Abstract
The enhanced degradation of methyl orange in aqueous solution was achieved by means of contact glow discharge electrolysis (CGDE) through adding a small amount of Fe2+ to the solution. Methyl orange was degraded and eventually decomposed into inorganic carbon when CGDE was carried out under an applied DC voltage of 480 V and a current of 65–80 mA. The oxidation process followed first-order kinetics; the degradation rate increased almost eight times in the presence of Fe2+. A reaction pathway is proposed based on analysis of the ultraviolet (UV) spectra of the solution and intermediate products from High Performance Liquid Chromatography-Mass Spectrometry (HPLC-MS). The addition of hydroxyl radicals promoted by the Fe2+ catalyst is a key step in enhancing the whole oxidation process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Water pollution caused by dyes has become a serious problem in developing countries. Dyes are used by many industries to color their products. The discharge of dye-containing wastewater not only causes eutrophication of receiving water bodies but incorporates the bio-refractory components as well, adding to the difficulty of treating and purifying the wastewater [1–3]. Contact glow discharge electrolysis (CGDE) is a technology that has been systematically studied since the 1960s [4]. In CGDE, plasma is generated locally between a platinum wire anode electrode and the surface of a liquid solution when the applied voltage is increased to a threshold value, normally 0.42 kV [4, 5]. The most distinct feature of CGDE is its non-faradaic chemical effects [6–10]: the yields obtained at the glow-discharge electrode are several times the faradaic value and the products are unique compared with conventional electrolysis. In the reaction zone, within the plasma at the anode, vapor phase H2O water molecules are ionized and activated, resulting in ion bombardment and hemolytic fission. Free OH and H radicals [11] are produced through energy transfer. At the same time, in the liquid-phase reaction zone near the plasma–anolyte interface, liquid phase H2O molecules are dissociated into H2, H2O2 and O2 as a result of bombardment by highly energized H2Ogas + from the anodic plasma [10]. A mixture of the active species OH•, H• and H2Ogas + diffuse across the primary zone (called the plasma layer) into the bulk electrolyte. These species are strong oxidizing agents. Thus, CGDE can be used in advanced oxidation processes (processes which are based on the generation of radical intermediates [12]). CGDE has been used for wastewater treatment by degradation of phenol [13], chloropheonols [14], 2,4-dichlorophenol [15], benzoic acid [16], aniline [17] and alkylbenzenesulfonates [18]. Gao et al have used this method to treat dyes such as acridine orange (OA) [19], α-naphthol [20], red B and flavine G [21]. Recently, we have applied the CGDE method to degrade methyl orange (MO). The degradation kinetics and mechanism were studied. However, the degradation rate was low (nearly 48.8% for 90 min) [22].
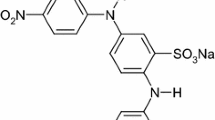
It is well known that the ferrous ion can react with hydrogen peroxide to produce hydroxyl radicals which can be used to oxidize organic pollutants. In this work, the catalytic effect of the ferrous ion on the degradation of MO using CGDE has been studied. To our knowledge there are no reported data on the degradation of MO using CGDE with Fe2+ as catalyst. The structures of MO in acidic and basic solution are shown in Scheme 1.
2 Experimental
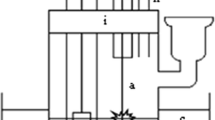
The apparatus is illustrated in Fig. 1. The reaction vessel was made up using two cylindered glass tubes (4 cm diameter) connected by a sintered glass disk with medium porosity at the bottom. The anode consisted of a platinum wire (0.5 mm diameter) held in a brass support. The cathode was a stainless steel plate (4 cm2 in area) placed in another glass tube. Both the anode and the cathode were immersed into the solution during the reaction. During degradation argon gas was passed through the solution to remove air. The anode was immersed into the solution to a depth of approximately 2 mm. The power supply was a DH1722-6 direct current power unit which provided voltages of 0–1000 V and a maximum output of 300 mA. The experiments are performed under the conditions listed in Table 1 unless otherwise stated. Considerable heat was produced in the discharge during the reaction therefore the cell was placed in an ice-water bath to ensure a long-time reaction.
The solution most frequently used was 200 mL with varying concentrations of MO dissolved in sodium sulfate (0.015 M). Before each run, 1 mL of 2 × 10−3 M Fe2+ was added to the solution and a magnetic stir bar was used at the bottom of the anodic side of the vessel to ensure that the solution mixed well. The concentration of the reactant obtained was determined by a UNICO UV-2102 spectrophotometer. The solution pH was measured using an REX PHSJ-3F pH meter. At the end of the reaction the products in solution, as well as starting materials, were analyzed by means of high performance liquid chromatography-mass spectroscopy (HPLC-MS) HP1100LC-MS (Agilent Integrity System).
HPLC conditions included a C18 4-μm column (4.6 × 150 mm) used for separations. The mobile phase was a mixture of 80% solvent A (methanol) and 20% solvent B (H2O). The flow rate was 0.7 mL min−1. The detector wavelength was 220 nm. The injection volume was 5 μL. For mass spectrometric analysis, electrospray ionization (ESI) mass spectra were obtained. The mass spectrometer was operated in positive ion mode. The detailed conditions of MS were as follows: capillary voltage was 3,500 V, nebulizer pressure was 25 psi and the skimmer was 70 V. The flow rate of the drying gas was 9 L min−1 and the temperature of the drying gas was maintained at 350 °C.
3 Results and discussion
3.1 Relationship of current and voltage during the course of reaction
Figure 2 shows the different current (I) and voltage (U) values during the electrolysis reaction to attain CGDE in the presence of Fe2+. When the voltage changed from 0 to 260 V, the current value changed correspondingly from 0 to 160 mA The relationship between I and U obeys Ohm’s law. Small bubbles produced from the pointed side of the anode were observed. When the applied voltage was higher than 420 V, the current fell to its lowest value indicating a turning point in the whole change of I and U. During the reaction, keeping the U value above 260 V for a few minutes, the voltage value began increasing abruptly from 260 to 300 V and then kept rising to 420 V where the current fell quickly to its lowest point at 40 mA. Simultaneously, a weak glow spot on the pointed side of the anode appeared. Adjusting the voltage to 480 and 500 V caused an increase in current and a brightening of the glow spot with sparks was observed. Its normal size was less than 1 cm2 from the anode across the anolyte. In comparison to the work of Hickling and Ingram [4] and Sengupta [10], it is clear that the reaction is significantly enhanced under CGDE.
The UV absorption spectra of MO in the presence of 2 × 10−3 M Fe2+ at different degradation times are shown in Fig. 3. Before the reaction, the absorption curve had maximum absorption at 507 nm. Lowering of the peak at 507 nm was observed with increased reaction time. Meanwhile, there was absorption at about 420 nm after half an hour’s degradation. The peak at 420 nm may be attributed to N,N dimethyl-4-nitroaniline, which is an intermediate of MO degradation [23].
3.2 Degradation rate changes for MO with and without addition of Fe2+
For these studies the electrolytes were prepared at a MO concentration of 4.15 mg L−1 in the presence of 2 × 10−3 M Fe2+ and in the absence of Fe2+. Each of the above solutions was treated under CGDE conditions. The change in MO concentration was determined by monitoring the UV absorption peak at 507 nm at different reaction times.
The degradation behavior of MO (4.15 mg L−1) with and without Fe2+ ions at an initial pH of 3 at different reaction times is shown in Fig. 4. For the solution in the absence of Fe2+ ions, the concentration of MO (Fig. 4a) decreases gradually. The longer the reaction time the more MO is degraded. The degradation of MO (Fig. 4b) is only 27.7% for MO solution over the course of 40 min. However, for the solution in the presence of Fe2+ ions, the concentration of MO decreases much faster. The degradation is nearly 91.6% for MO aqueous solution in the presence of Fe2+ over 40 min.
3.3 Dynamic behavior analysis
The data of Fig. 4 was fitted to the following equation:
where c0 is the initial concentration of MO, c t is the concentration of MO at reaction time t, k is the first order rate constant in min−1 and t is the time in minutes. In the absence of Fe2+ ions a linear relationship with correlation coefficient Ra 2 = 0.9981 was obtained. In the presence of Fe2+ ions a linear relationship with correlation coefficient R 2a = 0.994 was obtained. From Fig. 5 it is clear that MO degradation follows pseudo-first order kinetics with or without Fe2+ ions in solution. However, the first-order rate constant corresponding to MO solution in the presence of Fe2+ ions is almost eight times larger than that in the absence of Fe2+ ions.
3.4 pH changes
pH changes after each degradation are shown in Fig. 6. A decrease of nearly one pH unit was observed after degradation. The decrease in pH can be attributed to acidic intermediates produced during the reaction which is in agreement with the presence of organic acids as stable products.
3.5 Mechanism analysis
Previous investigations of the reaction mechanism of CGDE [5–10] propose that charged species in the plasma (present in the discharge gap or sheath of vapor around the anode) are accelerated due to the steep potential gradient and enter the air–liquid interface with an energy that may be greater than 100 eV [8]. In the reaction zone, within the plasma, around the anode, vapor H2O molecules are ionized or activated. They are bombarded by each other and broken up by an energy transfer mechanism, which results in free ·OH and ·H radicals [11], and additional non-faradaic yields of H2 and O2[10]. In the liquid-phase reaction zone near the plasma-anolyte interface, several liquid H2O molecules are broken up into H2 and H2O2. Additionally, O2 that is bombarded by H2Ogas + can diffuse across the bulk electrolyte and interact with itself or any active substrate in the solution. The overall reaction is as follows [5, 10]:
In the presence of ferrous iron the degradation of methyl orange is accelerated as shown above. Fe2+ reacts with hydrogen peroxide in the CGDE process acting as a Fenton—like reagent. The oxidation process is represented by the following reactions:
The ferrous iron initiates and catalyses the decomposition of hydrogen peroxide, resulting in the generation of hydroxyl radicals. The generation of the radicals involves a complex reaction sequence in aqueous solution [12].
Moreover, the newly yielded ferric ions may catalyse the decomposition of hydrogen peroxide into water and oxygen [12]. The reactions are shown in Eqs. 11–14
Hydroxyl radicals can also oxidize organic compounds (RH) by abstraction of protons producing organic radicals (R·), which are highly reactive and can be further oxidized [24],
The R• may be oxidized by Fe3+ according to the following reactions [25],
From the above analysis, more •OH free radicals and Fe3+ are produced in the presence of Fe2+ and accelerate the degradation reaction.
3.6 Intermediate products analysis
The HPLC-MS results of the anolyte of MO, whose initial concentration is 4.15 mg L−1 after a reaction time of 40 min are shown in Figs. 7 and 8 respectively.
Based on the MS results of Fig. 8, the main reaction products in aqueous solution (pH = 3.0) in the presence of 2 × 10−3 M Fe2+during CGDE were determined. A mechanistic scheme is presented in Fig. 9 which illustrates the reaction intermediate products and pathways of MO in aqueous solution (pH = 3.0). Figure 9 shows that the intermediate product of MO is N, N dimethyl-4-nitroaniline (m/z = 168.1), which is consistent with the results of the UV–vis spectra.
4 Conclusion
The enhanced degradation of MO in aqueous solution was achieved using CGDE through the addition of Fe2+ to the solution. The methyl orange was degraded and eventually decomposed into inorganic carbon. The oxidation process followed first-order kinetics and the degradation rate increased eight fold in the presence of Fe2+ compared with the absence of Fe2+. The increase in hydroxyl radicals produced by the catalysis of Fe2+ was considered to be the key step in the oxidation enhancement. The process may be applicable in practical dye pollutant treatment.
References
Konstantinou IK, Albanis TA (2004) Appl Catal B-Env 49:1
Georgiou D, Melidis P, Aivasidis A, Gimouhopoulos K (2002) Dyes Pigments 52:69
Chacon JM, Leal MT, Sanchez M, Bandala ER (2006) Dyes Pigments 69:144–150
Hickling A, Ingram MD (1964) Trans Faraday Soc 60:783
Hickling A (1971) Mod Aspects Electrochem 6:329
Sengupta SK, Singh OP (1991) J Electroanal Chem 301:189
Sengupta SK, Singh OP (1994) J Electroanal Chem 369:113
Sengupta SK, Singh R, Srivastava AK (1998) Indian J Chem Sect A 37:558
Sengupta SK, Singh R, Srivastava AK (1995) Indian J Chem Sect A 34:459
Sengupta SK, Singh R, Srivastava AK (1998) J Electrochem Soc 145:2209
Bullock AT, Gavin DL, Ingram MD (1980) J Chem Soc Faraday Trans I 76:648
Neyens E, Baeyens J (2003) J Hazard Mater B 98:33
Amano R, Tomizawa S, Tezuka M (2004) Electrochem 72:836
Tezuka M, Iwasaki M (1998) Thin Solid Films 316:123
Lu QF, Yu J, Gao JZ (2006) J Hazard Mater 136:526
Ogata A, Miyamae K, Mizuno K, Kushiyama S, Tezuka M (2002) Plasma Chem Plasma Process 22:537
Tezuka M, Iwasaki M (2001) Thin Solid Films 386:204
Tezuka M, Amano R (2006) Water Res 40:1857
Gao JZ, Hu ZG, Wang XY, Hou JG, Lu XQ, Kang JW (2001) Thin Solid Films 390:154
Gao JZ, Pu LM, Yang W, Yu J, Li Y (2004) Plasma Process Polym 1:171
Gao JZ, Wang XY, Hu ZG, Deng HL, Hou JG, Lu XQ, Kang JW (2003) Water Res 37:267
Gong JY, Cai WM (2007) Plasma Sci Technol 9:190
Moussa D, Doubla A, Georges KY, Brisset JL (2007) IEEE Trans Plasma Sci 35:444
Walling C (1975) Acc Chem Res 8:125
Tang WZ, Tassos S (1997) Water Res 31:1117
Acknowledgements
This work was supported by the Doctoral Degree Science Research Foundation from the Ministry of Education,China (No.20050248015). The authors are grateful to Elizabeth Whitsitt and Jonathan Brege of Rice University, Houston Texas for proofreading and language help.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gong, J., Wang, J., Xie, W. et al. Enhanced degradation of aqueous methyl orange by contact glow discharge electrolysis using Fe2+ as catalyst. J Appl Electrochem 38, 1749–1755 (2008). https://doi.org/10.1007/s10800-008-9626-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-008-9626-z