Abstract
Background
Targeted anti-IL-1β therapy may be a valuable option for the management of gouty arthritis. The present meta-analysis has evaluated the effect of canakinumab, an anti-IL-1β monoclonal antibody in gouty arthritis.
Methods
A standard meta-analysis protocol was developed and after performing a comprehensive literature search in MEDLINE, Cochrane, and International Clinical Trial Registry Platform (ICTRP), reviewers assessed eligibility and extracted data from three relevant articles. A random-effects model was used to estimate the pooled effect size as the mean difference in Visual Analouge Scale (VAS) score, serum hsCRP, serum Amyloid A, and risk ratio for global assessment between the groups. Quality assessment was done using the risk of bias assessment tool and summary of findings was prepared using standard Cochrane methodology with GradePro GDT.
Results
Treatment with canakinumab showed a mean reduction of VAS score by 14.59 mm [95% CI − 19.42 to − 9.77], serum hsCRP by 15.36 mg/L [95% CI 1.62–29.11], serum Amyloid A by 67.18 mg/L [95% CI 17.06–117.31], and improvement in patient global assessment (RR = 1.478; 95% CI 1.29–1.67) and physician global assessment (RR = 1.44; 95% CI 1.28–1.61). The probability that future studies may have a mean difference in VAS score less than zero has been calculated to be 27.3% using a cumulative distribution function (CDF) calculator.
Conclusion
This meta-analysis shows the beneficial effect of canakinumab over triamcinolone by reducing VAS score, serum hsCRP, serum amyloid A, and improvement in global assessments in acute gouty arthritis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gout is an autoinflammatory disease associated with increased blood levels of urate and subsequent deposition of monosodium urate crystals in and around joints (Martinon 2010). The burden of gout is substantial as it is one of the most common causes of inflammatory arthritis with an increasing prevalence rate in industrialised countries (Arromdee et al. 2002; Trifiro et al. 2013). Gout is also an independent risk factor for cardiac and all-cause morbidity and mortality (Clarson et al. 2015; Kleinman et al. 2007; Kuo et al. 2010; Scire et al. 2013). The symptoms and chronicity of the disease deeply impact patients' quality of life and workability. Monosodium urate crystals can trigger the release of pro-inflammatory cytokines, in particular, interleukin-1β (IL-1β) (Martinon et al. 2006; So 2008). Acute gout is traditionally treated with NSAIDs, corticosteroids, and colchicine. However, these patients have multiple comorbidities that limit the use of some conventional therapies (Khanna et al. 2014). Chronically, urate-lowering therapies (ULTs) reduce urate stores and, when combined with anti-inflammatory prophylaxis, reduce the risk of new flares (Harrold et al. 2009; Neogi 2011). The comorbidities associated with the anti-inflammatory agents used for the treatment such as hypertension, diabetes mellitus, renal insufficiency and cardiovascular disease may worsen the disease by frequent flares and persistent inflammation which ultimately contribute to the joint destruction and quality of life (Riedel et al. 2004). Current treatment is first based on lifestyle measures and then on a pharmacological approach (Khanna et al. 2012; Zhang et al. 2006).
As IL-1β is the key mediator in gouty arthritis inflammation, targeted anti-IL-1β therapy may be a valuable option for the management of gouty arthritis (Busso and So 2010). Canakinumab, a fully human anti-IL-1β monoclonal antibody with a long plasma half-life (3–4 weeks), has shown superior efficacy (more rapid and sustained pain relief and a significant reduction in risk of new flares) to triamcinolone acetonide (TA) in dose-ranging studies in patients with acute gouty arthritis (Alten et al. 2008; So et al. 2010).
Despite many treatment options available for management of acute gouty arthritis, incomplete pain relief and the chance of new flare has led to the exploration of alternative therapies. Canakinumab, a human anti-IL-1β monoclonal antibody, is being studied as one of the options for treatment of acute gouty arthritis. Although a few clinical trials have been conducted in this regard with results favouring canakinumab, a meta-analysis will help to quantify the magnitude of the effect of Canakinumab in patients of acute gouty arthritis (Schlesinger et al. 2012, 2011a, 2011b; So et al. 2010). Hence, the present meta-analysis has been planned to evaluate the effect of canakinumab on clinical and biochemical parameters in gouty arthritis.
Materials and methods
Development and registration of protocol
We developed and followed a standard meta-analysis protocol following preferred reporting items for systematic reviews and meta-analysis-protocol (PRISMA-P) 2015 guidelines (Moher et al. 2015) and registered the protocol in International Prospective Register of Ongoing Systematic Reviews (CRD42020160758). This meta-analysis has been conducted and reported conforming to the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement (Moher et al. 2009). The Cochrane handbook was used as a methodological reference (Moriya et al. 2011).
Literature search
A systematic literature search was performed independently by three review authors (M.J., A.T., and R.M.) using PubMed, Cochrane, and the WHO International Clinical Trials Registry Platform (ICTRP) databases for randomised clinical trials on the use of Canakinumab in acute gouty arthritis published till March 2020. Studies published in English only were included for the meta-analysis; however, the search strategy was not restricted by the date of publication. PICO scheme was followed for developing the inclusion criteria. Key elements that were used in our search are “P” (Arthritis, Gout, Gouty arthritis/Arthritides, Gouty/Gouty Arthritides), “I” (Canakinumab, immunoglobulin G1, anti-(human interleukin 1beta) (human clone ACZ885 heavy chain V region)/ilaris/ACZ-885/ACZ885), the “C” (Dexamethasone/Dexasone/Triamcinolone/Acetonide, Triamcinolone/Colchicine/NSAIDs/Anti-inflammatory Agents, Non-Steroidal NSAIDs/Non-Steroidal Anti-Inflammatory Agents/Analgesics, Anti-Inflammatory/Anti-Inflammatory Analgesics/Aspirin-Like Agents/Aspirin-Like Agents), and the “O” (Analog Scale, Visual/Analog Scales, Visual/Scale, Visual Analog/Scales, Visual Analog/Visual Analog Scales).
Study selection criteria
Types of studies
Randomised clinical trials that have visual analogue scale (VAS), serum hsCRP, and serum amylase A levels as outcome measures were included in this meta-analysis. Retrospective comparative studies, review articles, letter to the editor, foreword, comments, case series, case reports, and studies where it was impossible to retrieve or calculate data of interest were excluded from this meta-analysis.
Types of participants
Adult human subjects of both sexes, irrespective of age with a diagnosis of gouty arthritis, were included in the meta-analysis. In all the included studies, the following exclusion criteria were followed: use of other NSAIDs, systemic or intraarticular steroids, colchicine before screening, tumour necrosis factor inhibitor in the 24 h before the screening, diagnosis of rheumatoid, infectious or septic or other inflammatory arthritis, and a risk factor for tuberculosis.
Types of interventions
Experimental intervention: Canakinumab 150 mg subcutaneous injection with or without standard medical therapy was considered the intervention in this meta-analysis.
Control intervention: Triamcinolone acetonide 40 mg intramuscular injection was the control intervention in this meta-analysis.
Type of outcome measures
Primary: Change in visual analogue scale (VAS) after drug administration.
Secondary: Change in serum high sensitivity C-reactive protein (hsCRP), serum amyloid A (SAA) protein, and global assessment to treatment (both physician and patient global assessment to treatment) were compared between canakinumab and triamcinolone group.
Study selection and data collection
Selection of studies
The selection of relevant studies was done in a stepwise manner. First, three review authors (M.J., A.T., and R.M.) independently screened the titles, abstracts, and keywords of all references retrieved. Then, full text from all selected studies was obtained and assessed by the authors and those studies which met the inclusion criteria were included in the meta-analysis. Reasons for exclusion of any study were duly noted and any disagreement was resolved through discussion with clinical pharmacologist cum statistical advisor (A.M.).
Data extraction and management
For this update, three review authors (A.T., M.J., and R.M.) independently collected data and assessed the quality using guidelines published by the Cochrane Collaboration (Moriya et al. 2011). Any disagreement between the authors was resolved by consensus or in consultation with the clinical pharmacologist cum statistical advisor (A.M.). Extracted data included publication type and source; trial design including timing, follow-up, sequence generation and allocation concealment; participants including selection criteria; interventions including canakinumab used along with its dose; outcome measures including VAS score (0–100 mm), serum hsCRP (mg/L), SAA (mg/L), and physician and patient global assessment to treatment.
Data analysis
The effect of individual studies was pooled in the random-effect model for this meta-analysis using package “meta” in R software (Version 4.0) (Balduzzi et al. 2019; Schwarzer 2007).
Assessment of risk of bias in included studies
Three review authors (M.J., A.T., and R.M.) independently assessed the internal validity of eligible studies according to the Cochrane Collaboration’s risk of bias tool (Moriya et al. 2011), resolving any disagreement by discussion until consensus obtained. Authors described the risk of bias and judged it as high, low, or unclear in the domains like random sequence generation; allocation concealment; blinding of participants, personnel; blinding of outcome assessment; incomplete outcome data; selective reporting; and other sources of bias.
Measures of treatment effect
In this meta-analysis, the outcome measures of interest were VAS score (0–100 mm), serum hsCRP (mg/L), and SAA (mg/L) which are continuous variables and global assessment to treatment which is categorical. The standardised mean difference was calculated to estimate the effect size to assess the difference in outcome measures between experiment and control groups for continuous variables. The difference in odds ratio was used to assess the difference in physician and patient global assessment to treatment. Random effect model was used for overall between-group analyses. The outcome has been depicted as a point estimate with 95% confidence interval as well as prediction interval. The probability of the future studies showing an effect exactly opposite to that perceived in present meta-analysis has been calculated using the cumulative distribution function (CDF) calculator for the t-distribution (S, 2020).
Assessment of heterogeneity
Keeping in mind the fact that statistical heterogeneity is inevitable due to clinical and methodological diversity in clinical studies, it is important to consider the extent of the inconsistency or to quantify inconsistency across the included studies. The chi-squared test was used to assess whether observed differences in results were compatible with chance alone. A low P value (or a large chi-squared statistic relative to its degree of freedom) provided evidence of heterogeneity of intervention effects (variation in effect estimates beyond chance). I2 statistics which describes the percentage of the variability in effect an estimate that is due to heterogeneity was calculated for quantifying inconsistency.
Sensitivity analysis
Sensitivity analysis was undertaken to test the robustness of the results obtained in the present meta-analysis. In the case of high heterogeneity, the forest plots were obtained again after excluding individual studies, one at a time, and observing the effect of the exclusion of a particular study on individual parameters.
Assessment of publication bias
The publication bias across studies has been assessed using Begg and Mazumdar rank correlation test using the meta-essentials workbook (Suurmond et al. 2017).
Summary of findings
The certainty of the evidence was established following five grade considerations, viz., risk of bias, inconsistency, imprecision, indirectness, and publication bias in the methodology and outcomes of the included studies. Summary of findings was prepared using standard Cochrane methodology with GradePro GDT (2015).
Results
Description of included studies
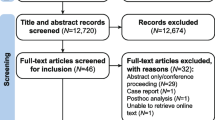
The literature search with appropriate search terms revealed a total of 32 publications. Twenty-six articles were excluded as 17 were review articles, 4 were case reports, 3 articles in non-English languages, one foreword, and one in-vitro study. The screening and selection process of the studies for present meta-analysis is depicted in PRISMA flowchart (Fig. 1). Once the full-text records of the remaining six publications were retrieved and screened, three articles were further excluded. Two of the excluded studies had different outcome measures than the ones intended to be included in the present study and one study was a duplicate of an already included study. Finally, three studies were included in the meta-analysis, which were published as full-text articles between 2010 and 2012 (Tables 1, 2). One of the included studies reported the outcomes of two different studies and was the results of two separate trials and was assessed as independent study units. Thus, a total of four randomised clinical trials have been reported in this meta-analysis. The study subjects, who were patients of acute gouty arthritis, were administered either canakinumab (test drug) or triamcinolone acetate (standard treatment) and the effects were compared. Risk of bias for all included studies is summarised in Table 3.
Effects of intervention
The effect of canakinumab as compared to triamcinolone acetate on VAS score, serum hsCRP, serum amyloid A levels and physician and patient global assessment to treatment in patients of acute gouty arthritis was evaluated in this meta-analysis. The effect sizes of included studies were compared in R, version 3.5 (Package meta), using a random-effect model (Balduzzi et al. 2019).
VAS score
This outcome was reported in all the four included studies. The test for heterogeneity was statistically significant (heterogeneity: χ2 = 39.56, df = 3 (P < 0.001); I2 = 92%; n = 622). The random model analysis of VAS score resulted in a mean reduction of VAS score by 14.59 mm [95% CI − 19.42 to − 9.77; prediction interval − 37.09 to 7.90] [Z = 5.93 (P < 0.001)] in the patients treated with canakinumab as compared to those treated with triamcinolone acetate (Fig. 2a). The significant reduction of VAS score as witnessed in the canakinumab group reflects the better efficacy of the drug in pain reduction in acute gouty arthritis as compared to triamcinolone acetate. The prediction interval in this forest plot includes the null effect as well as the opposite effect from the results obtained in this meta-analysis. The probability that future studies may have a mean difference in VAS score less than zero has been calculated to be 27.3% using the cumulative distribution function (CDF) calculator for the t-distribution.
a Forest plot of the included studies pooled together using a random-effects model for assessing the change in VAS score at 72 h. Randomised-controlled trials are indicated by the first author and year of publication. The size of each box is proportional to the weight of the corresponding study in the analysis, and the lines represent its 95% CI. Each open diamond represents the pooled mean difference, and its width represents the corresponding 95% CI. The straight line below diamond depicts the prediction interval. b The sensitivity analysis excluded the study of Schlesinger et al. (2011a, b)
Sensitivity analysis indicated that on removing the VAS score reported by Schlesinger et al. (2011a, b), the heterogeneity dropped considerably (heterogeneity: χ2 = 9.29, df = 2 (P = 0.01); I2 = 78%; n = 539). The mean reduction in VAS score of 12.66 mm [95% CI 8.39–16.93] [Z = 5.81 (P < 0.001)] (Fig. 2b) was still significantly lower in the canakinumab group as compared to the triamcinolone acetate group.
Serum hsCRP
This outcome was reported in all the included studies of the meta-analysis. The test of heterogeneity was statistically significant (heterogeneity: χ2 = 28.16, df = 3 (P < 0.00001); I2 = 89%; n = 622). Random model analysis of the variable depicted a reduction of 15.36 mg/L [95% CI 1.62–29.11; prediction interval: − 49.17 to 79.90] [Z = 2.19 (P = 0.03)] (Fig. 3a) in hsCRP levels in the canakinumab-treated patients as compared to the triamcinolone acetate-treated patients. This statistically significant lowering of hsCRP in the canakinumab-treated group depicts a larger lowering of CRP, a pro-inflammatory protein, therefore exerting an anti-inflammatory action in acute gouty arthritis.
a Forest plot of the included studies pooled together using a random-effects model for assessing the change in serum hsCRP levels. Randomised-controlled trials are indicated by the first author and year of publication. The size of each box is proportional to the weight of the corresponding study in the analysis, and the lines represent its 95% CI. Each open diamond represents the pooled mean difference, and its width represents the corresponding 95% CI. The straight line below diamond depicts the prediction interval. b The sensitivity analysis excluded the study of So et al. (2011)
Sensitivity analysis was performed and the result after removal of the study by So et al. indicated that the level of heterogeneity was reduced which was not statistically significant (heterogeneity: χ2 = 0.42, df = 2 (P = 0.81); I2 = 0%; n = 537). The mean reduction of hsCRP levels was 8.28 mg/L which was still statistically significant [95% CI 3.31–13.24] [Z = 3.27 (P = 0.001) (Fig. 3b).
Serum amyloid A (SAA)
SAA levels were reported in all the four randomised-controlled trials. The test for heterogeneity was significant (heterogeneity: χ2 = 18.99, df = 3 (P = 0.0003); I2 = 84%; n = 622). Random model analysis of their results revealed a mean decrease of 67.18 mg/L [95% CI 17.06–117.31; prediction interval: − 161.51 to 295.87] [Z = 2.63 (P = 0.008)] in the canakinumab group as compared to the triamcinolone acetate group (Fig. 4a). This indicated that there was a significantly better reduction in SAA, which also is a pro-inflammatory marker, in patients of acute gouty arthritis treated with canakinumab.
a Forest plot of the included studies pooled together using a random-effects model for assessing the change in serum Amyloid A levels. Randomised-controlled trials are indicated by the first author and year of publication. The size of each box is proportional to the weight of the corresponding study in the analysis, and the lines represent its 95% CI. Each open diamond represents the pooled mean difference, and its width represents the corresponding 95% CI. The straight line below diamond depicts the prediction interval. b The sensitivity analysis excluded the study of So et al. (2011)
Sensitivity analysis was performed for SAA levels and it was observed that after removal of the study by So et al., the heterogeneity reduced, although it was not statistically significant (heterogeneity: χ2 = 2.38, df = 2 (P = 0.3); I2 = 16%; n = 537). There was a mean decrease of 43.27 mg/L [95% CI 12.54–74.01] [Z = 2.76 (P = 0.006)] in SAA levels in the canakinumab group as compared to the triamcinolone acetate group that was still statistically significant (Fig. 4b).
Patient global assessment
This outcome, used to assess the overall severity of the disease as reported by the patient, was reported in all the four included studies. The test for heterogeneity was non-significant (heterogeneity: χ2 = 2.48, df = 3 (P = 0.48); I2 = 0%; n = 622). There was a statistically significant improvement in the severity score (RR = 1.478) [95% CI 1.29–1.67; prediction interval 1.11–1.95] [Z = 5.83 (P < 0.001)] in the canakinumab-treated group, thus suggesting a better improvement of symptoms (Fig. 5a).
a Forest plot of the included studies pooled together using a random-effects model for assessing the change in patient global assessment. Randomised-controlled trials are indicated by the first author and year of publication. The size of each box is proportional to the weight of the corresponding study in the analysis, and the lines represent its 95% CI. Each open diamond represents the pooled relative risk, and its width represents the corresponding 95% CI. The straight line below diamond depicts the prediction interval. b Forest plot of the included studies pooled together using a random-effects model for assessing the change in physician global assessment. Randomised-controlled trials are indicated by the first author and year of publication. The size of each box is proportional to the weight of the corresponding study in the analysis, and the lines represent its 95% CI. Each open diamond represents the pooled relative risk, and its width represents the corresponding 95% CI. The straight line below diamond depicts the prediction interval
Physician global assessment
This is a scoring system used to assess the disease severity as reported by the physician. Three of the four included randomised-controlled trials reported this outcome. The test for heterogeneity was non-significant (heterogeneity: χ2 = 0.62, df = 2 (P = 0.73); I2 = 0%; n = 539). The reduction in the severity of disease score was better in the canakinumab group as compared to the triamcinolone acetate group (RR = 1.44) [95% CI 1.28–1.61; prediction interval 0.67–3.08] [Z = 6.02 (P < 0.001)], thereby suggesting the better action of canakinumab in relieving the symptoms of acute gouty arthritis (Fig. 5b).
Potential biases in the review process
There were no obvious biases within the review process. We conducted extensive literature searches and included full-text publications. Two review authors independently identified and extracted data from the studies. The relevance of each paper was also carefully assessed. The assessment of publication bias using Begg and Mazumdar rank correlation test was performed using meta-essential workbook, which showed Kendall’s tau value of 0.67 with a two-tailed P value of 0.17 which is not significant (Suurmond et al. 2017).
Summary of findings
Overall summary for each outcome measure has been represented in the summary of finding table (Table 1). The certainty grade has been downgraded for VAS score and serum hsCRP levels from high grade to moderate grade due to the presence of potential biases, and thus, further studies are needed for canakinumab in gouty arthritis to derive any conclusion for these endpoints (Table 4).
Discussion
Anti-inflammatory agents form an important component in the management of gouty arthritis apart from urate-lowering therapy. Although the conventional anti-inflammatory treatment options like non-steroidal anti-inflammatory drugs (NSAIDs), colchicine, or corticosteroids like triamcinolone acetate produce rapid pain relief and resolution of flares (Jordan et al. 2007; Neogi 2011; Zhang et al. 2006), their use can be limited by comorbidities like hypertension, diabetes mellitus, renal sufficiency, and cardiovascular disease (Riedel et al. 2004) or intolerance and unresponsiveness to therapy (Schlesinger et al. 2012). Under these circumstances, canakinumab, a human anti-IL-1β monoclonal antibody seems a valuable opinion. Although there have been a few clinical trials that have measured the efficacy of canakinumab in patients of acute gouty arthritis (Schlesinger et al. 2012, 2011a; So et al. 2010), this meta-analysis would be the first attempt to summarise together and quantify the net effect of canakinumab over triamcinolone acetate (a conventional anti-inflammatory drug) in reducing pain and inflammation in these patients.
Visual analogue scale (VAS) showed a statistically significant decrease at 72 h in patients treated with canakinumab as compared to those that were treated with triamcinolone acetate. This result was similar to all the individual clinical trials that were included in the meta-analysis (Schlesinger et al. 2012, 2011a; So et al. 2010). Patient and physician global assessment of disease severity are crucial components of the measurement of overall disease activity and response to treatment (Challa et al. 2017; Pascoe et al. 2015). They are measured on a 5-point Likert scale. The current meta-analysis showed a significantly higher odds of achieving a good or excellent score in global assessment in patients treated with canakinumab as opposed to triamcinolone acetate. The improvement seen in patient global assessment at 72 h was similar to the result of all the four individual clinical trials that were part of the meta-analysis (Schlesinger et al. 2012, 2011a; So et al. 2010). On the other hand, physician global assessment to disease severity was reported only in three of the four studies included in the meta-analysis and the individual studies reported better assessment in the canakinumab group compared to the triamcinolone acetate group as indicated in the meta-analysis (Schlesinger et al. 2012; So et al. 2010). The rapid improvement in VAS and the global assessment scores at 72 h thus shows a rapid relief in pain symptoms in patients treated with canakinumab as compared to triamcinolone acetate.
Serum hsCRP and SAA are pro-inflammatory markers and their serum values give an objective assessment of the level of inflammation. The present meta-analysis reported a statistically significant decrease in the levels of the two markers at 72 h and this was consistent with the findings reported in the individual studies as well (Schlesinger et al. 2012; So et al. 2010). Since canakinumab is an IL-1β antibody, reduction in hsCRP and SAA levels indicates a role of IL-1β in production of these proteins (Berger et al. 2002). Suppression of IL-1β and associated inflammatory markers may slow joint destruction and, therefore, disease progression in patients of gouty arthritis, as well (Schlesinger and Thiele 2010).
Random effects model was used to justify the high heterogeneity that could be encountered in the assessment of various parameters. This model is based on the assumption that the various effects that have been used in the participating studies (mean difference in VAS, hsCRP, and SAA) of the meta-analysis are not identical and follow a particular distribution. This, therefore, provides a likely explanation for the high heterogeneity of the meta-analysis. Sensitivity analysis was conducted to address high heterogeneity (evidenced by high I2 value) in VAS, hsCRP, and SAA. There was a significant reduction in heterogeneity in hsCRP and SAA levels after excluding the study by So et al., whereas exclusion of the study by Schlesinger et al. (2011a, b) reduced the high heterogeneity in VAS.
The present meta-analysis had a few limitations like it did not analyse the results for long-term follow-up and compared the effect of canakinumab only against triamcinolone acetate and no other conventional anti-inflammatory agents due to paucity of data. Analysis of long-term effects on canakinumab would give an idea on the chronic use of the drug as gouty arthritis is a chronic condition and warrants long-term use of drugs. Similarly, comparison with the other anti-inflammatory agents would give a clear picture regarding the efficacy of canakinumab as compared to all the other conventional anti-inflammatory agents.
In conclusion, the present meta-analysis demonstrated the efficacy of canakinumab over triamcinolone acetate in reducing pain, as seen by a reduction in VAS and global assessment of disease severity at 72 h in patients of acute gouty arthritis. Canakinumab also proved to be an effective anti-inflammatory agent as it significantly reduced serum hsCRP and SAA levels thus implying its role in the reduction of disease severity. Therefore, the present meta-analysis reaffirms the role of canakinumab in treating patients of acute gouty arthritis that have contraindications or are intolerant or unresponsive to conventional pain relief modalities. However, prediction interval and cumulative distribution function project that effects opposite to that achieved in the present analysis are possible in future studies and the certainty of evidence table advocates need of a greater number of randomised-controlled trials to conclude the superiority of canakinumab over triamcinolone.
References
Alten R, Gram H, Joosten LA, van den Berg WB, Sieper J, Wassenberg S et al (2008) The human anti-IL-1 beta monoclonal antibody ACZ885 is effective in joint inflammation models in mice and in a proof-of-concept study in patients with rheumatoid arthritis. Arthritis Res Ther 10:R67
Arromdee E, Michet CJ, Crowson CS, O'Fallon WM, Gabriel SE (2002) Epidemiology of gout: is the incidence rising? J Rheumatol 29:2403–2406
Balduzzi S, Rucker G, Schwarzer G (2019) How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health 22:153–160
Berger P, McConnell JP, Nunn M, Kornman KS, Sorrell J, Stephenson K et al (2002) C-reactive protein levels are influenced by common IL-1 gene variations. Cytokine 17:171–174
Busso N, So A (2010) Mechanisms of inflammation in gout. Arthritis Res Ther 12:206
Chakraborty A, Van LM, Skerjanec A, Floch D, Klein UR, Krammer G, Sunkara G, Howard D (2013) Pharmacokinetic and pharmacodynamic properties of canakinumab in patients with gouty arthritis. J Clin Pharmacol 53(12):1240–1251
Challa DNV, Crowson CS, Davis JM 3rd (2017) The patient global assessment of disease activity in rheumatoid arthritis: identification of underlying latent factors. Rheumatol Ther 4:201–208
Clarson LE, Chandratre P, Hider SL, Belcher J, Heneghan C, Roddy E et al (2015) Increased cardiovascular mortality associated with gout: a systematic review and meta-analysis. Eur J Prev Cardiol 22:335–343
Cumulative Distribution Function (CDF) (2020) Calculator for the t-Distribution [Software]. [Online]. https://www.danielsoper.com/statcalc
GRADEpro GDT (2015) GRADEpro Guideline Development Tool [Software]. [Online]. https://gradepro.org
Harrold LR, Andrade SE, Briesacher BA, Raebel MA, Fouayzi H, Yood RA et al (2009) Adherence with urate-lowering therapies for the treatment of gout. Arthritis Res Ther 11:R46
Hirsch JD, Gnanasakthy A, Lale R, Choi K, Sarkin AJ (2014) Efficacy of Canakinumab vs. triamcinolone acetonide according to multiple gouty arthritis-related health outcomes measures. Int J Clini Pract 68 (12):1503–1507
Jordan KM, Cameron JS, Snaith M, Zhang W, Doherty M, Seckl J et al (2007) British Society for Rheumatology and British Health Professionals in Rheumatology guideline for the management of gout. Rheumatology (Oxford) 46:1372–1374
Khanna D, Khanna PP, Fitzgerald JD, Singh MK, Bae S, Neogi T et al (2012) 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken) 64:1447–1461
Khanna PP, Gladue HS, Singh MK, FitzGerald JD, Bae S, Prakash S et al (2014) Treatment of acute gout: a systematic review. Semin Arthritis Rheum 44:31–38
Kleinman NL, Brook RA, Patel PA, Melkonian AK, Brizee TJ, Smeeding JE et al (2007) The impact of gout on work absence and productivity. Value Health 10:231–237
Kuo CF, See LC, Luo SF, Ko YS, Lin YS, Hwang JS et al (2010) Gout: an independent risk factor for all-cause and cardiovascular mortality. Rheumatology (Oxford) 49:141–146
Martinon F (2010) Mechanisms of uric acid crystal-mediated autoinflammation. Immunol Rev 233:218–232
Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J (2006) Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440:237–241
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P et al (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 4:1
Moriya M, Slade N, Brdar B, Medverec Z, Tomic K, Jelakovic B et al (2011) TP53 mutational signature for aristolochic acid: an environmental carcinogen. Int J Cancer 129:1532–1536
Neogi T (2011) Clinical practice. Gout N Engl J Med 364:443–452
Pascoe VL, Enamandram M, Corey KC, Cheng CE, Javorsky EJ, Sung SM et al (2015) Using the Physician Global Assessment in a clinical setting to measure and track patient outcomes. JAMA Dermatol 151:375–381
Riedel AA, Nelson M, Wallace K, Joseph-Ridge N, Cleary M, Fam AG (2004) Prevalence of comorbid conditions and prescription medication use among patients with gout and hyperuricemia in a managed care setting. J Clin Rheumatol 10:308–314
Schlesinger N, Thiele RG (2010) The pathogenesis of bone erosions in gouty arthritis. Ann Rheum Dis 69:1907–1912
Schlesinger N, De Meulemeester M, Pikhlak A, Yucel AE, Richard D, Murphy V et al (2011a) Canakinumab relieves symptoms of acute flares and improves health-related quality of life in patients with difficult-to-treat Gouty Arthritis by suppressing inflammation: results of a randomized, dose-ranging study. Arthritis Res Ther 13:R53
Schlesinger N, Mysler E, Lin HY, De Meulemeester M, Rovensky J, Arulmani U et al (2011b) Canakinumab reduces the risk of acute gouty arthritis flares during initiation of allopurinol treatment: results of a double-blind, randomised study. Ann Rheum Dis 70:1264–1271
Schlesinger N, Alten RE, Bardin T, Schumacher HR, Bloch M, Gimona A et al (2012) Canakinumab for acute gouty arthritis in patients with limited treatment options: results from two randomised, multicentre, active-controlled, double-blind trials and their initial extensions. Ann Rheum Dis 71:1839–1848
Schwarzer G (2007) meta: an R Package for meta-analysis, vol 7
Scire CA, Manara M, Cimmino MA, Govoni M, Salaffi F, Punzi L et al (2013) Gout impacts on function and health-related quality of life beyond associated risk factors and medical conditions: results from the KING observational study of the Italian Society for Rheumatology (SIR). Arthritis Res Ther 15:R101
So A (2008) Developments in the scientific and clinical understanding of gout. Arthritis Res Ther 10:221
So A, De Meulemeester M, Pikhlak A, Yucel AE, Richard D, Murphy V et al (2010) Canakinumab for the treatment of acute flares in difficult-to-treat gouty arthritis: results of a multicenter, phase II, dose-ranging study. Arthritis Rheum 62:3064–3076
Suurmond R, van Rhee H, Hak T (2017) Introduction, comparison, and validation of meta-essentials: a free and simple tool for meta-analysis. Res Synth Methods 8:537–553
Trifiro G, Morabito P, Cavagna L, Ferrajolo C, Pecchioli S, Simonetti M et al (2013) Epidemiology of gout and hyperuricaemia in Italy during the years 2005–2009: a nationwide population-based study. Ann Rheum Dis 72:694–700
Zhang W, Doherty M, Bardin T, Pascual E, Barskova V, Conaghan P et al (2006) EULAR evidence based recommendations for gout. Part II: management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis 65:1312–1324
Author information
Authors and Affiliations
Contributions
MJ concept and design, literature search, study selection and data collection, data extraction and management, data analysis and interpretation of data, critical revision of the manuscript for important intellectual content, and final approval of the manuscript. AT literature search, study selection and data collection, data extraction and management, and final approval of the manuscript. AM concept and design, data analysis and interpretation, drafting of the manuscript, and final approval of the manuscript. RM literature search, study selection and data collection, data extraction, critical revision of the manuscript for important intellectual content, and final approval of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
None to declare.
Consent for publication
Authors are responsible for the correctness of the statements provided in the manuscript.
Availability of data and material
All data generated or analysed during this study are included in this published article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jena, M., Tripathy, A., Mishra, A. et al. Effect of canakinumab on clinical and biochemical parameters in acute gouty arthritis: a meta-analysis. Inflammopharmacol 29, 35–47 (2021). https://doi.org/10.1007/s10787-020-00753-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-020-00753-z