Abstract
Aims
To explore the effect of intravenous tranexamic acid (IV-TXA) on inflammation and immune response following primary total knee arthroplasty (TKA).
Methods
Primary TKA patients (n = 125) were randomized into the following four groups: group A to receive placebo; group B to receive a single dose of 20 mg kg−1 IV-TXA and 20 mg of intravenous dexamethasone (IV-DXM); group C to receive six doses of IV-TXA (total dosage > 6 g); and group D to receive six doses of IV-TXA combined with three doses of IV-DXM (total dosage = 40 mg). The primary outcomes were C-reactive protein (CRP) and interleukin (IL)-6 levels and the secondary outcomes were complement C3 and C4 and T-cell subset levels, which were measured preoperatively and at 24 h, 48 h, 72 h, and 2 weeks postoperatively.
Results
The postoperative peak CRP and IL-6 levels in group C (93.7 ± 22.2 mg L−1, 108.8 ± 41.7 pg mL−1) were lower compared with those in group A (134.7 ± 28.8 mg L−1, P < 0.01; 161.6 ± 64.4 pg mL−1, P < 0.01). Groups B and D exhibited significantly lower CRP and IL-6 levels compared with groups A and C at 24 h, 48 h, and 72 h postoperatively (P < 0.05 for all). In group C, complement C3 and C4 levels were higher compared with those in group A at 48 h (0.967 ± 0.127 g L−1 vs. 0.792 ± 0.100 g L−1, P < 0.01; 0.221 ± 0.046 g L−1 vs. 0.167 ± 0.028 g L−1, P < 0.01) and 72 h (1.050 ± 0.181 g L−1 vs. 0.860 ± 0.126 g L−1, P = 0.01; 0.240 ± 0.052 g L−1 vs. 0.182 ± 0.036 g L−1, P < 0.01) postoperatively and CD3 and CD4 subset levels were higher compared with those in group B at 24 h postoperatively (66.78 ± 9.29% vs. 56.10 ± 12.47%, P < 0.05; 36.69 ± 5.78% vs. 28.39 ± 8.89%, P < 0.05).
Conclusion
Six doses of IV-TXA could attenuate the inflammatory effect, modulate the immune response, and reduce immunosuppression caused by DXM in patients after TKA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Total knee arthroplasty (TKA) is a safe, effective treatment option that can relieve pain and improve knee function for patients with end-stage knee joint diseases (Kehlet 2013). In patients undergoing TKA, the coagulation cascade and inflammatory response were activated following tissue injury, but consequent uncontrolled bleeding and infection still remain significant risk factors (Levi et al. 2004). To reduce bleeding, the most commonly used antifibrinolytic agent is tranexamic acid (TXA) (Zhang et al. 2019). As an analog of the amino acid lysine, TXA exerts its antifibrinolytic effect through competitively inhibiting plasminogen activation and plasmin binding to fibrin (McCormack 2012). Plasmin is the effector protease of the fibrinolytic system and it has also been well characterized as a potent modulator of inflammation and immunity (Draxler et al. 2017). Plasmin has been reported to act in a proinflammatory manner by triggering chemotaxis (Li et al. 2010) and cytokine release (Syrovets et al. 2001). Plasmin may also induce an immunosuppressive state in dendritic cells and reduce their ability to mount an allogeneic immune response (Borg et al. 2015). TXA may also have inflammatory and immune modulatory effects because it inhibits plasmin (Draxler et al. 2017). Although the impact of TXA on the coagulation system has been studied extensively, the effects on inflammation and immune system activation remain unclear. A better understanding of the effects of TXA on inflammation and immune system activation helps us to use it reasonably and effectively.
Although several in vivo studies on TXA’s anti-inflammatory properties have been published (Xie et al. 2016; Zeng et al. 2018), few placebo-controlled comparisons have been reported. The aim of this study was to explore the possibility that the plasmin-mediated high inflammation status and immunosuppression in patients undergoing TKA can be reversed by intravenous TXA (IV-TXA). We, therefore, investigated pre- and postoperative inflammatory makers and immune indices in patients who were selected from a randomized controlled trial. These results from patients who received combined intravenous dexamethasone (IV-DXM) were also compared to quantify the effects.
Materials and methods
Study design
After approval was obtained from the institutional review board at West China Hospital of Sichuan University (2012-268), this prospective, placebo-controlled, double-blind randomized clinical trial was registered in the Chinese Clinical Trial Registry (ChiCTR-INR-17011500). This trial was performed in accordance with the provisions of the Declaration of Helsinki, as revised in 2013 (World 2013). The protocol was drafted and the study was conducted and reported in accordance with the Consolidated Standards of Reporting Trials (CONSORT) statement (Bian and Shang 2011). Before inclusion and randomization, written informed consent and research authorizations were obtained from all participants. No changes were made to the trial design during the study.
Study population
From May 2017 to April 2018, consecutive adult patients who were scheduled to receive a primary unilateral TKA for end-stage osteoarthritis at our hospital were screened for recruitment into this study. Patients were excluded if they had flexion deformity ≥ 30°, varus/valgus deformity ≥ 30°, preoperative anemia (haemoglobin [Hb] < 12 g dL−1 for women, < 13 g dL−1 for men) (World 2001), preoperative C-reactive protein (CRP) in serum > 10 mg L−1, known allergic reactions to TXA or DXM, preoperative abnormal immune function or combined with immune-related diseases, preoperative hepatic or renal dysfunction, cardiac or cerebrovascular problems, previous history of deep venous thrombosis or pulmonary embolism, congenital or acquired clotting disorders, known inflammatory disease, or preoperative use of corticosteroids.
Treatment regimens, blinding, and randomization
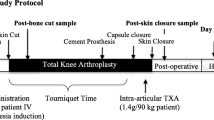
The recruited patients were randomized into one of four groups using a computer-generated randomization list (Table 1). Group A was set as the placebo control group and these patients received no TXA or DXM. Group B received a single dose of 20 mg kg−1 of IV-TXA (100 ml: 1 g; Chongqing Lummy Pharmaceutical Co., Ltd., Chongqing, China) 5–10 min before the skin incision. Additionally, a single dose of 20 mg of IV-DXM (1 ml: 5 mg, Tianjin Kingyork group, Co., Ltd., Tianjin, China) was injected just after general anesthesia was administered. Group C received a dose of 20 mg kg−1 of IV-TXA 5–10 min before the skin incision and 1 g of IV-TXA was administered 3, 7, 12, 18, and 24 h later, without using DXM. Group D received a dose of 20 mg kg−1 of IV-TXA 5–10 min before the skin incision and 1 g of IV-TXA was administered 3, 7, 12, 18, and 24 h later. Additionally, a dose of 20 mg of IV-DXM was injected just after the general anesthesia was administered and 10 mg of IV-DXM was administered 24 and 48 h after the first dose.
The preoperative doses were administered by anesthetists and the postoperative protocol was performed by nurses. A random allocation sequence concealed in opaque sealed envelopes was opened just before surgery. The anesthetists and nurses were not involved in this trial. The patients, surgeons, data controller, and analyst were blinded to allocation until the final data analysis.
Surgery and postoperative protocol
As the previous study reported (Xie et al. 2016), all surgical procedures were performed by one senior surgeon and a midline skin incision using the medial parapatellar approach was performed in all cases. All TKA procedures were conducted using a cemented posterior-stabilized prosthetic design, with no tourniquet or vacuum wound drainage. General anesthesia was selected by the anesthetists and blood pressure was controlled within 90–110 mmHg/60–70 mmHg (systolic/diastolic blood pressure) throughout the procedure. The intraoperative intravenous crystalloid fluid volume was controlled within 400–600 mL.
All patients were managed in accordance with the same perioperative protocols (Xie et al. 2016). A combination of mechanical and chemical prophylaxis was adopted to prevent venous thromboembolism. All patients were offered the usual standardized anesthesia, which consisted of intraoperative periarticular injection with ropivacaine (0.2%) and oral administration of enteric-coated diclofenac sodium (50 mg twice daily; Novartis, Basel, Switzerland) for postoperative pain management. The criterion of blood transfusion was set at an Hb level of < 7 g dL−1 or 7–10 g dL−1 with symptomatic anemia, in accordance with the National Ministry of Health guidelines (Zhang et al. 2018).
Study parameters
Patient demographics and preoperative characteristics were obtained before TKA. The primary outcomes were inflammatory markers including CRP and interleukin (IL)-6, which were measured preoperatively and at 24 h, 48 h, 72 h, and 2 weeks postoperatively. CRP levels were measured using standard commercial rate nephelometry kits (high-sensitivity C-reactive protein reagent, Beckman Coulter, Inc., CA, USA), which were analyzed using IMMAGE immunochemical system software (Beckman IMMAGE 800, Beckman Coulter, Inc., CA, USA) and the normal range was < 5 mg L−1. Each estimation of IL-6 was performed using standard electrochemiluminescence immunoassay kits (specific mAb R1 regent, Roche Group, Basel, Switzerland) and analyzed on a validated Modular Analytics platform (Roche E170, Roche Group, Basel, Switzerland). The normal range of IL-6 was 0.00–7.00 pg mL−1.
The secondary outcomes were immune indices including complement C3 and C4 and T-cell subsets, which were measured preoperatively and at 24 h, 48 h, 72 h, and 2 weeks postoperatively. Complement C3 (normal range 0.785–1.520 g L−1) and C4 (normal range 0.145–0.360 g L−1) were measured using standard rate nephelometry kits, as described for CRP. T-cell subsets were assessed using standard flow cytometry analysis kits (BD Biosciences, San Jose, CA, USA) by BD FACS Canto™ II flow cytometers, including the percentage of CD3 subsets (normal range 66.9–83.1%), the percentage of CD4 subsets (normal range 33.19–47.85%), and the percentage of CD8 subsets (normal range 20.4–34.7%). All measurements were made in strict accordance with the instructions and performed at the Department of Laboratory Medicine, certified by Clinical American Pathology.
To monitor for adverse events, patients were followed for a period of 1 year. During follow-up, patients were assessed for the incidence of thromboembolic events, wound complications, hospital readmission, and mortality. Thromboembolic events were defined as deep vein thrombosis (DVT), symptomatic pulmonary embolism (PE), myocardial infarction (MI), or stroke. Doppler ultrasound was used routinely to detect DVT at the time of discharge, and at the 3-month and 1-year follow-ups, or when DVT was suspected. PE was diagnosed using clinical symptoms and an enhanced chest computed tomography scan. MI was diagnosed using an electrocardiogram and cardiac enzymes and stroke was confirmed using brain computed tomography or magnetic resonance imaging. Wound complications were defined as swelling, exudate, or deep or superficial infection. No changes were made to the outcomes during the study.
Statistical analysis
Sample size calculations were performed using PASS 2011 (NCSS, LLC, Kaysville, Utah, USA) software with a one-way analysis of variance (ANOVA) design for the randomized controlled trial. Based on the results of the previous study (Xie et al. 2016), the IL-6 levels were 145.5 ± 33.4 pg mL−1 on postoperative day 1 after TKA, with a single dose of IV-TXA (20 mg kg−1) that was administered just before the skin incision. We assumed a difference of 30 pg mL−1 for IL-6 levels on postoperative day 1, a power of 0.90, a significance level of 0.05, and a 20% exclusion rate, and it was determined that a minimum of 28 patients per arm were required.
All the data analyses were performed using SPSS version 24.0 (IBM Corp, Armonk, NY, USA). The Pearson Chi-square test and Fisher’s exact test were performed to analyze the qualitative variables. One-way ANOVA and Tukey’s post hoc test were performed to analyze the parametric samples, while the Kruskal–Wallis H test and Mann–Whitney U test were used for non-parametric data. A p value less than 0.05 was considered to be statistically significant.
Results
Patient demographic characteristics
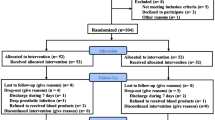
One hundred and sixty patients were assessed for eligibility. Among them, 32 were excluded for the following reasons: 30 were ineligible and 2 declined to participate. The remaining 128 patients were included and randomized equally into four groups. 3 patients in groups A, B, and D did not receive the allocated intervention. Thus, 125 patients (105 women, 20 men) were included in the final analysis (Fig. 1), with 31 patients in group A, 31 patients in group B, 32 patients in group C, and 31 patients in group D. There were no statistical differences among the four groups in terms of baseline demographic data and preoperative clinical characteristics (Table 2).
Inflammatory markers
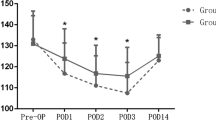
According to the inflammatory marker analysis, CRP levels increased gradually after surgery and peaked at 48 h or 72 h postoperatively (Fig. 2a). The CRP levels in group C were lower compared with group A at 48 h and 72 h postoperatively (P < 0.05). Groups B and D exhibited significantly lower CRP levels compared with groups A and C at 24 h, 48 h, and 72 h postoperatively (P < 0.05). IL-6 levels increased rapidly after surgery and peaked at 24 h postoperatively (Fig. 2b). The IL-6 levels in group C were lower compared with group A at 24 h, 48 h, and 72 h postoperatively (P < 0.05). Groups B and D exhibited significantly lower IL-6 levels compared with groups A and C at 24 h, 48 h, and 72 h postoperatively (P < 0.05). Moreover, group D exhibited lower IL-6 levels compared with group B at 48 h and 72 h postoperatively (P < 0.05). CRP and IL-6 levels gradually decreased to their lowest points at 2 weeks postoperatively and no difference was detected among the four groups (P > 0.05; see details in Table 4in Appendix).
Perioperative inflammatory marker levels of CRP (A) and IL-6 (B) in serum. The error bars indicate standard deviation. # indicates a significant difference (P < 0.05) between Groups A and B, * indicates a significant difference (P < 0.05) between Groups A and C, § indicates a significant difference (P < 0.05) between Groups A and D, ## indicates a significant difference (P < 0.05) between Groups B and C, ** indicates a significant difference (P < 0.05) between Groups B and D, and §§ indicates a significant difference (P < 0.05) between Groups C and D. CRP, C-reactive protein; IL-6, interleukin 6; Pre preoperative, H hours, W weeks
Immune indices
In group A, complement C3 and C4 levels dropped to the nadir value at 24 h or 48 h postoperatively (Fig. 3a, b). Complement C3 levels in group C were higher compared with group A at 48 h and 72 h postoperatively (P < 0.05). Groups C and D exhibited significantly higher complement C4 levels compared with group A at 48 h and 72 h postoperatively (P < 0.05; see details in Table 5 in Appendix).
Perioperative immune indices in serum: complement C3 (Fig. 3a), complement C4 (Fig. 3b), CD3 subsets (Fig. 3c), CD4 subsets (Fig. 3d), and CD8 subsets (Fig. 3e). The error bars indicate standard deviation. # indicates a significant difference (P < 0.05) between Groups A and B, * indicates a significant difference (P < 0.05) between Groups A and C, § indicates a significant difference (P < 0.05) between Groups A and D, and ## indicates a significant difference (P < 0.05) between Groups B and C. Pre preoperative, H hours, W weeks
CD3 and CD4 subsets dropped to the nadir value at 24 h postoperatively in group A (Fig. 3c,d), while there were no significant change in CD8 subsets after surgery (Fig. 3e). The CD3 subset levels in group C were higher compared with group B at 24 h postoperatively (P < 0.05). Groups A and C exhibited significantly higher CD4 subset levels compared with group B at 24 h postoperatively (P < 0.05; see details in Table 6 in Appendix).
Adverse events
No differences in adverse outcomes including thromboembolic events, wound complications, hospital readmissions, and mortality were found between groups during the 1-year follow-up period (Table 3). There were two DVTs (6.5%) in group A and one DVT (3.2%) in group B, and no episodes of PE, MI, or stroke. One patient in group C had a superficial wound infection and was readmitted to the hospital to receive the appropriate treatment. No episodes of deep wound infection or death occurred in the study groups.
Discussion
TXA is an anti-fibrinolytic agent that blocks plasmin formation. Because plasmin is known to promote inflammatory and immunosuppressive responses, we explored the possibility that plasmin-mediated high inflammation status and immunosuppression in patients undergoing TKA can be reversed by TXA. To investigate the inflammatory and immune modulatory effects of IV-TXA compared with placebo, six doses of IV-TXA (total dosage > 6 g) were administered to patients who underwent primary unilateral TKA without a tourniquet from the beginning of the procedure and through the following 24 h, based on the duration of postoperative fibrinolysis and IV-TXA pharmacokinetics (Andersson et al. 1978; Blanie et al. 2013). To quantify the effects, we compared these results in patients who received combined IV-DXM. We found that six doses of IV-TXA could attenuate the acute inflammatory reaction after TKA compared with placebo. However, this anti-inflammatory effect was weaker compared with IV-DXM. Additionally, six doses of IV-TXA may modulate the immune response by improving complement levels postoperatively and alleviating the immunosuppressive action of IV-DXM on T-lymphocytes.
Several studies have reported that TXA administration could achieve a remarkable decrease of blood loss in patients undergoing TKA, which was attributed to effective inhibition of postoperative fibrinolysis (Wang et al. 2018; Xie et al. 2016; Zhang et al. 2019). However, few studies have focused on its anti-inflammatory effects. Xie et al. conducted a randomized controlled trial in which 151 patients undergoing primary TKA were recruited to receive a single bolus of IV-TXA (20 mg kg−1), and either a dosage of 20 mg kg−1 and one additional dose of 10 mg kg−1 of IV-TXA, or a dosage of 20 mg kg−1 and two additional doses of 10 mg kg−1 of IV-TXA. The additional bolus of IV-TXA resulted in lower serum CRP and IL-6 levels. However, there was no placebo-controlled comparison (Xie et al. 2016). Moreover, in a similar prospective study that evaluated the anti-inflammatory effect of multiple oral TXA doses, Wang et al. demonstrated a significant reduction in CRP and IL-6 levels with multi-dose regimens compared with placebo (Wang et al. 2018). However, the administration of corticosteroids and their potential mechanism were not investigated in the study. Unlike Wang’s study (Wang et al. 2018), we used a regimen of six doses of IV-TXA because the intravenous route is the most widely used route of administration and it is more controllable. In our study, the CRP level at 48 h and 72 h postoperatively and the IL-6 level at 24 h, 48 h, and 72 h postoperatively were lower after administering six doses of IV-TXA compared with placebo, which showed that IV-TXA can also attenuate the acute inflammatory reaction after TKA.
The possible anti-inflammatory mechanisms of IV-TXA could be explained by the following research evidence. First, plasmin is a potent proinflammatory activator of monocytes that stimulates nuclear factor-κB (NF-κB) and AP-1, resulting in the production of proinflammatory cytokines such as tumor necrosis factor-α (TNF-α), IL-1α, IL-1β, and tissue factor (Syrovets et al. 2001). However, the recent in vivo study by Teng et al. demonstrated that TXA has anti-inflammatory effects by suppressing the NF-κB pathway signaling, which was similar to p38 MAPK inhibitors (Teng et al. 2018; Carter et al. 2019). A previous study investigated the inflammatory properties of plasmin-mediated C5a generation and they found that TXA treatment may have inhibited plasmin generation of C5a (Wu et al. 2017). In addition to inhibiting direct plasmin-mediated inflammation, TXA might also promote anti-inflammatory effects by preventing fibrinogen, fibrin, and the generation of fibrin degradation products, which are known to initiate inflammation (Schuliga 2015; Szaba and Smiley 2002). In our previous study, we have showed that administering six doses of IV-TXA could effectively reduce fibrin degradation product and D-dimer levels at 24 h, 48 h, and 72 h postoperatively (Zhang et al. 2019), which was consistent with the reduction of CRP and IL-6 levels in this study. Additionally, Later et al. found that the use of TXA may alter inflammatory pathways at the genomic expression level. In their study, TXA altered gene expression of three genes compared with placebo. This finding was interesting and provided new ideas for us to explain the possible mechanism (Later et al. 2013).
Although TXA is known to have anti-inflammatory effects, its clinical value is still unclear and it has not been accurately assessed. Therefore, we compared the anti-inflammatory effects of TXA with those of a known inflammatory modulating therapy, intravenous DXM administration, and the effects seem remarkable. In the current study, a single dose of IV-DXM in group B and three doses of IV-DXM in group D were combined with IV-TXA. We found that five additional doses of IV-TXA could reduce the peak of CRP and IL-6 levels to more than one-third of a single dose of IV-DXM and the additional doses of IV-DXM achieved continuous reduction of the IL-6 levels at 48 h and 72 h postoperatively. To the best of our knowledge, no study exists that compared the anti-inflammatory effects of TXA with a placebo-treated patient group and patients receiving DXM in TKA patients. DXM potentiated a much wider anti-inflammatory effect through inhibition of several key pro-inflammatory mediators and stimulation of anti-inflammatory mediators (Xu et al. 2018a).
Because of the transient immunosuppressive status after TKA (Slade et al. 1975), and because DXM may aggravate the immunosuppressive status, we monitored the changes in the T-cell subsets as cellular immune indicators after TKA. Unsurprisingly, we found that the percentages of CD3 and CD4 subsets decreased to the minimum at 24 h postoperatively and the percentages of CD4 subsets when DXM was administered in group B were significantly lower compared with placebo in group A. However, the percentages of the CD3 and CD4 subsets improved with the administration of multiple doses of IV-TXA in group C compared with those in group B, which suggested that the immune response suppressed by DXM can be improved by TXA. A possible explanation could be that TXA alleviated the effects that were mediated by plasmin, which promotes the immunosuppressive response (Draxler et al. 2017). Boudreau et al. found that traumatic brain injury (TBI) mice treated with TXA demonstrated increased naive CD4 + cell counts compared with TBI saline controls (Boudreau et al. 2017), which was consistent with its immune-enhancing effects. Another explanation could be that TXA reduced the loss of immune cells while reducing blood loss, but the detailed mechanism requires further study.
The complement system plays an important role in humoral immunity, but it can be influenced by coagulation factors such as plasmin. Various studies demonstrated that plasmin is a complement-activating enzyme that mediates generation of the chemotactic agents C3a (Amara et al. 2010) and C5a (Amara et al. 2010; Foley et al. 2016), while others demonstrated that plasmin is a complement inhibitor (Barthel et al. 2012). In the current study, complement C3 and C4 were measured preoperatively and at 24 h, 48 h, 72 h, and 2 weeks postoperatively. The results showed that complement C3 and C4 levels were higher with administration of multiple IV-TXA doses in group C compared with placebo in group A. This suggests that TXA may have a potential role in modulating humoral immunity. However, few studies have focused on the effect of TXA on the complement system. Further studies need to be performed to confirm our findings.
Postoperative thromboembolic events are the major concern for TKA patients who receive multiple doses of TXA. Although many surgeons are hesitant to use TXA, a basic scientific study demonstrated that TXA is not thrombogenic, but rather, that it prevents the degradation of existing clots (Benoni et al. 1997). In the current trial, we adopted an “earlier anticoagulation” strategy based on the previous study (Xie et al. 2016), and combined with physical approaches and early rehabilitation activities, we detected a low incidence of thromboembolic events during a 1-year follow-up. This was comparable with studies on the safety of multiple TXA doses (Wang et al. 2018; Xie et al. 2016; Zeng et al. 2018). Because DXM may aggravate the immunosuppressive status, postoperative wound complications are of particular concern. During the 1-year follow-up, only one patient in group C had a superficial infection, which may have resulted from improper discharged wound care. The patient was readmitted to our hospital and received appropriate treatment. Despite multiple doses of DXM (total dosage = 40 mg) that were used in group D, no increase in wound infection or death occurred. This is in agreement with previous studies that evaluated the safety of multi-dose and high-dose DXM (Xu et al. 2018a, b).
This study has some limitations. First, because of the strict inclusion criteria, the sample size may not have been large enough to determine significance for all variables. However, as a result, there was less external impact to the results, which had better accuracy and authenticity. Additionally, although there were no statistical differences among the four groups in terms of baseline demographic data, the population was mostly elderly women, which reflects the population characteristics of patients with osteoarthritis, but this is not representative of the entire population. It is unclear whether the findings apply to other populations. Finally, few cytokines and immune indicators were included in the current study and the effects of TXA on pro-inflammatory cytokines were not explored. It has been shown that the balance between pro- and anti-inflammatory cytokines is vital to the understanding of inflammation and the inflammatory response (Robertshaw 2008). Therefore, future studies should include more cytokines and immune indicators and explore their possible molecular mechanisms.
In summary, this randomized clinical trial found that six doses of IV-TXA could attenuate the acute inflammatory reaction after TKA compared with placebo. However, this anti-inflammatory effect was weaker compared with that of IV-DXM. Six doses of IV-TXA may modulate the immune response by improving postoperative complement levels and alleviating IV-DXM immunosuppression on T-lymphocytes. This finding has profound clinical implications that could potentially broaden the scope of TXA usage. Future studies should focus on the effect of TXA on the immune response by including more cytokines and immune indicators and exploring their possible molecular mechanisms.
References
Amara U et al (2010) Molecular intercommunication between the complement and coagulation systems. J Immunol 185:5628–5636
Andersson L, Eriksson O, Hedlund PO, Kjellman H, Lindqvist B (1978) Special considerations with regard to the dosage of tranexamic acid in patients with chronic renal diseases. Urol Res 6:83–88
Barthel D, Schindler S, Zipfel PF (2012) Plasminogen is a complement inhibitor. J Biol Chem 287:18831–18842
Benoni G, Lethagen S, Fredin H (1997) The effect of tranexamic acid on local and plasma fibrinolysis during total knee arthroplasty. Thromb Res 85:195–206
Bian ZX, Shang HC (2011) CONSORT 2010 statement updated guidelines for reporting parallel group randomized trials. Ann Intern Med 154:290–291 (author reply 291–292)
Blanie A, Bellamy L, Rhayem Y, Flaujac C, Samama CM, Fontenay M, Rosencher N (2013) Duration of postoperative fibrinolysis after total hip or knee replacement: a laboratory follow-up study. Thromb Res 131:e6–e11
Borg RJ et al (2015) Dendritic cell-mediated phagocytosis but not immune activation is enhanced by plasmin. PLoS ONE 10:e0131216
Boudreau RM et al (2017) Impact of tranexamic acid on coagulation and inflammation in murine models of traumatic brain injury and hemorrhage. J Surg Res 215:47–54
Carter DW et al (2019) Tranexamic acid suppresses the release of mitochondrial DAMPs and reduces lung inflammation in a murine burn model. J Trauma Acute Care Surg 86:617–624
Draxler DF, Sashindranath M, Medcalf RL (2017) Plasmin: a modulator of immune function. Semin Thromb Hemost 43:143–153
Foley JH et al (2016) Complement activation in arterial and venous thrombosis is mediated by plasmin. EBioMedicine 5:175–182
Kehlet H (2013) Fast-track hip and knee arthroplasty. Lancet 381:1600–1602
Later AF, Sitniakowsky LS, van Hilten JA, van de Watering L, Brand A, Smit NP, Klautz RJ (2013) Antifibrinolytics attenuate inflammatory gene expression after cardiac surgery. J Thorac Cardiovasc Surg 145(1611–1616):1616.e1611–1614
Levi M, van der Poll T, Buller HR (2004) Bidirectional relation between inflammation and coagulation. Circulation 109:2698–2704
Li X, Syrovets T, Genze F, Pitterle K, Oberhuber A, Orend KH, Simmet T (2010) Plasmin triggers chemotaxis of monocyte-derived dendritic cells through an Akt2-dependent pathway and promotes a T-helper type-1 response. Arterioscler Thromb Vasc Biol 30:582–590
McCormack PL (2012) Tranexamic acid: a review of its use in the treatment of hyperfibrinolysis. Drugs 72:585–617
Robertshaw HJ (2008) An anti-inflammatory role for tranexamic acid in cardiac surgery? Crit Care 12:105
Schuliga M (2015) The inflammatory actions of coagulant and fibrinolytic proteases in disease. Mediators Inflamm 2015:437695
Slade MS, Simmons RL, Yunis E, Greenberg LJ (1975) Immunodepression after major surgery in normal patients. Surgery 78:363–372
Syrovets T, Jendrach M, Rohwedder A, Schule A, Simmet T (2001) Plasmin-induced expression of cytokines and tissue factor in human monocytes involves AP-1 and IKKbeta-mediated NF-kappaB activation. Blood 97:3941–3950
Szaba FM, Smiley ST (2002) Roles for thrombin and fibrin(ogen) in cytokine/chemokine production and macrophage adhesion in vivo. Blood 99:1053–1059
Teng Y, Feng C, Liu Y, Jin H, Gao Y, Li T (2018) Anti-inflammatory effect of tranexamic acid against trauma-hemorrhagic shock-induced acute lung injury in rats. Exp Anim 67:313–320
Wang D et al (2018) The antifibrinolytic and anti-inflammatory effects of multiple doses of oral tranexamic acid in total knee arthroplasty patients: a randomized controlled trial. J Thromb Haemost 16:2442–2453
World HO (2001) Iron deficiency anaemia: assessment prevention and control. A guide for programme managers, vol 21. WHO, Geneva
World MA (2013) World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 310:2191–2194
Wu X, Dubick MA, Schwacha MG, Cap AP, Darlington DN (2017) Tranexamic acid attenuates the loss of lung barrier function in a rat model of polytrauma and hemorrhage with resuscitation. Shock 47:500–505
Xie J, Ma J, Yao H, Yue C, Pei F (2016) Multiple boluses of intravenous tranexamic acid to reduce hidden blood loss after primary total knee arthroplasty without tourniquet: a randomized clinical trial. J Arthroplast 31:2458–2464
Xu B, Ma J, Huang Q, Huang ZY, Zhang SY, Pei FX (2018a) Two doses of low-dose perioperative dexamethasone improve the clinical outcome after total knee arthroplasty: a randomized controlled study. Knee Surg Sports Traumatol Arthrosc 26:1549–1556
Xu H, Zhang S, Xie J, Lei Y, Cao G, Pei F (2018b) Multiple doses of perioperative dexamethasone further improve clinical outcomes after total knee arthroplasty: a prospective, randomized, controlled study. J Arthroplast 33:3448–3454
Zeng WN, Liu JL, Wang FY, Chen C, Zhou Q, Yang L (2018) Low-dose epinephrine plus tranexamic acid reduces early postoperative blood loss and inflammatory response. J bone Jt Surg: Am 100:295–304
Zhang S, Huang Q, Xu B, Ma J, Cao G, Pei F (2018) Effectiveness and safety of an optimized blood management program in total hip and knee arthroplasty: a large, single-center, retrospective study. Medicine 97:e9429
Zhang S, Xie J, Cao G, Lei Y, Huang Q, Pei F (2019) Six-dose intravenous tranexamic acid regimen further inhibits postoperative fibrinolysis and reduces hidden blood loss following total knee arthroplasty. J Knee Surg. https://doi.org/10.1055/s-0039-1694768
Acknowledgements
The authors sincerely acknowledge the entire staffs of the Department of Orthopedic Surgery, West China Hospital, Sichuan University, who offered assistance in the coursing of this study. This study was funded by the National Health and Family Planning Commission of the People's Republic of China (CN) program (201302007). We thank Jodi Smith, PhD, from Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest, financial or otherwise.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, S., Xu, H., Xie, J. et al. Tranexamic acid attenuates inflammatory effect and modulates immune response in primary total knee arthroplasty: a randomized, placebo-controlled, pilot trial. Inflammopharmacol 28, 839–849 (2020). https://doi.org/10.1007/s10787-020-00695-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-020-00695-6