potential until −1.4 V (SCE). The reductive current density was than maintained at a relatively steady value over the remaining potential range. Upon electrode rotation, the reductive current density increased significantly. Moreover, the current density increased with the increasing electrode rotating speed. Furthermore, with the positive shift of applied potential, the reductive current density decreased, then experienced a plateau at approximately −1.1 V ~ –1.0 V (SCE), and finally decreased rapidly to zero when the potential approached corrosion potential. The current density plateau phenomenon was more apparent at a higher electrode rotating speed.
3.2 Potentiostatic cathodic current transient measurements of Al RDE
Figures 2 and 3 show the cathodic current transient curves measured on Al RDE at −0.7 V and −0.75 V (SCE), respectively. The two potential values were within the cathodic Tafel region, as seen in Fig. 1. It is seen that, at both applied potentials, the cathodic current density dropped rapidly to a more negative value upon the increase of electrode rotating speed, and then reached a steady value. Moreover, with the increase in electrode rotating speed, the steady cathodic current density increased.
3.3 Anodic voltammogram measurements
Figure 4 shows the anodic voltammograms of Al RDE as a function of electrode rotating speed. It is seen that anodic current density increased with the positive shift of potential. To further investigate the dependence of anodic current density on electrode rotating speed, potentiostatic measurements were performed. Figure 5 shows the potentiostatic anodic polarization measurements of Al RDE at −0.5 V (SCE) under the various electrode rotating speeds. It is apparent that the steady state current intensity decreased with the increase of electrode rotating speed, with some irregular changes of current density in the first 400 s.
4 Discussion
4.1 Purely diffusion-controlled cathodic reaction kinetics
The cathodic process during Al corrosion in aerated ethylene glycol–water solution is dominated by diffusion of the dissolved oxygen towards the Al electrode surface and then the reduction of oxygen by:
Apparently, an increase of electrode rotating speed would accelerate the diffusion of oxygen towards the electrode for cathodic reduction reaction, resulting in an increase of cathodic reductive current density (Fig. 1). The presence of a current density plateau in cathodic polarization curve indicates that diffusion of oxygen towards the electrode surface acts as the rate-controlling step of the cathodic reaction. According to Levich equation [8], a purely diffusion-controlled electrode reaction process occurring on a RDE surface is characterized as:
where i L is the steady-state limiting diffusive current density, n is stoichiometric number of electrons exchanged in the reaction, A is electrode area, F is Faraday constant, D is diffusion coefficient, ω is angular rotating velocity of RDE, ν is kinematic viscosity of the solution, and C 0 is the bulk concentration of the electroactive species. It is worthy pointing out that the angular velocity of RDE should be limited in the range of 10 s−1 < ω < 1000 s−1 since the Levich equation applies for the laminar flow only [9]. It is apparent from the equation that the cathodic limiting diffusive current density is proportional to the square root of angular velocity for a purely diffusion-controlled reaction.
The measured limiting diffusive current density at the individual electrode rotating speed (in both rpm and rad s−1) is shown in Table 1. Figure 6 shows the relationship between the measured i L and ω1/2. A well-fitted linear relationship is observed. It is further demonstrated that cathodic process in Al corrosion is a purely diffusion-controlled process.
From the slope of the fitted straight line, diffusion coefficient of oxygen in the test solution can be obtained. The values and unites of input parameters in the present system are listed in Table 2. Since the calculated diffusivity is based on the fitting of the measured cathodic polarization curve, and the cathodic reaction is dominated by reduction of oxygen in aerated, near-neutral pH solution, it is thus ascertain that the diffusivity of O2 is measured in this work. The experimentally fitted value of diffusion coefficient (D) of oxygen in ethylene glycol–water solution is 3.0 × 10−8 cm2 s−1.
In addition to diffusivity of oxygen, some other important hydrodynamic parameters that are essential to cathodic and anodic reactions can be determined from the measurements of cathodic limiting diffusive current density, such as mass-transfer coefficient (k m), thickness of diffusion layer (δ), Sherwood number (Sh) and Schimidt number (Sc). The formulas for calculation of the parameters are shown as [8]
where r is the radius of RDE. Furthermore, Reynolds number (Re) is usually used to quantify the fluid flow condition, and is determined by the geometry of electrode, fluid velocity and kinematic viscosity of solution by
The values of all parameters are determined and listed in Table 3.
Experimental Sherwood dimensionless group correlation for RDE is defined as:
Re-organize Eq. 8:
Therefore, there is a linear relationship between the logarithm of \( \left( {\frac{Sh}{{Sc^{0.33} }}} \right) \) and the logarithm of Re. Use the determined values in Table 3 and make a plot in Fig. 7, and the coefficients, a and b, can be obtained from the line: a = 0.636, and b = 0.497. Therefore, the Sherwood dimensionless group correlation for mass-transfer of oxygen in glycol–water solution is determined as
Furthermore, the fitted values of a (0.636) and b (0.497) in this work are comparable to the theoretical values according to Levich equation, where a = 0.62 and b = 0.5 [9, 10]. The approximation of the constants supports the conclusion that the plateau in cathodic polarization curve is due to diffusion of cathodic reactant, i.e., oxygen.
4.2 Cathodic reaction kinetics under mixed control
With the positive shift of cathodic potential, the cathodic process shows a mixed-controlled behavior, which is confirmed by potentialstatic current transient measurements in Figs. 2 and 3. When RDE is used to investigate a mixed-controlled reaction, the Koutecky–Levich equation is usually applied to separate the measured currents that are attributed to mass-transfer step and charge-transfer step, respectively:
where i K is the current intensity in the absence of mass-transfer effect, k 0 is the potential-independent electrochemical rate constant, α is charge-transfer coefficient, R is the molar gas constant, T is temperature, and η is over-potential. Other parameters have the identical meaning to the previous description. z in Eq. 11 is the stoichiometric number of electrons exchanged in charge-transfer step. According to the reported works [11, 12], the charge-transfer controlled step for oxygen reduction is a one-electron transfer process:
Thus, the stoichiometric number of electrons exchanged, z, is equal to 1. As shown in Fig. 8, the purely charge-transfer controlled current density, i K, can be obtained by extrapolating i −1 to an infinitely high mass-transfer rate, where charge-transfer is expected to dominate the cathodic process. It is seen from Fig. 8 that, when potential is shifted positively from −0.75 V (SCE) to −0.70 V (SCE), the purely charge-transfer controlled current density decreases, indicating that the oxygen reduction is inhibited at a less negative potential. According to Eq. 11, determination of i K upon the extrapolation of i −1 at various potentials allows calculations of k 0. The electrochemical reaction rate constant, k 0, is determined to be 1.1 × 10−9 cm s−1.
At potentials less than the plateau potentials on the cathodic curve in Fig. 1, the cathodic reaction is mainly reduction of hydrogen ions. It is a typical activation-controlled cathodic process.
4.3 Effects of cathodic oxygen reduction on corrosion of Al alloy
Anodic behavior of Al alloy in aerated ethylene glycol–water solution is presented in Fig. 4, where the extremely low current density region up to about −0.35 V (SCE) refers to passivity of Al alloy. It has been acknowledged [13] that the good corrosion resistance of Al alloys is due to the formation of a layer of oxide film on electrode surface by
In the presence of chloride ions, they may penetrate into the defects of Al2O3 film and replace oxygen, generating cation vacancies at the film/solution interface [14]. A flux of cation vacancies across the film to the film/metal interface is thus resulted in. If the cation vacancy flux is so high that the cation vacancies cannot be annihilated at the metal/film interface, a cation vacancy condensate will be formed, causing the thinning of film or the local detachment from the metal. A pit is then generated at the location where chloride ions absorb. At low anodic potentials, the pit initiation and its repassivation compete each other, causing current oscillation, as shown in Fig. 4. At a sufficiently high anodic potential, metastable pitting develops into stable pitting, resulting in a significant increase of anodic current density.
There is little effect of electrode rotation on anodic behavior of Al alloy during stable pitting. However, fluid hydrodynamics play a significant role in the formation of oxide film. As seen in Fig. 5, at a high electrode rotating speed, a low anodic current density is resulted in. Apparently, an enhanced electrode rotation would increase the solution hydrodynamics, and thus the oxygen diffusion towards the electrode surface for reduction reaction [15, 16]. The generated hydroxide ions are favorable to the formation of Al oxide film on electrode surface. The initial irregularity of current density in the first 400 s in Fig. 5 is probably attributed to the slight difference of electrode surface status at the different tests. After a steady-state is achieved, the initial difference in electrode surface condition will be offset by a stable electrode state, which depends on the various electrode rotating speeds.
5 Conclusions
An increase of electrode rotating speed would accelerate the diffusion of oxygen towards the electrode surface for cathodic reduction reaction, resulting in an increase in cathodic reductive current density. The cathodic reaction is a purely diffusion-controlled process in aerated, ethylene glycol–water solution. The experimentally fitted diffusion coefficient of oxygen is 3.0 × 10−8 cm2 s−1. The dependence of cathodic current on rotation speed was in quantitative agreement with Levich equation. The Sherwood dimensionless group correlation for mass-transfer of oxygen is Sh = 0.636Re 0.497 Sc 0.33.
At potentials more positive than the diffusion controlled region, the cathodic process was controlled by both diffusion and electrochemical kinetics. The electrochemical reaction rate constant, k 0, is determined to be 1.1 × 10−9 cm s−1.
There is little effect of electrode rotation on anodic behavior of Al alloy during stable pitting. However, fluid hydrodynamics play a significant role in formation of the oxide film and the Al alloy passivity. An enhanced electrode rotation would increase the oxygen diffusion towards the electrode surface for reduction reaction. The generated hydroxide ions are favorable to the formation of Al oxide film on electrode surface.
References
Szklarska-Smialowska Z (1999) Corros Sci 41:1743
Frankel GS (1998) J Electrochem Soc 145:2186
Miller WS, Zhuang L, Bottema J, Wittebrood AJ, De Smet P, Haszler A, Vieregge A (2000) Mater Sci Eng A280:37
De Micheli SM (1978) Corros Sci 18:605
Baumgartner M, Haesche H (1990) Corros Sci 31:231
Niu L, Cheng YF (2007) J Mater Sci 42:8613
Niu L, Cheng YF (2008) Wear 265:367
Levich VG (1962) Physicochemical hydrodynamics. Prentice-Hall, Englewood Cliffs, NJ
Bard AJ, Faulkner FR (2004) Electrochemical methods: fundamentals and applications. Kluwer Academic Publishers, NJ
Greef R, Peat R, Peter LM (1985) Instrumental methods in electrochemistry. Ellis Horwood Ltd, Chichester, UK
King F, Quin MJ, Litke CD (1995) J Electroanal Chem 385:45
Kear G (2007) Electrochim Acta 52:1889
Pourbaix M, Deltombe E, Vanleugenhaghe C (1966) Atlas of electrochemical equilibria in aqueous solutions. Pergamon Press, Oxford
Macdonald DD (1992) J Electrochem Soc 139:3434
Raja KS, Jones DA (2006) Corros Sci 48:1623
Zhang GA, Xu LY, Cheng YF (2008) Electrochim Acta 53:8245
Acknowledgements
This work was supported by Canada Research Chairs Program, Natural Science and Engineering Research Council of Canada (NSERC) and Dana Canada Corporation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Y., Cheng, Y.F. Cathodic reaction kinetics and its implication on flow-assisted corrosion of aluminum alloy in aqueous ethylene glycol solution. J Appl Electrochem 39, 1267–1272 (2009). https://doi.org/10.1007/s10800-009-9794-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-009-9794-5