Abstract
Both oral and intraocular routes have been recommended for medication administration in toxoplasmic retinochoroiditis; however, available data, in support or against, are scarce. The objective of this study was to compare the efficacy of intravitreal clindamycin plus dexamethasone (IVCD) and conventional oral therapy (COT) including pyrimethamine, sulfadiazine, folinic acid and prednisone in active toxoplasmic retinochoroiditis. In this prospective randomized single-blind clinical trial, patients with active toxoplasmic retinochoroiditis received either IVCD (n = 32), or COT (n = 34) for 6 weeks. Changes of best-corrected visual acuity, retinal lesion size, and vitreous inflammation before and after treatment, as well as complications/side-effects and recurrence rate within at least 2 years of follow-up were compared between groups. Although all the variables improved significantly at 6 weeks within each group, changes were comparable between the IVCD and COT receivers. There was only one case with hepatotoxicity in the COT group which responded favorably to drug change. No injection-related complication was observed. Recurrence rates were 12.5 and 14.7 % in the IVCD and COT groups, respectively (p = 0.54). In conclusion, both IVCD and COT are equally effective against active toxoplasmic retinochoroiditis but the former is apparently safer and more convenient.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Toxoplasmic retinochoroiditis (TRC) is the most common cause of posterior uveitis [1] and is regarded as the most frequent underlying etiology of posterior segment infection which may lead to unilateral vision loss. The infection manifests as a focal necrotizing retinitis usually associated with vitritis. Perivasculitis and vascular narrowing may be observed in the involved region. Presence of a chorioretinal scar adjacent to the lesion is a pathognomonic finding [2].
Small extramacular lesions can be followed up without any specific treatment [3]; however, when the lesion threatens the optic nerve, macula or main retinal vessels, or when a peripheral lesion is accompanied with severe vitritis, therapy is indicated [4].
Various systemic treatments have been proposed in patients with TRC, but the conventional one is a combination of pyrimethamine, sulfadiazine and folinic acid for 5–6 weeks [5]. To alleviate ocular inflammation, oral prednisone may be administered at a dosage of 30–50 mg/day for 2–3 weeks in combination with antibiotics [6].
Systemic medications, however, may not be efficacious enough to control the infection in all cases of TRC. In addition, they may be potentially accompanied by serious complications such as hepatotoxicity [7]. With the introduction of new combinations such as pyrimethamine and azithromycin the hazard of systemic side-effects is decreased but not completely resolved [8].
On the other hand, alternative routes have been proposed for administration of medications in TRC patients such as subconjunctival [9] or intravitreal injection of clindamycin plus dexamethasone [10].
Uncontrolled confounding factors such as Toxoplasma gondii infection (congenital or postnatally acquired) and location of lesions, as well as methodological shortcomings such as inappropriate blinding are the main limitations of a previous similar study [11].
Here we aimed to compare the efficacy of intravitreal clindamycin plus dexamethasone (IVCD) versus conventional oral therapy (COT) in patients with active TRC.
Materials and methods
Study design and participants
In this prospective randomized single-blind clinical trial, patients with active TRC received IVCD or COT after approval by the review board/ethics committee of Tabriz University of Medical Sciences. The patients were recruited from two referral teaching eye centers (Nikookari and Alavi) from January 2009 through to the end of July 2011.
After explanation of the study protocol and its probable safety and efficacy, an informed written consent was obtained from each participant.
TRC was diagnosed when there were clinical findings of retinal necrosis, retinitis or vitreitis in the absence of other identifiable causes, as well as positive serum titers of antibody (immunoglobulin [Ig] G or M) to T. gondii.
The clinical presentations were decreased visual acuity/blurred vision in all patients plus ocular flutter in 61 cases (92.4 %). In clinical examination there was a focus of retinitis along with edema, infiltration of inflammatory cells and vitreitis in the same location, without old scars in the primary cases and adjacent to old scars (n = 32, 48.5 %) in the remaining.
In equivocal cases (n = 2) appropriate tests were performed to exclude tuberculosis, sarcoidosis, collagen-vascular disease, and metastasis. Patients with previous therapies for TRC, history of allergic reaction to the drugs used in this study, or presence of other ocular diseases were not included.
Intervention and variables
At baseline, the best-corrected visual acuity (BCVA) was measured and fundus photography (nonmydriatic retinal camera TRC NW 200; Topcon, Tokyo, Japan) was performed. The BCVA was expressed in logarithm of the minimum angle of resolution (logMAR) scale.
The retinal lesion size was reported as ≤1 disc area (DA), <1 and ≤2 DA, and <2 and ≤3 DA [12]. ‘Improvement’ in retinal lesion size was considered when a lesion size changed from one group to a lower one.
Based on the location of the retinal lesion, the eyes were categorized as ‘juxtamacular’ or ‘juxtapapillary’. The degree of cell and flare in the anterior chamber and vitreous (inflammation) was determined on a previously described scale from 0 to 4 [13, 14]. The serum titers of antibody (IgG or IgM) to T. gondii were measured by enzyme-linked immunosorbent assay.
Patients were assigned randomly to one of these two treatment groups: (1) The IVCD group received an intravitreal injection of 1 mg clindamycin plus 400 μg dexamethasone. The injection was performed using a 30-gauge needle 3.5 mm from the limbus following topical anesthesia and anterior chamber paracentesis under sterile conditions in the operating room. All the patients were phakic at the time of injection. (2) The COT group received pyrimethamine (initial dose of 75 mg/day for 2 days followed by 25 mg/day for 6 weeks), sulfadiazine (initial dose of 2 g/day for 2 days followed by 1 g four times/day for 6 weeks), folinic acid (5 mg/day for 6 weeks), and oral prednisone (50 mg/day for 3 weeks starting from the third day of therapy).
Patients were re-examined weekly for up to 6 months after initiation of the treatments. The BCVA, grade of inflammation, and lesion size, as well as emergence of any systemic or ocular complication were investigated at each session. The BCVA was rated as ‘improved’ when two or more Snellen lines were gained. Inflammation was regarded as ‘resolved’ when a ‘grade 0’ or ‘trace’ in the anterior chamber was reported and the vitreous inflammation was resolved.
Randomization, group assignation and masking procedure
The participants were randomly allocated to two equal groups by a computer random number generation. Randomization numbers were kept in a sealed document by a care provider not involved in the survey until the end of the study. The patients were allocated to each group by the same person. The outcome variables were evaluated by a masked skilled retina specialist at baseline and at follow-ups.
Statistical analysis
With an assumption of a 25 % difference in lesion size reduction between groups, a standard deviation equal to 30 %, an alpha equal to 0.05, and a power of 90 %, 32 samples were required in each group. Hence, 35 patients (eyes) were included in each group. Analysis of data was performed with SPSS for Windows V 18.0 (SPSS Inc., IL, USA). Based on the results of the Shapiro–Wilk W test and the quantile–quantile plot (Q–Q plot), all quantitative data were distributed normally. Statistical methods included the Chi-squared test, Fisher’s exact test, McNemar test, Wilcoxon signed-rank test, paired or independent samples t tests, 2-way analysis of variance, and an ordinal regression model. p values ≤0.05 were considered as significant.
Results
Out of the initial 70 patients with TRC, four cases were lost-to-follow-up, leaving 32 patients (eyes) in the IVCD group and 34 patients (eyes) in the COT group.
General data including patient’s age (p = 0.19, independent samples t test) and gender (p = 0.64, Chi-squared test), location of retinal lesion (p = 0.22, Chi-squared test), positive serum IgG and IgM titers to T. gondii (p = 0.98, Chi-squared test), and follow-up (p = 0.29, independent samples t test) were comparable between the two groups (Table 1).
Baseline and 6-month BCVA, vitreous inflammation and retinal lesion size are outlined in Table 2.
The two groups were comparable in terms of baseline datasets (independent samples t test for BCVA, Chi-squared test for vitreous inflammation and retinal lesion size).
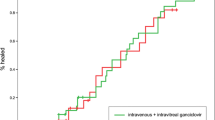
The mean BCVA improved significantly within each group (p < 0.001, paired test); whereas the mean reduction of BCVA was not significantly different between the IVCD and COT groups (0.38 ± 0.35 logMAR vs. 0.35 ± 0.29 logMAR, respectively; p = 0.31, independent samples t test). Improved visual acuity was documented in 27 cases in the IVCD group and in 28 cases in the COT group with no significant difference between the two (p = 0.83, Chi-squared test; see Fig. 1).
Vitreous inflammation was grade 0 or trace at 6 months in 20 cases in each group. The inflammation improved significantly within each group (p < 0.001, Wilcoxon signed-rank test) with no significant difference between the two (p = 0.43, ordinal regression test; see Table 2).
The retinal lesions improved significantly in terms of size after 6 months compared with baseline data within each group (p < 0.001, McNemar test), with comparable improvements between the two groups (p = 0.86, Chi-squared test, Table 2).
The mean BCVA change was comparable between the IgM-negative (0.35 ± 0.31 logMAR) and IgM-positive (0.39 ± 0.36 logMAR) cases in the IVCD group (p = 0.38, independent samples t test). A similar result was obtained between the two groups in the COT receivers (0.35 ± 0.27 logMAR vs. 0.34 ± 0.27 logMAR, respectively; p = 0.87, independent samples t test). These findings were also comparable between the corresponding subgroups among the treatment groups (p = 0.93 for the IgM-negative subgroup, p = 0.48 for the IgM-positive subgroup, independent samples t test). Based on a 2-way analysis of variance, the cross-product (interaction) effect of IgM and treatment group on BCVA improvement was not statistically significant (p = 0.41, see Table 3).
Comparing the rates of improvement for the retinal lesions size between the IgM-negative and IgM-positive cases in the IVCD group did not show a statistically significant difference (p = 0.53, Chi-squared test). In the COT group these values were also comparable between the IgM-negative and IgM-positive subgroups (p = 0.39, Chi-squared test). Between-group analysis did not disclose significant differences between the subgroups (p = 0.56 for the IgM-negative subgroup, Chi-squared test, p = 0.46 for the IgM-positive subgroup, Fisher’s exact test, see Table 3).
Based on amount of change, the BCVA improved significantly better in the cases with juxtapapillary retinal lesions than in the cases with juxtamacular retinal lesions both in the IVCD (0.35 ± 0.31 logMAR vs. 0.40 ± 0.29 logMAR; p = 0.02, independent samples t test) and COT (0.33 ± 0.26 logMAR vs. 0.38 ± 0.29 logMAR; p = 0.01, independent samples t test) groups. The corresponding subgroups were comparable in this regard between the two treatment groups (p = 0.78 for the juxtapapillary retinal lesion subgroup, p = 0.82 for the juxtamacular retinal lesion subgroups, independent samples t test). Based on a 2-way analysis of variance, the cross-product effect of retinal lesion location and treatment group on BCVA improvement was not statistically significant (p = 0.69, see Table 4).
Comparing the rate of improvement for retinal lesion size between the cases with juxtapapillary retinal lesions and the cases with juxtamacular retinal lesions cases in the IVCD group did not show a statistically significant difference (p = 0.89, Chi-squared test). In the COT group these values were also comparable between the cases with juxtapapillary retinal lesions and the cases with juxtamacular retinal lesions subgroups (p = 0.92, Chi-squared test). Between-group analysis did not reveal significant differences between the subgroups (p = 0.97 for the cases with juxtapapillary retinal lesions, p = 0.77 for the cases with juxtamacular retinal lesions, Chi-squared test, see Table 4).
One episode of recurrence was observed in four cases (12.5 %) in the IVCD group and in five cases (14.7 %) in the COT group without statistically significant difference (p = 0.54, Fisher’s exact test). They occurred at 2, 8, 11 and 13 months in the IVCD group and at 3, 11 (2 cases), 12 and 14 months in the CT group after the initial treatments. After retreatment with the same regimens the symptoms were resolved completely.
Overall, in the IVCD group 28 cases (87.5 %) received one injection and 4 cases (12.5 %) received two injections.
In the course of treatment, one case (2.9 %) was diagnosed with hepatotoxicity in the COT group. The condition resolved after changing pyrimethamine–sulfadiazine to azithromycin. No other major adverse drug reaction or injection-related complication was encountered during the period of follow-up.
Discussion
In the present clinical trial, the IVCD and COT were both almost equally effective in treatment of TRC in terms of lesion size reduction, improvement of visual acuity, resolution of vitreous inflammation and recurrence rate. Different systemic treatments have been suggested in these patients with variables outcomes, side-effects and complications [15].
Clindamycin, an antibiotic which interferes with protein synthesis in microorganisms, is an effective drug against T. gondii [16, 17] via intravenous, intravitreal, oral and subconjunctival routes. However, this drug penetrates poorly into cerebrospinal fluid (CSF) and vitreous [18]. This is also true for most of the current systemic antibiotics routinely used in TRC patients [19–21]. For example, concentration of pyrimethamine in CSF and intraocular fluids is only 10 % of its simultaneous concentration in serum [22, 23].
On the other hand, systemic use of these medications may potentially cause systemic complications, such as bone marrow suppression after pyrimethamine use; hemolytic or aplastic anemia, thrombocytopenia, icterus, nausea and akin rash associated with sulphonamides; gastrointestinal dysfunction, skin rash and photosensitivity due to minocycline use; and clindamycin-associated pseudomembranous colitis. These side-effects may lead to discontinuation of the medications [24, 25].
Although the subconjunctival route could be regarded as a surrogate method for delivering the drugs [9], in the case of clindamycin, reaching optimal levels in vitreous would not be expected because a significant portion of the administered drug is released into tear film through the tract which is created by the injecting needle and absorbed transcorneally into the anterior chamber [26]. Furthermore, there is a report of conjunctival necrosis following subconjunctival injection of clindamycin [27].
Accumulation of clindamycin in the retina and choroid following intramuscular and subconjunctival injections has been also observed in rabbits [28]; however, with intravitreal injection of the drug a more inhibitory effect on toxoplasma is achieved. The intravitreal concentration following 1 mg injection of the drug is ≥1.6 μg/ml for about 40 h which is higher than 50 % inhibitory concentration for T. gondii [29].
Potential risks of intravitreal injections include inadvertent retinal damage and retinal detachment, infectious endophthalmitis and damage to the posterior capsule of lens [30, 31]. However, these complications can be prevented by avoiding reattachment of a syringe with antibiotics to the original vitreous biopsy needle, following the guidelines for intravitreal injection of drugs and sterilization procedures [32].
There is evidence that immune mechanisms may play a role in pathogenesis of TRC-related tissue damage including reactivity of peripheral lymphocytes to retinal S-antigen (S-Ag) [33], anti-S-Ag antibodies [34], and anti-photoreceptor antibodies other than antibodies against S-Ag [35].
In a survey, it was demonstrated that 82 % of patients with TRC who had appropriate response to treatment had used systemic corticosteroids [5]. In a similar study, dexamethasone at the dosage of 1 mg was injected intravitreally for treatment of endophthalmitis [36] and because of concomitant injection of clindamycin the corticosteroid did not aggravate the disease [30].
The IVCD has been claimed to be safe [37] and effective in treatment of TRC [38]. We confirmed the effectiveness and safety of the IVCD in treatment of TRC by within-group analysis. Similar results were also attained in the COT group. This is in line with previous reports [7, 39].
In a very similar series by Soheilian et al. [11], the two groups of IVCD and COT receivers were comparable with regard to retinal lesion size, BCVA improvement, side-effects and recurrence rate. They concluded that intravitreal injection of clindamycin plus dexamethasone may be an acceptable alternative to the classic treatment in ocular toxoplasmosis because of higher convenience for patients, safer profile, greater availability, and fewer follow-up visits and hematologic evaluations. A unique finding of this study was that a better response in terms of lesion size reduction to COT was observed in IgM-positive cases and to intravitreal injection of clindamycin plus dexamethasone in IgM-negative patients.
Although the majority of our results are in conformity with those in the mentioned report, no significant difference was observed between the two groups in terms of retinal lesion size reduction and visual acuity stratified by the status of serum IgM. A small number of IgM-positive cases in Soheilian’s series (16.2 % of patients) was a serious limitation in this regard (this rate was 47 % in our study).
The retinal lesion location is an important factor in measuring the effect of treatment on visual acuity [40]. The improvement of visual acuity in our patients was significantly higher in the cases with juxtapapillary lesions in comparison with the eyes affected with juxtamacular lesions in both treatment groups with no significant difference between the two groups. This finding was expected due to the anatomic and physiologic importance of the macula than the blind spot. Intraocular clindamycin has not shown any retinal toxicity in different studies [41].
There were no significant intravitreal injection-related side-effects in the IVCD group; however, one case (2.9 %) was diagnosed with hepatotoxicity in the COT group. The exact rate of drug-related side-effects is not clear in patients receiving classic treatment, ranging between 3 and 64 % in different settings and with various doses of medications [11]. The recurrence rate was 12.5 % in the IVCD group versus 14.7 % in the COT receivers within a minimum of 24 months of follow-up. The rate of recurrence varies greatly in different studies, ranging from 5.9 to 25 %. The period of follow-up, host factors, and pathogenicity of the organism are main determinants in this regard [11].
In conclusion, the present study suggests that an intravitreal injection of clindamycin and dexamethasone is as effective as conventional oral therapy in the treatment of TRC with fewer systemic side-effects. Neither status of the serum IgM, nor the location of retinal lesion could affect the decision of choosing between IVCD and COT. Conducting further studies with larger sample sizes, with focus on immunocompromised patients, and in eyes with lesions inside the fovea is recommended. Comparing the results with other available and common regimens such as clindamycin plus Bactrim plus prednisone would be clinically valuable.
References
Henderly DE, Genstler AJ, Smith RE et al (1987) Changing patterns of uveitis. Am J Ophthalmol 103:131–136
Tabbara KF (1995) Ocular toxoplasmosis: toxoplasmic retinochoroiditis. Int Ophthalmol Clin 35:15–29
American Academy of Ophthalmology (2011) Focal points: clinical modules for ophthalmologists. American Academy of Ophthalmology, San Francisco
Nussenblatt RB, Whitcup SM, Palestine AG (1995) Uveitis: fundamentals in clinical practice. Mosby, St. Louis
Engstrom RE Jr, Holland GN, Nussenblatt RB et al (1991) Current practices in the management of ocular toxoplasmosis. Am J Ophthalmol 111:601–610
Wertheim MS, Smith JR, Cunningham ET et al (2004) Toxoplasmosis can test diagnostic skills. Rev Ophthalmol 11:140
Rothova A, Meenken C, Buitenhuis HJ et al (1993) Therapy for ocular toxoplasmosis. Am J Ophthalmol 115:517–523
Bosch-Driessen LH, Verbraak FD, Suttorp-Schulten MS et al (2002) A prospective, randomized trial of pyrimethamine and azithromycin vs pyrimethamine and sulfadiazine for the treatment of ocular toxoplasmosis. Am J Ophthalmol 134:34–40
Ferguson JG Jr (1981) Clindamycin therapy for toxoplasmosis. Ann Ophthalmol 13:95–100
Martinez CE, Zhang D, Conway MD et al (1998–1999) Successful management of ocular toxoplasmosis during pregnancy using combined intraocular clindamycin and dexamethasone with systemic sulfadiazine. Int Ophthalmol 22:85–88
Soheilian M, Ramezani A, Azimzadeh A et al (2011) Randomized trial of intravitreal clindamycin and dexamethasone versus pyrimethamine, sulfadiazine, and prednisolone in treatment of ocular toxoplasmosis. Ophthalmology 118:134–141
Dodds EM, Holland GN, Stanford MR et al (2008) Intraocular inflammation associated with ocular toxoplasmosis: relationships at initial examination. Am J Ophthalmol 146:856–865
Kanski JJ (2007) Clinical ophthalmology: a systematic approach. Butterworth–Heinemann, Oxford
Kimura SJ, Thygeson P, Hogan M (1959) Signs and symptoms of uveitis. II. Classification of the posterior manifestations of uveitis. Am J Ophthalmol 47:171–176
Lam S, Tessler HH (1993) Quadruple therapy for ocular toxoplasmosis. Can J Ophthalmol 28:58–61
Guldsten H (1983) Clindamycin and sulphonamides in the treatment of ocular toxoplasmosis. Acta Ophthalmol (Cph) 61:51–57
Tabbara KF, Nozik RA, O’Connor GR (1974) Clindamycin effects on experimental ocular toxoplasmosis in the rabbit. Arch Ophthalmol 92:244–247
Zimmerman TJ, Kooner KS, Sharir M, Fechtner RD (1997) Text book of ocular pharmacology. Lippincott Williams & Wilkins, Philadelphia
Norrby R (1978) A review of the penetration of antibiotics into CSF and its clinical significance. Scand J Infect Dis Suppl 14:296–309
Keusch GT, Present DH (1976) Summary of a workshop on clindamycin colitis. J Infect Dis 133:578–587
Reeves DS, Wilkinson PJ (1979) The pharmacokinetics of trimethoprim and trimethoprim/sulphonamide combinations, including penetration into body tissues. Infection 7:S330–S341
Weiss LM, Harris C, Berger M et al (1988) Pyrimethamine concentrations in serum and cerebrospinal fluid during treatment of acute Toxoplasma encephalitis in patients with AIDS. J Infect Dis 157:580–583
Kaufman HE (1961) The penetration of daraprim (pyrimethamine) into the monkey eye. Am J Ophthalmol 52:402–404
Katlama C, De Wit S, O’Doherty E et al (1996) Pyrimethamine–clindamycin vs. pyrimethamine–sulfadiazine as acute and long-term therapy for toxoplasmic encephalitis in patients with AIDS. Clin Infect Dis 22:268–275
Luft BJ, Hafner R, Korzun AH et al (1993) Toxoplasmic encephalitis in patients with the acquired immunodeficiency syndrome. N Engl J Med 329:995–1000
Wine NA, Gornall AG, Basu PK (1964) The ocular uptake of subconjunctivally injected C14 hydrocortisone. 1. Time and major route of penetration in a normal eye. Am J Ophthalmol 58:362–366
Benzina Z, Chaabouni S, Hentati N et al (2005) Recurrent toxoplasmic retinochoroiditis after clindamycin treatment. J Fr Ophtalmol 28:958–964
Tabbara KF, O’Connor GR (1975) Ocular tissue absorption of clindamycin phosphate. Arch Ophthalmol 93:1180–1185
Fichera ME, Bhopale MK, Roos DS (1995) In vitro assays elucidate peculiar kinetics of clindamycin action against Toxoplasma gondii. Antimicrob Agents Chemother 39:1530–1537
Peyman GA, Schulman JA (1994) Intravitreal surgery: principles and practice. Appleton & Lange, Norwalk
Peyman GA, Meffert SA, Chou F, Conway MD (2001) Vitreoretinal surgical techniques. Taylor & Francis, London
Lee PJ, Seal DV, Peyman GA (2004) Endophthalmitis: diagnosis and management. Informa Healthcare, London
Nussenblatt RB, Gery I, Ballintine EJ et al (1980) Cellular immune responsiveness of uveitis patients to retinal S-antigen. Am J Ophthalmol 89:173–179
Abrahams IW, Gregerson DS (1982) Longitudinal study of serum antibody responses to retinal antigens in acute ocular toxoplasmosis. Am J Ophthalmol 93:224–231
Whittle RM, Wallace GR, Whiston RA et al (1998) Human antiretinal antibodies in toxoplasma retinochoroiditis. Br J Ophthalmol 82:1017–1021
Kishore K, Conway MD, Peyman GA (2001) Intravitreal clindamycin and dexamethasone for toxoplasmic retinochoroiditis. Ophthalmic Surg Lasers 32:183–192
Pague JT, Peyman GA (1974) Intravitreal clindamycin phosphate in the treatment of vitreous infection. Ophthalmic Surg 5:34–39
Sobrin L, Kump LI, Foster CS (2007) Intravitreal clindamycin for toxoplasmic retinochoroiditis. Retina 27:952–957
Soheilian M, Sadoughi MM, Ghajarnia M et al (2005) Prospective randomized trial of trimethoprim/sulfamethoxazole versus pyrimethamine and sulfadiazine in the treatment of ocular toxoplasmosis. Ophthalmology 112:1876–1882
Lasave AF, Díaz-Llopis M, Muccioli C et al (2010) Intravitreal clindamycin and dexamethasone for zone 1 toxoplasmic retinochoroiditis at twenty-four months. Ophthalmology 117:1831–1838
Soheilian M, Rafati N, Peyman GA (2001) Prophylaxis of acute posttraumatic bacterial endophthalmitis with or without combined intraocular antibiotics: a prospective, double-masked randomized pilot study. Int Ophthalmol 24:323–330
Conflict of interest
The authors report no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baharivand, N., Mahdavifard, A. & Fouladi, R.F. Intravitreal clindamycin plus dexamethasone versus classic oral therapy in toxoplasmic retinochoroiditis: a prospective randomized clinical trial. Int Ophthalmol 33, 39–46 (2013). https://doi.org/10.1007/s10792-012-9634-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-012-9634-1