Abstract
Background
Beta thalassemia (β-thalassemia) is a hereditary disease caused by defective globin synthesis and can be classified into three categories of minor (β-TMi), intermedia (β-TI), and major (β-TM) thalassemia. The aim of our study is to investigate the effects of β-thalassemia and its treatment methods on different parts of the eye and how early-diagnostic methods of ocular complications in this disorder would prevent further ocular complications in these patients by immediate treatment and diet change.
Methods
We developed a search strategy using a combination of the words Beta thalassemia, Ocular abnormalities, Iron overload, chelation therapy to identify all articles from PubMed, Web of Science, Scopus, and Google Scholar up to December 2018. To find more articles and to ensure that databases were thoroughly searched, the reference lists of selected articles were also reviewed.
Results
Complications such as retinopathy, crystalline lens opacification, color vision deficiency, nyctalopia, depressed visual field, reduced visual acuity, reduced contrast sensitivity, amplitude reduction in a-wave and b-wave in Electroretinography (ERG), and decrease in the Arden ratio in Electrooculography (EOG) have all been reported in β-thalassemia patients undergoing chelation therapy.
Conclusion
Ocular problems due to β-thalassemia may be a result of anemia, iron overload in the body tissue, side effects of iron chelators, and the complications of orbital bone marrow expansion.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Thalassemia is one of the most common inherited blood disorders which its typical characteristic is abnormality in the production of hemoglobin chains. It occurs as a result of various abnormalities in hemoglobin genes and might be manifested in any of the hemoglobin chains such as alpha, beta, gamma, and delta. Beta thalassemia (β-thalassemia) occurs due to the missing or reduced synthesis of beta chains [1].
There are three sub-types of β-thalassemia categorized based on severity:
- 1.
β-thalassemia minor (β-TMi) or β-thalassemia carrier/β-thalassemia trait: in this condition, hemoglobin chain deficiency is not severe enough to cause malfunction.
- 2.
β-thalassemia intermedia (β-TI): this is a condition in which beta chain deficiency in hemoglobin is severe enough to cause severe anemia and considerable disorder in the patients’ health, such as skeletal deformities and spleen enlargement.
- 3.
β-thalassemia major (β-TM)/Mediterranean anemia/Cooley’s anemia: in β-TM patients, lack of β-chain in hemoglobin contributes to a life-threatening anemia and the patient requires regular blood transfusions and permanent medical care [2].
Despite rapid activation of bone marrow in the body of β-TM patients for erythropoiesis, these patients are still anemic because of ineffective erythropoiesis and early destruction of red blood cells. Since this hyperactivity in the bone marrow causes skeletal deformities, immediate blood transfusion should be initiated as soon as the diagnosis of β-TM has been confirmed. Regular transfusion regimen is required to maintain hemoglobin concentration in normal range. This will prevent chronic hypoxia of the body organs and decrease the hyperactivity of bone marrow [2, 3].
Multiple transfusions result in iron overload in the body. Iron is vital for a great amount of metabolic processes in the body, and many enzymes require iron. It is a crucial element of cytochrome protein and is essential for ATP production, though excessive iron level can lead to oxidative stress. Iron accumulation might result in problems in heart, liver, glands, and eyes and ultimately might lead to death. To remove excessive iron, chelators’ drugs are used to reduce iron accumulation. Iron chelator should selectively bind the iron but no other important elements such as cooper and zinc [4].
Deferoxamine (DFO) is the oldest iron chelator, and if consumed regularly and sufficiently, it prevents iron overload complications and increases the survival in thalassemia patients. The main problem is that this drug is administered through injection, and in the case of excessive infusion of DFO to patients who do not have excessive iron overload, visual and auditory complications may occur [5]. In recent years, oral chelators have been introduced to the market in two forms of Deferasirox (DFX) and Deferiprone (DFP). These oral regiments do not have injection problems of DFO, and it has been claimed that they have less side effects [6, 7].
Thalassemia and its management strategies can lead to many ocular problems. Various studies have reported ocular problems caused by variety of underling factors such as anemia, iron overload, chelation therapy and orbital bone marrow expansion [8].
Table 1 lists the prevalence of ocular problems in these patients reported by previous studies [9,10,11,12,13,14,15,16,17,18,19,20,21]. It has been reported that the prevalence of ocular abnormalities in β-thalassemia patients is between 10.5 and 74% [9,10,11,12,13,14,15,16,17,18,19,20,21]. The discrepancy in the reports can be attributed to different parameters that had been considered, different age-groups of the participants, diverse treatment methods, frequency of blood transfusions, and the type and dosage of iron chelator agents.
Moreover, most of the studies have worked on β-TM patients, while less attention has been paid to β-TI and β-TMi patients. The findings reported ocular complications such as retinopathy, crystalline lens opacification, color vision deficiency, nyctalopia, depressed visual field, reduced visual acuity, reduced contrast sensitivity, some alterations in the results of electrophysiological tests in β-TM patients undergoing chelation therapy [9,10,11,12,13,14,15,16,17,18,19,20,21]. The effects of this disorder and its treatment methods in different parts of the eye will be discussed in the following section.
Ocular abnormalities in β-thalassemia major patients under chelation therapy
There are quite a lot of studies investigating the ocular problems of patients with β-TM and adverse effects of chelating agents, especially DFO. The literature recommends that these patients require regular examinations for ocular problems.
Refractive error and ocular biometric components
As mentioned before, lack of regular transfusion in β-thalassemia patients leads to severe anemia, and this results in dramatic expansion of bone marrow 30–40 times more than normal subjects. Therefore, regular transfusion can prevent anemia complications and compensatory bone marrow expansion [22]. However, sometimes we observe that despite normalizing hemoglobin concentration with regular blood transfusion and taking chelation therapy to eradicate excess iron, skeletal abnormalities such as craniofacial anomalies and long bone deformities can be observed in these patients [23, 24]. As ocular growth depends on the growth of the adjacent bony orbit, craniofacial changes can result in abnormal orbital growth and consequently could cause some changes in biometric parameters of the eye and therefore ocular refraction [16, 25]. Studies have demonstrated that these patients generally have impaired weight and height due to growth hormone deficiency. According to several studies, patients with growth hormone deficiency have shorter axial length, thicker crystalline lens, and steeper cornea in comparison with normal population [26,27,28]. Table 2 displays the results of previous studies working on the biometric parameters and refractive errors in thalassemic patients [16, 25, 29, 30]. As illustrated in Table 2, all studies revealed a shorter axial length in thalassemic patients compared to the normal and a possible explanation, for this might be orbital bone changes or growth hormone deficiency [16, 25, 29, 30].
Because of shorter axial length reported in majority of prior studies, a higher prevalence of hyperopia than other forms of refractive errors is expected. Khalaj et al. [29] investigated the prevalence of refractive errors in β-thalassemia major patients and found that the prevalence of hyperopia was considerably higher (68%) in thalassemia group compared to the normal population in the control group (5.6%), being similar in age and gender.
Two other studies conducted in Iran demonstrated that mean degree of refractive errors in β-thalassemic patients was slightly more positive in comparison with normal population, though the difference between the two groups was not statistically significant. The reason is compensatory mechanism of steeper cornea and thicker crystalline lens [16, 25]. Elkitkat et al. [30] stated that in emmetropization process of β-thalassemic children, their cornea get steeper and their crystalline lens get thicker as a compensatory mechanism for greater risk of hyperopia that is due to shorter axial length and if these changes occur severely, myopic shift emerges.
Dry eye
Dry eye in β-thalassemia patients have been explored using a variety of techniques. Analyzing the quality of tear film using tear break-up time test (TBUT), studies have shown that tear break-up time occurs in less than 10 s in 13.3%, 33%, and 46.15% of β-thalassemic patients [17, 18, 31].
Gartaganis et al. have reported that in 56.73% of β-thalassemic patients, the moistened area in the Schirmer strip was less than 10 mm within 5 min and rose bengal staining was abnormal in 20% of the patients [31]. They also utilized conjunctival cytology by cytobrush to recognize the effective factors in ocular surface disorders. Their investigations revealed goblet cell loss and squamous metaplasia that are normally observed in patients with dry eye. It can be inferred that fewer goblet cells which are responsible for mucin production may be the underlying factor for shorter time in TBUT results in these patients [31].
Deficiency in trace elements and lack of vitamins can be other factors affecting the ocular surface changes in β-thalassemic patients. Vitamin E is an effective antioxidant, and studies have demonstrated that its level is low in the blood plasma of β-thalassemic patients. This could be effective on oxidant/antioxidant balance and make the ocular surface more sensitive to in vitro oxidative modifications [31,32,33].
Jafari et al. revealed that there is a relationship between dry eye and increase in the serum ferritin level. Since increase in iron deposition in glands creates cytotoxic effects and could cause endocrine and exocrine dysfunction and since lacrimal glands are typical tubuloacinar exocrine glands, iron overload could damage their exocrine secretions and subsequently interfere with tear film production [17, 31, 34].
Cataract
The prevalence of cataract in β-thalassemic patients, as reported in different studies, varies from 6.3 to 45.7% [9,10,11, 13,14,15, 17, 18]. A diverse sub-type of crystalline lens opacity has been reported among β-thalassemic patients, such as posterior subcapsular haze, posterior cortical haze, streaks in posterior capsule, and peripheral punctate lens opacity at the cortex [9,10,11, 13,14,15, 17, 18]. The contributory factor to the development of cataract in β-thalassemic patients might be free radical damage due to iron overload [35]. In these patients, the iron overload causes greater oxidative stress and eventually cataract formation [36,37,38]; thereby, several studies have claimed that chelating drugs could have a protective effect by decreasing iron overload [35].
On the other hand, some studies have indicated that cataract development can be the result of chelating agents [14]. They reveal that there is a relationship between crystalline lens opacity and consuming chelators. Yet there are studies that have demonstrated that the cataract formation could be observed in β-thalassemic patients who did not undergo chelation therapy. Hence, chelation therapy [9], per se, is not responsible for cataract formation in these patients.
Few studies have addressed the prevalence of cataract in β-thalassemic patients undergoing different chelation agents [14, 16, 17]. Jafari et al. reported that the prevalence of cataract was higher among the patients who were on DFP. In this study, the prevalence of cataract in patients who were on DFO, combinations of DFO and DFP, and DFX was 6.7%, 13.7%, and 7.7%, respectively [17]. In a study conducted by Nowroozzadeh et al., the prevalence of cataract formation in a group on DFP was greater (18.8%) than the group on DFO (10.7%) [16]. Similarly, Mahdizadeh et al. [39] in a case report stated that cataract occurred in two of their patients who were on DFP therapy. Contrary to the findings of these studies, Taneja et al. [14] revealed that the prevalence of cataract in patients who were on DFO was higher than patients who were on DFP. Overall, since in most of the studies most patients undergoing DFP or Deferasirox were previously treated by DFO, it cannot be certainly concluded which chelator was responsible for these changes.
However, studies have displayed that antioxidants could reduce exudative reactions and consequently could maintain crystalline lens clarity. Considering antioxidants’ role in maintaining crystalline lens clarity, several studies have reasoned that crystalline lens opacity in these patients is due to nutritional deficiency [36].
Retinal abnormalities
Different studies have reported retinal disorders in β-thalassemic patients such as retinal pigment epithelium (RPE) degeneration and mottling, peripheral and central retinal thinning, venous tortuosity and engorgement, retinal hemorrhage, retinal edema, cup-to-disc ratio enlargement, and macular scar [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21, 40,41,42,43,44,45]. The prevalence of retinal disorders reported in different studies is illustrated in Table 1.
Retinal disorders in β-thalassemic patients can be classified into two general groups: pseudoxanthoma elasticum (PXE)-like and non-PXE-like retinal abnormalities [41]. PXE is a progressive genetic disorder caused by mutations in the ABCC6 gene, and its principal characteristic is calcium fragmentation and mineralization of elastic fibers. In this disorder, calcium and other minerals are deposited in the elastic fibers of blood vessels, skin, and Bruch’s membrane beneath the RPE layer [46]. These changes in hemoglobinopathies were originally reported in 1950 in a patient with sickle cell [47], and since then they were frequently reported in different types of hemoglobinopathies, including β-thalassemia [41]. These acquired changes, which are similarly observed both in β-thalassemic patients and in PXE ones, are called PXE-like syndrome [48]. It is well known that the development of PXE-like manifestations including angioid streaks (AS), peau d’orange, and optic disc drusen is actually acquired [20, 48, 49]. Furthermore, there are studies that reported pattern dystrophy-like macular changes including vitelliform maculopathy and butterfly-shaped macular lesions in patients undergoing prolonged DFO treatment [42,43,44,45]. Some researchers proposed iron overload of tissues and associated oxidative stress due to hemolytic disorders as a potential cause of elastic fiber damage in β-thalassemia patients [20, 48, 50]. While some others believe that even if iron contributes to changes in the elastic fibers, it cannot explain the clinical and structural similarities with inherited PXE [51]. It is reported that the level of ABCC6/Abcc6 transport activity is an influential modulator of calcification and could be the pathomechanism for elastic fiber mineralization. Martin et al. [51] in their animal study found a progressive decrease in Abcc6 production in mice. This down-regulation mediated by changes in the transcriptional regulation of Abcc6. Because of similarity of transcriptional regulators in human and mouse ABCC6/Abcc6 genes [52], it can be speculated that similar to mice, molecular changes could lead to ABCC6 endowment and increased susceptibility to dystrophic mineralization in ocular tissue and progressive development of PXE manifestations in some of these patients [51].
In AS, structural alterations are formed as tears in elastic lamina Bruch’s membrane and afterward RPE undergoes degeneration and atrophy [53]. The incidence of AS has been reported in different studies [45, 53,54,55,56]. This disorder is usually asymptomatic, but if the lesion expands to the fovea or causes choroidal neovascularization (CNV), hemorrhage, and scar in macula, it might result in progressive vision loss [20, 41]. Even in some cases, vision loss has been reported due to the RPE atrophy without CNV formation [57].
Small confluent dark yellowish lesion in RPE is called peau d’orange usually observed prior to the manifestation of AS [20]. These lesions are usually observed at the posterior pole very early in the disease and more peripheral in later stages [58].
Vascular tortuosity is one of the non-PXE-like anomalies in β-thalassemia patients. Mild and chronic anemia between transfusion sessions are the causes of this anomaly and lead to tissue hypoxia and retinal tortuosity [59, 60]. Moreover, it has been found that there is a relationship between iron overload and patient’s age with vascular tortuosity formation. Another risk factor for retinal disorders is iron overload due to repeated transfusion. RPE is the site of accumulation and deposition of iron in retina; thereby, these disorders are usually observed in RPE [4, 61]. Indeed, the presence of iron in retina becomes challenging when ferrous iron (Fe2+), which could produce hydroxyl radicals from hydrogen peroxide during a Fenton reaction, causes oxidative damages to retina and RPE [62, 63]. To decrease iron overload and its complications, chelating agents should be utilized. A large number of studies have reported that chelation therapy could prevent retinal damage caused by iron overload. However, the amount of drug consumption and dosage should be properly controlled to prevent adverse effects of excessive iron and other associated minerals removal [63].
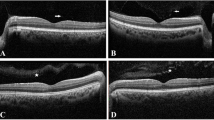
The most common oldest chelating agent in β-thalassemic patients is intravenous DFO therapy. DFO toxicity has led to nyctalopia, color vision deficiency, visual field defects, visual acuity impairment, and RPE changes [41, 64]. There is significant correlation between DFO dosage and its ocular side effects. In most studies, when patients were on DFO with dosage of less than 50 mg/kg/d, mild or no ocular toxicities have been reported [17, 65]. Some studies have revealed that the retinal toxicity due to intravenous DFO therapy is reversible to some extent after discontinuing the drug [66, 67]. Therefore, it is essential to perform ocular examinations by implementing different methods such as electrodiagnostic procedures (especially ERG), visual field testing, color vision testing, confocal near-infrared (NIR) reflectance imaging, fundus fluorescein angiography (FFA), late-phase indocyanine green (ICG) angiography, fundus autofluorescence (FAF), and spectral domain optical coherence tomography (SD-OCT). Table 3 summarizes previous retinal findings with different imaging techniques in β-thalassemia patients who were under chelation therapy [14, 42, 45, 68,69,70,71,72,73,74].
Viola et al. [45] used multimodal imaging such as confocal scanning laser ophthalmoscope (cSLO), NIR, FAF, and SD-OCT to investigate and follow up clinical course of β-thalassemia patients with DFO retinopathy. They found pattern dystrophy-like changes in half of their patients. Follow-up examinations of their patients during a 40-month period revealed a progressive development of RPE atrophy in most of their cases. Therefore, in cases requiring long-term treatment with chelator drugs, observing early changes such as pattern dystrophy-like alterations in deep structures of the retina could predispose sight-threatening complications such as RPE atrophy [45]. Therefore, early diagnosis and long-term follow-up of retinal findings, either PXE-like or related to DFO, are essential and would be helpful in the decision to discontinue or switch therapy to prevent sight-threatening ocular problems.
Oral DFP and DFX are the alternative drugs to intravenous DFO therapy in these patients [7, 75, 76]. Most of the studies on animals showed that DFP is an effective retinal iron chelator. DFP could pass through blood retinal barrier and chelate intracellular iron without inducing any retinal toxicity. Consequently, in most studies, DFP has been introduced as retinal protected iron chelator [77, 78]. In contrast, a number of studies reported that RPE degeneration had been developed in patients on DFP [12, 17]. Jafari et al. in a study comparing retinal abnormalities in thalassemic patients undergoing different chelating drugs concluded that the prevalence of RPE degeneration was higher in a group who consumed DFP + DFO [17]. Similarly, Taher et al. confirmed the prevalence of RPE degeneration in patients undergoing DFP was four times more than the patients who were on DFO [12]. Since in all of these studies, prior to taking DFP, the patients were on DFO therapy, we could not certainly claim that the side effects are due to DFP therapy or DFO usage.
Furthermore, studies have shown that DFX is an effective systemic chelator, though there is no evidence confirming its penetration to the retina [41].
Contrast sensitivity
Scant research has been conducted on contrast sensitivity in β-thalassemic patients [79,80,81]. In 2000, Gartaganis et al. investigated contrast sensitivity using CSV-1000 test in patients with thalassemia major receiving regular transfusion and being treated by subcutaneous infusions of DFO. They found out that contrast sensitivity in all frequencies was less than that of the normal participants [79]. In 2010, Spyridon et al. investigated contrast sensitivity using B-VAT II-SG Mentor Video Acuity Tester in β-TM patients who were regularly transfused and cured by subcutaneous infusions of DFO. They observed a significant difference in contrast sensitivity of these patients and the control group in several spatial frequencies studied (2,3, 6 cycle/degrees). Conclusively, they stated that DFO administration could make changes in the retina causing contrast sensitivity reduction in β-TM patients; therefore, these patients should be regularly monitored for ocular complications [81].
In 2017, Ghazanfari et al. compared contrast sensitivity in β-TM patients who were treated by DFO and DFX with normal population using Freiberg visual acuity and contrast test (FrVACT). The results of their study indicated that contrast sensitivity in thalassemic patients who were treated by DFO was significantly lower than that of the normal control group in all spatial frequencies studied (1, 5, and 15 cycle/degrees). Furthermore, it was revealed that contrast sensitivity in the group treated by DFX was similarly lower in all frequencies, though the difference was not statistically significant [80].
There are quite a lot of studies investigating the contrast sensitivity evaluation in the early diagnosis of ocular disorders. Most of them have demonstrated that the contrast sensitivity test is more sensitive than visual acuity test [82, 83]. Most researchers believe that patients with lower contrast sensitivity require a more meticulous examination in the follow-up sessions to prevent more ocular problems [83]. The results of a study conducted by Ghazanfari et al. also confirmed that contrast sensitivity test is more sensitive than visual acuity test in the diagnosis of ocular disorders in β-thalassemic patients. It also showed that contrast sensitivity was affected before the manifestation of other complications in these patients. Therefore, it is recommended to perform contrast sensitivity test in the regular ocular examination of these patients [80].
Visual field defects
The prevalence of visual field defects in β-thalassemic patients is between 33.7 and 74%, and the most common type of visual field defect in these patients is general depression [11, 17]. Researchers believe that visual field defect might be due to toxic effects of chelating agent and that there is a strong relationship between chelating agent dosage and visual field loss. Research has shown that visual field defect occurs in patients receiving chelator with the dose of over 40–50 mg/kg/d [13, 17, 72].
Electrodiagnostic findings
Practically, ocular electrodiagnostic evaluations are used to measure the function of the retina and optic nerve and can detect various functional defects and retinal degeneration as well as visual-related neurological defects [84,85,86]. Many previous studies on β-thalassemic patients have implemented flash-electroretinography (FERG), pattern-ERG, multifocal-ERG, electrooculography (EOG), and visual evoked potential (VEP) [11, 13, 15, 19, 65, 87,88,89,90,91,92].
A series of studies has indicated that the changes in electrophysiology tests are related to toxic effects of chelation therapy. Although the findings related to these changes are highly controversial, there is general agreement that chelating agents especially DFO have a substantial effect on various electrodiagnostic tests [65, 87, 89, 90]. Table 4 demonstrates the results of some previous studies that used these methods to investigate adverse effects of chelation therapy on electrophysiological tests [65, 87,88,89,90,91,92]. The results of studies demonstrated that ERG and VEP findings in children with thalassemia are slightly similar to the findings of these tests in SIDERosis bulbi cases [18].
Most of the studies that have utilized pattern VEP (PVEP) test of β-thalassemic patients to evaluate neurophysiologic manifestations of the disease have reported greater latency time in PVEP test in these patients [65, 92,93,94]. However, there are several studies showing no significant difference in PVEP of β-thalassemic patients compared to the normal population [88]. Overall, the results of these studies revealed that in β-thalassemic patients, neurologic abnormalities influencing VEP responses are due to several underlying factors, such as chronic hypoxia, intraocular iron overload, bone marrow expansion, and toxicity of DFO [95, 96].
In some studies, neurotoxicity due to DFO has been highlighted as the most important cause of VEP changes in these patients [91, 92, 94].
It is remarkable that in most cases neurophysiological manifestations are not associated with any clinical signs and symptoms. Arden conducted a study on flash-ERG, pattern-ERG, and EOG in 43 patients with β-TM and β-TI who had no clinical symptoms. He pointed out that pattern-ERG was influenced to a greater extent in comparison with flash-ERG and EOG. Although the findings of this study showed reduced Arden ratio in EOG test and some abnormalities in flash-ERG, these changes were not considerable. Altogether, he did not find any correlation between chelating agent dosage, blood ferritin level, and neurophysiological changes. Thus, he proposed that these changes are attributed to β-cells damage caused by iron deposition in pancreas, followed by diabetes mellitus and retinal disorder [97]. Jiang et al. conducted a study on older patients with β-thalassemia and reported that the function of rod cells and accordingly scotopic activity of the retina became interrupted as the result of progressive iron accumulation. The authors also documented that the toxicity of chelation agents was not so effective. Indeed, effectiveness of chelating agents appears in long-term consumption and causes more severe functional complications [98]. Furthermore, Gelmi et al. worked on thalassemic patients who were not previously exposed to higher dosages of DFO. They came to the conclusion that in older patients, the amplitude of N1-P1 in VEP test was greater than normal values, and this can be attributed to iron overload. Furthermore, they stated that abnormalities found in ERG test results increased with age and defects due to iron overload, included mostly in b-wave. Based on their findings, iron overload was more effective than DFO toxicity in these conditions [90]. However, some studies showed contradictory results and worked on DFO toxicity effect on cone responses, though they found that the results related to rod cells were normal [44, 70]. Even in several studies, the findings indicated reduction in both rod and cone responses [99].
A closer look at the literature on ERG and EOG, however, reveals a number of gaps and shortcomings. To date, this is not clearly stated in the literature whether ERG and EOG tests could show any signs before FFA and SD-OCT clinical manifestations. However, it is prudent to pay attention to the results of these tests and use them as a clinical guideline before retinal manifestations [41, 100]. Many studies have highlighted the importance of EOG test. They have emphasized that decline in Arden ratio in EOG is extremely beneficial since majority of complications related to iron overload occur in RPE layer [100, 101]. In contrast, some other studies have reported normal EOG findings in β-thalassemic patients [91]. Kertes et al. introduced multifocal-ERG as an effective method in the diagnosis of maculopathies resulted from DFO toxicity [102]. They reported depression of responses, especially in central areas, and the results were consistent with previous research [89, 102]. Studies have made a comparison between oral iron chelator and DFO. It was found that electrodiagnostic findings related to DFO show a greater decrease than oral iron chelator group. It was observed more in the figures related to wave latency in comparison with wave amplitude [89].
Although the results of quantitative studies on electrodiagnostic test in β-thalassemic patients are not consistent, electrophysiological results in most of the studies, even in the absence of ocular clinical signs and symptoms, have been a warning for the onset and progressing of clinical manifestations in these patients. Therefore, it can be concluded that regular electrophysiological tests in thalassemic patients can be helpful in controlling drug consumption and in early diagnosis of ocular disorders, particularly macular degeneration, when there are no signs or symptoms.
Ocular abnormalities in β-thalassemia minor (β-TMi)
Scant research has been conducted on the ocular disorders of β-TMi. In 1953, Rudd et al. conducted a longitudinal case study. They followed up a patient with β-TMi for several years. At first, no ocular disorder was observed, though they observed visual acuity as a result of retinal hemorrhages several years later [103]. In 1986, Kinsella and Mooney reported manifestations of retinal AS in a 42-year-old patient with minor thalassemia. The patient was − 3.5 diopter myopic suffering from a long-term anemia with blood transfusion in her pregnancy. AS were observed in both of her eyes which could be because of iron deposition during blood transfusion in her three pregnancies along with development of defects in Bruch’s membrane as a result of myopia [56].
Ocular abnormalities in β-thalassemia intermedia (β-TI)
Similar to β-TMi, few studies have been conducted on the ocular disorders in β-TI patients. In 1989, Aessopos et al. carried out a study and employed fundus examination on 100 patients with β-thalassemia (62 patients with β-TM and 38 patients with β-TI). They observed AS in 20% of patients (9 patients with β-TM and 11 patients with β-TI). They also concluded that there was a positive relationship between the age of patients and the occurrence of AS. None of their patients who were under 20 manifested AS [54]. In 2008, another study was conducted by Aessopos et al., and they presented two cases that were both suffered from progressive decline in visual acuity due to AS. As expected, elastic fibers in these patients were gradually degenerated and caused PXE-like anomalies [57]. Barteselli et al. (2014) evaluate 255 Italian patients with β-thalassemia, (153 β-TM and 102 β-TI). In order to characterize their ocular phenotype, they used confocal scanning laser ophthalmoscope (cSLO) in NIR reflectance imaging and other imaging modalities. They found PXE-like fundus changes in 11.1% of β-TM and 52% of β-TI patients. PXE-like lesions in β-TI patients were peau d’orange (42.2%), AS (31.4%), pattern dystrophy-like changes (7.8%), and optic disc drusen (2.0%). Similar to previous reports, their results showed that TI patients carry a higher risk of developing ocular PXE-like lesions than TM, especially in older cases, in cases of previous splenectomy and in cases that needed transfusions and treatment with iron-chelating agents. In order to determine the relationship between fundus alterations and multiple hematologic parameters and the reason for higher prevalence of ocular changes in TI patients compare to TM ones, they compare hematologic parameters such as hemoglobin (Hb) level, liver iron concentration (LIC), and serum ferritin level between two groups and found that except age, Hb level, and LIC, no other differences were found between β-TM and β-TI groups [20]. Since 90% of excess iron is deposited in the liver, the iron concentration in the liver can be indicative of iron levels throughout the body and widely used to determine the necessary dose and the efficacy of a chelation regimen [104, 105]. Musallam et al. (2012) report that in spite of lower serum ferritin levels in β-TI patients, in comparison with β-TM, iron liver concentration is higher in these patients [105]. Therefore, longer age and longer chronic hypoxia due to non-transfusion-dependent anemia in β-TI patients can be the reason for the higher incidence of PXE-like ocular problems in them [20, 106]. Another risk factor for the development of ocular PXE-like lesions in β-thalassemia patients is splenectomy [20]. Since the intact spleen may be a reservoir of excess iron, splenectomized patients may show a higher rate of complications related to iron overload [107]. In consistent with these findings, Barteselli et al. [20] found a strong correlation between splenectomy and PXE-like fundus changes. In general, as PXE-like lesions are age dependent and their prevalence is higher in patients over 30, their development is more likely in β-TI patients who normally live longer than the major thalassemic patients [48, 57].
Management strategies
The first step in management process of β-thalassemic patients is regular blood transfusion. Although it prevents anemia, it results in excessive iron accumulation in the body and failure in organs, such as heart, liver and the eye. Hence, these patients inevitably require undergoing iron chelation therapy to remove excessive iron from the body [4].
Ophthalmic abnormalities in these patients might be as a result of several underlying factors, such as the disease itself, iron accumulation, and toxicity of chelating agents [8]. Consequently, these patients should repeatedly undergo eye examinations so that if each of these complications occurs, the patient is referred to hematologist for further necessary decisions and chelation therapy modification to inhibit or prevent more adverse effects.
Several techniques can be recommended to immediately detect ocular disorders in β-thalassemia patients. As discussed above, contrast sensitivity evaluation is one of the most suitable methods that is affected before the manifestation of symptoms and clinical signs like visual acuity loss and even pathological complications [82, 83]. Since contrast sensitivity tests are inexpensive and available, they could be included in routine eye examination of β-thalassemic patients. Likewise, prior research suggests that electro-diagnostic tests, especially ERG and EOG, could be practical in the early diagnosis of the effects of β-thalassemia and its treatment plans [76].
Another clinical test for the investigation of early ophthalmic manifestations of β-thalassemia and its management strategies is FAF. Abnormal fluctuations in FAF have been reported in these patients prior to observable changes in fundus examination. This is an effective, fast, and noninvasive method for monitoring retinal involvement in these patients [43, 45, 74]. FAF using short-wavelength light mainly originates from the RPE and is useful for detecting secondary changes within this layer [45].
Other methods that could be particularly helpful in the early diagnosis of β-thalassemia complications are visual field investigation particularly by utilizing advanced technologies like micro-perimeter devices, SD-OCT and confocal near-infrared (NIR) reflectance imaging [43, 45, 70, 108]. Long-wavelength light used in NIR reflectance helps to visualize early alterations occurring within Bruch’s membrane underneath the RPE, which absorbs shorter-wavelengths lights [108]. Since most of the complications related to β-thalassemia are age dependent and appear in people over 20, it would be suggested that regular ocular checkups be performed in their second decade of life. This issue is largely important when PXE-like lesions especially AS are observed in the process of the disease. Moreover, if CNV is formed, we should take treatment actions to prevent retinal hemorrhages and vision loss.
Conclusion
The literature review has shown that β-thalassemia can give rise to various ocular abnormalities as a result of anemia, repeated blood transfusion, iron overload, and chelation therapy. However, a number of challenges remain to be addressed concerning which factors are more effective in development of ocular complications. There is little information about the adverse effects of different chelating agents. Some claim that chelating drugs cause complications; on the other hand, others state that they play a protective role. With the advent of new iron chelators, it is necessary to plan future studies with greater sample sizes in different thalassemic groups who have used just one type of chelating medication since the start of treatment to better understand the complications of different chelators.
Since life expectancy in β-thalassemic patients has improved over time, the possibility of ocular abnormalities increases. It is demonstrated that primary fundus alterations in β-thalassemia patient’s eyes usually occur in the Bruch membrane and RPE. Abnormalities of these retinal layers are easily detectable using novel noninvasive imaging modalities such as cSLO, NIR reflectance, and FAF imaging even before symptoms are manifested in these patients. Therefore, long-term follow-up of β-thalassemia patients with these new multimodal imaging techniques, especially those who need long-term DFO treatment, is essential and could help to prevent development of further sight-threatening ocular problems by chelation therapy modification.
References
Provan D, Singer CR, Baglin T, Dokal I (2009) Oxford handbook of clinical haematology. Oxford University Press, Oxford
Cao A, Galanello R (2010) Beta-thalassemia. Genet Med 12(2):61
Olivieri NF, Nathan DG, MacMillan JH et al (1994) Survival in medically treated patients with homozygous β-thalassemia. N Engl J Med 331(9):574–578
He X, Hahn P, Iacovelli J et al (2007) Iron homeostasis and toxicity in retinal degeneration. Prog Retin Eye Res 26(6):649–673
Brittenham GM, Griffith PM, Nienhuis AW et al (1994) Efficacy of deferoxamine in preventing complications of iron overload in patients with thalassemia major. N Engl J Med 331(9):567–573
Bollig C, Schell LK, Rücker G et al (2017) Deferasirox for managing iron overload in people with thalassaemia. Cochrane Database Syst Rev 8:CD007476
Olivieri NF, Brittenham GM, Matsui D et al (1995) Iron-chelation therapy with oral deferiprone in patients with thalassemia major. N Engl J Med 332(14):918–922
Liaska A, Petrou P, Georgakopoulos CD et al (2016) β-Thalassemia and ocular implications: a systematic review. BMC Ophthalmol 16(1):102
Gartaganis S, Ismiridis K, Papageorgiou O, Beratis NG, Papanastasiou D (1989) Ocular abnormalities in patients with β-thalassemia. Am J Ophthalmol 108(6):699–703
Gaba A, D’Souza P, Chandra J, Narayan S, Sen S (1998) Ocular changes in β-thalassemia. Ann Ophthalmol Glaucoma 30(6):357–360
Shahriari H, Ghasemzadeh F, Eshghi P, Masoomian B (2006) Ocular side effects of desferal in patients with β-thalassemia. Bina J Ophthalmol 11:519–523
Taher A, Bashshur Z, Shamseddeen WA et al (2006) Ocular findings among thalassemia patients. Am J Ophthalmol 142(4):704–705
Rahiminejad M, Rahiminejad S, Rahimi M et al (2009) Ocular complication and visual evoked potential in β-thalassemia patients on desferal therapy. Res J Biol Sci 4(8):928–932
Taneja R, Malik P, Sharma M, Agarwal MC (2010) Multiple transfused thalassemia major: ocular manifestations in a hospital-based population. Indian J Ophthalmol 58(2):125
Dewan P, Gomber S, Chawla H, Rohatgi J (2011) Ocular changes in multi-transfused children with β-thalassaemia receiving desferrioxamine: a case-control study. S Afr J Child Health 5(1):11–14
Nowroozzadeh MH, Kalantari Z, Namvar K, Meshkibaf MH (2011) Ocular refractive and biometric characteristics in patients with thalassaemia major. Clin Exp Optom 94(4):361–366
Jafari R, Heydarian S, Karami H et al (2015) Ocular abnormalities in multi-transfused beta-thalassemia patients. Indian J Ophthalmol 63(9):710
Kumble D, Sekhon PK (2017) Ocular involvement in beta thalassemia major: a prospective study in an Indian cohort. Int J Contemp Pediatr 4(3):780–782
Merchant RH, Punde H, Thacker N, Bhatt D (2017) Ophthalmic evaluation in beta-thalassemia. Indian J Pediatr 84(7):509–514
Barteselli G, Dell’arti L, Finger RP et al (2014) The spectrum of ocular alterations in patients with beta-thalassemia syndromes suggests a pathology similar to pseudoxanthoma elasticum. Ophthalmology 121:709–718
Saif AT, Saif PS, Dabous O (2017) Fundus changes in thalassemia in Egyptian patients. Delta J Ophthalmol 18(1):20
Voskaridou E, Terpos E (2004) New insights into the pathophysiology and management of osteoporosis in patients with beta thalassaemia. Br J Haematol 127(2):127–139
Jensen C, Tuck S, Agnew J et al (1998) High prevalence of low bone mass in thalassaemia major. Br J Haematol 103(4):911–915
Weatherall DJ, Clegg JB (2008) The thalassaemia syndromes. Wiley, Hoboken
Heydarian S, Jafari R, Karami H (2016) Refractive errors and ocular biometry components in thalassemia major patients. Int Ophthalmol 36(2):267–271
Parentin F, Tonini G, Perissutti P (2004) Refractive evaluation in children with growth defect. Curr Eye Res 28(1):11–15
Parentin F, Perissutti P (2005) Congenital growth hormone deficiency and eye refraction: a longitudinal study. Ophthalmologica 219(4):226–231
Bourla DH, Laron Z, Snir M, Lilos P, Weinberger D, Axer-Siegel R (1197) Insulinlike growth factor I affects ocular development: a study of untreated and treated patients with Laron syndrome. Ophthalmology 113(7):e1–e5
Khalaj M, Mahyar A, Jahan Hashemi H, Godsi F (2009) Assessing the refractive errors in beta-thalassemia major patients. J Guilan Univ Med Sci 17(68):42–49
Elkitkat RS, El-Shazly AA, Ebeid WM, Deghedy MR (2018) Relation of anthropometric measurements to ocular biometric changes and refractive error in children with thalassemia. Eur J Ophthalmol 28(2):139–143
Gartaganis S, Georgakopoulos C, Exarchou A et al (2003) Alterations in conjunctival cytology and tear film dysfunction in patients with β-thalassemia. Cornea 22(7):591–597
Arcasoy A, Cavdar AO (1975) Changes of trace minerals (serum iron, zinc, copper and magnesium) in thalassemia. Acta Haematol 53(6):341–346
De Luca C, Filosa A, Grandinetti M, Maggio F, Lamba M, Passi S (1999) Blood antioxidant status and urinary levels of catecholamine metabolites in β-thalassemia. Free Radic Res 30(6):453–462
Borgna-Pignatti C, Cammareri V, De Stefano P, Magrini U (1984) The sicca syndrome in thalassaemia major. Br Med J (Clin Res Ed) 288(6418):668–669
Popescu C, Siganos D, Zanakis E, Padakis A (1998) The mechanism of cataract formation in persons with beta-thalassemia. Oftalmologia (Bucharest, Romania: 1990) 45(4):10–13
Athanasiadis I, Konstantinidis A, Kyprianou I, Robinson R, Moschou V, Kouzi-Koliakos K (2007) Rapidly progressing bilateral cataracts in a patient with beta thalassemia and pellagra. J Cataract Refract Surg 33(9):1659–1661
Dhawan V, KhR K, Marwaha R, Ganguly NK (2005) Antioxidant status in children with homozygous thalassemia. Indian Pediatr 42(11):1141–1145
Marsili S, Salganik RI, Albright CD et al (2004) Cataract formation in a strain of rats selected for high oxidative stress. Exp Eye Res 79(5):595–612
Mehdizadeh M, Nowroozzadeh MH (2009) Posterior subcapsular opacity in two patients with thalassaemia major following deferiprone consumption. Clin Exp Optom 92(4):392–394
Aksoy A, Aslan L, Aslankurt M et al (2014) Retinal fiber layer thickness in children with thalassemia major and iron deficiency anemia. Semin ophthalmol 29(1):22–26
Bhoiwala DL, Dunaief JL (2016) Retinal abnormalities in β-thalassemia major. Surv Ophthalmol 61(1):33–50
Genead MA, Fishman GA, Anastasakis A, Lindeman M (2010) Macular vitelliform lesion in desferrioxamine-related retinopathy. Doc Ophthalmol 121:161–166
Georgakopoulos CD, Tsapardoni F, Kostopoulou EV, Makri OE (2018) Pattern dystrophies in patients treated with deferoxamine: report of two cases and review of the literature. BMC Ophthalmol 18(1):246
Gonzales CR, Lin AP, Engstrom RE, Kreiger AE (2004) Bilateral vitelliform maculopathy and deferoxamine toxicity. Retina (Philadelphia, Pa) 24(3):464–467
Viola F, Barteselli G, Dell’Arti L et al (2014) Multimodal imaging in deferoxamine retinopathy. Retina (Philadelphia, Pa) 34(7):1428–1438
Finger RP, Issa PC, Ladewig MS et al (2009) Pseudoxanthoma elasticum: genetics, clinical manifestations and therapeutic approaches. Surv Ophthalmol 54(2):272–285
Goodman G, von Sallmann L, Holland MG (1957) Ocular manifestations of sickle-cell disease. AMA Arch Ophthalmol 58(5):655–682
Aessopos A, Farmakis D, Loukopoulos D (2002) Elastic tissue abnormalities resembling pseudoxanthoma elasticum in β-thalassemia and the sickling syndromes. Blood 99(1):30–35
Hamlin N, Beck K, Bacchelli B, Cianciulli P, Pasquali-Ronchetti I, Le Saux O (2003) Acquired Pseudoxanthoma elasticum-like syndrome in β-thalassaemia patients. Br J Haematol 122(5):852–854
Bunda S, Kaviani N, Hinek A (2005) Fluctuations of intracellular iron modulate elastin production. J Biol Chem 280(3):2341–2351
Martin L, Douet V, VanWart CM, Heller MB, Le Saux O (2011) A mouse model of β-thalassemia shows a liver-specific down-regulation of ABCC6 expression. Am J Pathol 178(2):774–783
Jiang Q, Matsuzaki Y, Li K, Uitto J (2006) Transcriptional regulation and characterization of the promoter region of the human ABCC6 gene. J Investig Dermatol 126(2):325–335
Georgalas I, Papaconstantinou D, Koutsandrea C et al (2009) Angioid streaks, clinical course, complications, and current therapeutic management. Ther Clin Risk Manag 5:81
Aessopos A, Stamatelos G, Savvides P et al (1989) Angioid streaks in homozygous β-thalassemia. Am J Ophthalmol 108(4):356–359
Gibson J, Chaudhuri P, Rosenthal A (1983) Angioid streaks in a case of beta thalassaemia major. Br J Ophthalmol 67(1):29
Kinsella FP, Mooney DJ (1988) Angioid streaks in beta thalassaemia minor. Br J Ophthalmol 72(4):303–304
Aessopos A, Floudas CS, Kati M et al (2008) Loss of vision associated with angioid streaks in β-thalassemia intermedia. Int J Hematol 87(1):35–38
Issa PC, Finger RP, Götting C, Hendig D, Holz FG, Scholl HP (2010) Centrifugal fundus abnormalities in pseudoxanthoma elasticum. Ophthalmology 117(7):1406–1414
Incorvaia C, Parmeggiani F, Costagliola C, Perri P, D’Angelo S, Sebastiani A (2003) Quantitative evaluation of the retinal venous tortuosity in chronic anaemic patients affected by β-thalassaemia major. Eye 17(3):324
Sorcinelli R, Sitzia A, Figus A, Lai M (1990) Ocular findings in beta-thalassemia. Metab Pediatr Syst Ophthalmol (New York, NY: 1985) 13(1):23–25
Moiseyev G, Chen Y, Takahashi Y, Wu BX, Ma J-X (2005) RPE65 is the isomerohydrolase in the retinoid visual cycle. Proc Natl Acad Sci 102(35):12413–12418
Błasiak J, Skłodowska A, Ulińska M, Szaflik J (2009) Iron and age-related macular degeneration. Klin Oczna 111(4–6):174–177
Mehta S, Dunaief JL (2012) The role of iron in retinal diseases. In: Studies on retinal and choroidal disorders. Humana Press, pp 259–275
Davies S, Hungerford J, Arden G, Marcus R, Miller M, Huehns E (1983) Ocular toxicity of high-dose intravenous desferrioxamine. Lancet 322(8343):181–184
Olivieri NF, Buncic JR, Chew E et al (1986) Visual and auditory neurotoxicity in patients receiving subcutaneous deferoxamine infusions. N Engl J Med 314(14):869–873
Baath JS, Lam WC, Kirby M, Chun A (2008) Deferoxamine-related ocular toxicity: incidence and outcome in a pediatric population. Retina (Philadelphia, Pa) 28:894–899
Haimovici R, D’Amico DJ, Gragoudas ES, Sokol S (2002) The expanded clinical spectrum of deferoxamine retinopathy. Ophthalmology 109:164–171
Wu C-H, Yang C-P, Lai C-C, Wu W-C, Chen Y-H (2014) Deferoxamine retinopathy: spectral domain-optical coherence tomography findings. BMC Ophthalmol 14(1):88
Gelman R, Kiss S, Tsang SH (2014) Multimodal imaging in a case of deferoxamine induced maculopathy. Retin Cases Brief Rep 8(4):306
Van Bol L, Alami A, Benghiat FS, Rasquin F (2014) Spectral domain optical coherence tomography findings in early deferoxamine maculopathy: report of two cases. Retin Cases Brief Rep 8(2):97–102
Eleftheriadou M, Theodossiadis P, Rouvas A, Alonistiotis D, Theodossiadis G (2012) New optical coherence tomography fundus findings in a case of beta-thalassemia. Clin Ophthalmol (Auckland, NZ) 6:2119
Simon S, Athanasiov PA, Jain R, Raymond G, Gilhotra JS (2012) Desferrioxamine-related ocular toxicity: a case report. Indian J Ophthalmol 60(4):315
Viola F, Barteselli G, Dell’Arti L et al (2012) Abnormal fundus autofluorescence results of patients in long-term treatment with deferoxamine. Ophthalmology 119(8):1693–1700
Arora A, Wren S, Gregory Evans K (2004) Desferrioxamine related maculopathy: a case report. Am J Hematol 76(4):386–388
Meerpohl JJ, Antes G, Rücker G et al (2012) Deferasirox for managing iron overload in people with thalassaemia. Cochrane Database Syst Rev (2)
Galanello R (2007) Deferiprone in the treatment of transfusion-dependent thalassemia: a review and perspective. Ther Clin Risk Manag 3(5):795
Song D, Zhao L, Li Y et al (2014) The oral iron chelator deferiprone protects against systemic iron overload-induced retinal degeneration in hepcidin knockout mice. Invest Ophthalmol Vis Sci 55:4525–4532
Gartaganis SP, Zoumbos N, Koliopoulos JX, Mela EK (2000) Contrast sensitivity function in patients with beta-thalassemia major. Acta Ophthalmol Scand 78(5):512–515
Ghazanfari A, Jafarzadehpour E, Heydarian S, Dailami KN, Karami H (2018) Comparison of contrast sensitivity in β-thalassemia patients treated by deferoxamine or deferasirox. J Optom 12:168–173
Spyridon G, Ioannis A, Nikolaos C et al (2010) Contrast sensitivity in patients with beta-thalassemia major and sickle cell disease under regular transfusions and treatment with desferrioxamine. Open Ophthalmol J 4:39
Regan D, Neima D (1983) Low-contrast letter charts as a test of visual function. Ophthalmology 90(10):1192–1200
Woods RL, Tregear SJ, Mitchell RA (1998) Screening for ophthalmic disease in older subjects using visual acuity and contrast sensitivity1. Ophthalmology 105(12):2318–2326
Aminoff MJ (2012) Electrodiagnosis in clinical neurology. Elsevier, Amsterdam
Arden G, Fojas M (1962) Electrophysiological abnormalities in pigmentary degenerations of the retina: assessment of value and basis. Arch Ophthalmol 68(3):369–389
Scholl HP, Zrenner E (2000) Electrophysiology in the investigation of acquired retinal disorders. Surv Ophthalmol 45(1):29–47
Dettoraki M, Kattamis A, Ladas I et al (2017) Electrophysiological assessment for early detection of retinal dysfunction in beta-thalassemia major patients. Graefe’s Arch Clin Exp Ophthalmol 255(7):1349–1358
Economou M, Zafeiriou DI, Kontopoulos E et al (2006) Neurophysiologic and intellectual evaluation of beta-thalassemia patients. Brain Dev 28(1):14–18
El-Shazly AA, Ebeid WM, Elkitkat RS, Deghedy MR (2017) Electroretinographic and visual-evoked potential changes in relation to chelation modality in children with thalassemia. Retina (Philadelphia, Pa) 37(6):1168–1175
Gelmi C, Borgna-Pignatti C, Franchin S, Tacchini M, Trimarchi F (1988) Electroretinographic and visual-evoked potential abnormalities in patients with beta-thalassemia major. Ophthalmologica 196(1):29–34
Wong V, Li A, Lee A (1993) Neurophysiologic study of β-thalassemia patients. J Child Neurol 8(4):330–335
Zafeiriou DI, Kousi AA, Tsantali CT et al (1998) Neurophysiologic evaluation of long-term desferrioxamine therapy in beta-thalassemia patients. Pediatr Neurol 18(5):420–424
De Virgiliis S, Congia M, Turco M et al (1988) Depletion of trace elements and acute ocular toxicity induced by desferrioxamine in patients with thalassaemia. Arch Dis Child 63(3):250–255
Marciani M, Cianciulli P, Stefani N et al (1991) Toxic effects of high-dose deferoxamine treatment in patients with iron overload: an electrophysiological study of cerebral and visual function. Haematologica 76(2):131–134
Aarabi B, Haghshenas M, Rakeii V (1998) Visual failure caused by suprasellar extramedullary hematopoiesis in beta thalassemia: case report. Neurosurgery 42(4):922–925
Stamboulis E, Vlachou N, Drossou-Servou M et al (2004) Axonal sensorimotor neuropathy in patients with β-thalassaemia. J Neurol Neurosurg Psychiatry 75(10):1483–1486
Arden G, Wonke B, Kennedy C, Huehns E (1984) Ocular changes in patients undergoing long-term desferrioxamine treatment. Br J Ophthalmol 68(12):873–877
Jiang C, Hansen RM, Gee BE, Kurth SS, Fulton AB (1998) Rod and rod mediated function in patients with β-thalassemia major. Doc Ophthalmol 96(4):333–346
Orton R, Sulh H (1985) Ocular and auditory toxicity of long-term, high-dose subcutaneous deferoxamine therapy. Can J Ophthalmol 20(4):153–156
Haimovici R, D’Amico DJ, Gragoudas ES, Sokol S, Group DRS (2002) The expanded clinical spectrum of deferoxamine retinopathy. Ophthalmology 109(1):164–171
Ravelli M, Scaroni P, Mombelloni S et al (1990) Acute visual disorders in patients on regular dialysis given desferrioxamine as a test. Nephrol Dial Transpl 5(11):945–949
Kertes PJ, Lee TK, Coupland SG (2004) The utility of multifocal electroretinography in monitoring drug toxicity: deferoxamine retinopathy. Can J Ophthalmol 39(6):656–661
Rudd C, Evans PJ, Peeney A (1953) Ocular complications in thalassaemia minor. Br J Ophthalmol 37(6):353
Angelucci E, Brittenham GM, Mclaren CE et al (2000) Hepatic iron concentration and total body iron stores in thalassemia major. N Engl J Med 343(5):327–331
Musallam KM, Cappellini MD, Taher AT (2013) Iron overload in β-thalassemia intermedia: an emerging concern. Curr Opin Hematol 20(3):187–192
Musallam KM, Cappellini MD, Wood JC, Taher AT (2012) Iron overload in non-transfusion-dependent thalassemia: a clinical perspective. Blood Rev 26:S16–S19
Tavazzi D, Duca L, Graziadei G, Comino A, Fiorelli G, Cappellini MD (2001) Membrane-bound iron contributes to oxidative damage of β-thalassaemia intermedia erythrocytes. Br J Haematol 112(1):48–50
Varano M, Scassa C (1998) Scanning laser ophthalmoscope microperimetry. Semin ophthalmol 13(4):203–209
Issa PC, Finger RP, Holz FG, Scholl HP (2009) Multimodal imaging including spectral domain OCT and confocal near infrared reflectance for characterization of outer retinal pathology in pseudoxanthoma elasticum. Invest Ophthalmol Vis Sci 50(12):5913–5918
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Human and animal rights
Not applicable.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Heydarian, S., Jafari, R., Dailami, K.N. et al. Ocular abnormalities in beta thalassemia patients: prevalence, impact, and management strategies. Int Ophthalmol 40, 511–527 (2020). https://doi.org/10.1007/s10792-019-01189-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-019-01189-3