Abstract
Curcumin is a natural compound derived from the spice, turmeric, that has been extensively reported for its efficacy in controlling or treatment of several inflammatory diseases. There is a growing body of literature that recognizes the anti-inflammatory effects of curcumin in the immune system. On the other hand, the role of inflammatory signaling pathways has been highlighted in the pathogenesis of several inflammatory diseases, and signaling molecules involved in these pathways are considered as valuable targets for new treatment approaches. We aimed to provide a comprehensive overview of the modulatory effects of curcumin on inflammatory signaling pathways which leads to inhibition of inflammation in different types of immune cells and animal models. In this comprehensive review, we elaborate on how curcumin can effectively inhibit multiple signaling molecules involved in inflammation including NF-κB, JAKs/STATs, MAPKs, β-catenin, and Notch-1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Inflammation and inflammatory signaling pathways
Inflammation is one of the major types of immune responses. It has an important role in both innate and adaptive immunity and has a crucial role in the defense against many harmful stimuli, of both endogenous and exogenous origin (Bianchi 2007; Grivennikov et al. 2010). During the inflammatory process, several immune cells (such as leucocytes) and plasma proteins (such as cytokines, complement proteins) are brought into the site of infection or damage in tissues and subsequently activated (Dinarello 2000). These blood-derived components of the immune system mediate inflammation to eliminate invading pathogens (such as bacteria, viruses, and fungi) and also promote tissue repair (Medzhitov 2008, 2010). The immune system has evolved to recognize the molecular structures of both foreign and endogenous molecules [such as lipopolysaccharide (LPS), heat shock proteins (HSPs)] by receptors expressed by cells of the immune system such as macrophages, dendritic cells (DCs), endothelial cells, B cells, and T cells (Bianchi 2007; Mohammadi et al. 2018b; Takeuchi and Akira 2010). As a consequence of binding of these receptors to their ligands, intracellular signal transduction pathways are activated to initiate and promote inflammatory responses in immune cells against the above-mentioned agents (Gordon 2002; Mohammadi et al. 2018b; Palm and Medzhitov 2009). During the inflammatory response, several inflammatory mediators such as pro-inflammatory cytokines and chemokines are produced by immune cells (Dinarello 2000; Keyel 2014; Mohammadi et al. 2018b). The most important pro-inflammatory cytokines in the immune responses are tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-6, IL-12, interferon-γ (IFN-γ), and IL-8 (Dinarello 2000; Mohammadi et al. 2018b). In addition, the interaction of the aforementioned cytokines with their receptors on the surface of immune cells also activates inflammatory signaling cascades in a positive feedback loop. The three main signaling pathways that mediate the inflammatory response in immune cells include nuclear factor-κB (NF-κB) signaling pathway, Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathway, and mitogen-activated protein kinase (MAPK) signaling pathway (Kyriakis and Avruch 1996; Lawrence 2009; O’shea et al. 2013). Inflammation is a protective biological response of the host immune system and is carefully controlled by several mechanisms (Hanada and Yoshimura 2002; MacDonald et al. 2011; Medzhitov 2008). However, failure in these mechanisms which tightly regulate inflammatory signaling pathways leads to unabated inflammation and generation of immune-mediated inflammatory diseases such as inflammatory bowel disease (IBD), rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), atherosclerosis, and multiple sclerosis (MS) (Abou-Raya and Abou-Raya 2006; Barnes and Karin 1997; Brydges and Kastner 2006; Martinon and Tschopp 2004). Therefore, modulation of these signaling molecules in the inflammatory signaling pathways can effectively induce anti-inflammatory effects and could potentially be a valuable approach for the management of inflammatory diseases.
One of the natural compounds that have shown potential anti-inflammatory properties and promise in the management or control of several inflammatory diseases is curcumin. Herein, we provide a comprehensive overview of the modulatory effects of curcumin on the inflammatory signaling pathways which leads to inhibition of inflammation in different types of immune cells and animal models.
Curcumin and its immunomodulatory effects
Curcumin is a natural compound derived from Curcuma longa L. (also called turmeric, a member of Zingiberaceae family) that is being used extensively for the management of several diseases. Research supports the critical roles played by curcumin and its analogs such as antibacterial, antiviral, antifungal, antioxidant, anti-inflammatory, hepatoprotective and anti-tumor activities (Aggarwal et al. 2009; Jalili-Nik et al. 2018; Mohammadi et al. 2018a, 2017; Momtazi and Sahebkar 2016; Momtazi et al. 2016; Panahi et al. 2016b, 2017a, b; Sahebkar 2013; Teymouri et al. 2017). In addition, it is well established that curcumin is considered to be a safe natural compound (Aggarwal et al. 2009; Jurenka 2009). In recent years, there has been an increasing interest in using curcumin as an immunomodulatory agent in the immune system. The immunomodulatory effect of curcumin arises from its interaction with a wide range of immune cells such as macrophages, DCs, B, and T cells (Abdollahi et al. 2018; Gao et al. 2004). The anti-inflammatory properties of curcumin have been demonstrated in the human and animal models of several inflammatory disorders such as RA, SLE, MS, type 1 diabetes mellitus (T1DM), atherosclerosis, metabolic syndrome, periodontal disease, colitis and Alzheimer’s disease (Abdollahi et al. 2018; Momtazi-Borojeni et al. 2017; Sahebkar et al. 2016; Soltani et al. 2019). Interestingly, recent evidence suggests that curcumin can reduce the pro-inflammatory cytokines such as IFN-γ, TNF-α, IL-1 and IL-8 via interaction with several signaling and transcription molecules such as NF-κB, JAKs/STASs, MAPKs and β-catenin (Gonzales and Orlando 2008; Han et al. 2002; Momtazi-Borojeni et al. 2017; Soetikno et al. 2011; Yang et al. 2017; Zhao et al. 2016). In this narrative review, we demonstrate that curcumin interacts with various signaling molecules in the inflammatory signaling pathways, thereby acting as an anti-inflammatory agent.
Effect of curcumin on the NF-κB signaling pathway
NF-κB was first identified in the B cells as a nuclear protein that binds specifically to kappa enhancer motif sequences in the NF-kB target genes (Sen and Baltimore 1986). This master transcription factor plays an essential role in the inducible expression of many genes associated with the inflammatory responses in the immune system including antimicrobial peptides, chemokines and cytokines (Sha et al. 1995; Xiao and Ghosh 2005). NF-κB proteins are located in the cytoplasm of the cells and repressed by their inhibitory proteins that are known as the inhibitors of NF-κB (IκBs) (Sen and Baltimore 1986). In response to various stimuli, the IκB becomes phosphorylated by an active IκB kinase (IKK), which results in the dissociation of IκB from NF-κB (Xiao and Ghosh 2005). Subsequently, NF-κB is released, translocated to the nucleus and bind their DNA binding sites to regulate the transcription of a large number of genes (Sha et al. 1995; Xiao and Ghosh 2005).
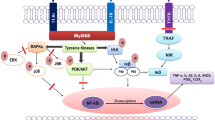
There is increasing evidence that the mode of action of curcumin involves modulating the NF-κB pathway, which may be considered as one of the key targets of curcumin (Fig. 1) (Jin et al. 2007; Kunnumakkara et al. 2007; Liu et al. 2017; Shakibaei et al. 2007; Suresh et al. 2018; Yekollu et al. 2011). The NF-κB network could be modulated at two stages: the inhibition of the NF-κB activation process, and by direct inhibition of NF-κB. In this regard, Brennan et al. reported that curcumin could inhibit NF-κB activation by inhibiting the degradation of IκB-α and reacting with the NF-kB itself in TNF-activated Jurkat T lymphoma cells (Brennan and O’Neill 1998). Curcumin may also interfere with the binding activity of NF-κB to the κB site in the IL-12p40 promoter, which significantly inhibits IL-12 production in LPS-activated macrophages (Kang et al. 1999b, c). In addition, curcumin treatment inhibited the NF-kB activation induced by oxygen–glucose deprivation in injured brain microvascular endothelial cells (BMECs) (Dong et al. 2014). Kim et al. reported that curcumin negatively regulates the production of pro-inflammatory cytokines (IL-1, IL-6, and TNF-α) from maturing DCs (Kim et al. 2005). In addition, the curcumin-treated DCs manifested an impaired induction of TH1 responses and a normal cell-mediated immune response (Kim et al. 2005). This indicates that the inhibitory effect of curcumin on DCs maturation, at least in part, could be derived from its actions on the NF-κB activation as a potential target (Kim et al. 2005).
A schematic view of curcumin’s modulatory effects on NF-κB, JAK/STAT, and MAPKs pathway. Curcumin suppresses activation and phosphorylation of JAKs and STATs proteins. Moreover, curcumin via both direct interactions with NF-κB and IκB suppresses activation of NF-κB. Finally, curcumin inhibits MAPK signaling pathway via its interaction with three main members of this pathway including JNK, p38, and ERK. As a result of curcumin’s modulatory functions, the pro-inflammatory process including infiltration of leukocyte into the site of inflammation, activation, maturation, and also the production of pro-inflammatory mediators by innate immune cells strongly was inhibited. On the other hand, curcumin suppresses acquired immune responses by its inhibitory effects on the activation, differentiation, and cytokine production of T cells
Further studies suggest that curcumin inhibits NF-κB signaling pathway by promoting the expression of IκB-α in activated human macrophages by influenza virus infection (Xu and Liu 2017). In addition, curcumin derivative BDMC33-treated macrophages showed an interrupted degradation of IκB, resulting in attenuation of NF-κB nuclear translocation (Lee et al. 2012). As a consequence of this event, the production of several pro-inflammatory mediators including NO, TNF-α, and IL-1β was suppressed by curcumin (Lee et al. 2012). Kumar and colleagues studied the effects of curcumin on the adhesion of monocytes to human umbilical vein endothelial cells (HUVECs) (Kumar et al. 1998). They demonstrated that the anti-inflammatory activity of curcumin may be due, in part, to the inhibition of leukocyte recruitment (Kumar et al. 1998). Curcumin blocked the TNF-induced adhesion of monocytes to HUVECs by inhibiting the expression of adhesion molecules and TNF-mediated activation of NF-κB (Kumar et al. 1998). Cho et al. reported that curcumin has an inhibitory effect on the expression of IL-1β and IL-6 expression induced in TNF-α-treated HaCaT cells (Cho et al. 2007). They suggested that curcumin exerts its anti-inflammatory and growth inhibitory effects by negative regulation of the NF-κB pathway (Cho et al. 2007). Bisdemethoxycurcumin, the active component of turmeric, suppresses the production of inflammatory cytokines including TNF-α, IL-8, and IL-6 by inhibiting the NF-κB activation and IκB degradation in pharmacologically induced inflammation in the human mast cells (Kong et al. 2018).
Pan et al. reported that a new synthetic curcumin analog (C66) decreased high glucose-induced over-expressions of intercellular adhesion molecule 1 (ICAM-1) or CD54 (an important ligand for β2 integrins), vascular cell adhesion molecule 1 (VCAM-1), and monocyte chemoattractant protein-1 (MCP-1). It also reduced renal macrophage infiltration and injury by suppressing NF-κB activation in diabetic mice (Pan et al. 2013).
Curcumin decreases the NF-κB activation in TCR-stimulated non-obese diabetic lymphocytes (Castro et al. 2014). Moreover, Soetikno et al. observed that the administration of curcumin protects against the development of diabetic nephropathy (Soetikno et al. 2011). Diabetic nephropathy is a major complication of diabetes and can be considered as an inflammatory disease (Gross et al. 2005). Monocytes/macrophages as the main source of pro-inflammatory mediators including TNF-α, IL-1β, and MCP-1 and are the key inflammatory cells involved in the pathogenesis of the diabetic nephropathy (Duran-Salgado and Rubio-Guerra 2014; Moreno et al. 2018). Macrophages infiltrating into the glomerulus are implicated in the development of glomerular injury (Duran-Salgado and Rubio-Guerra 2014). It has been indicated that curcumin could reduce macrophage infiltration by suppressing the activation of the NF-κB pathway in diabetic rat models (Soetikno et al. 2011). In accordance with this finding, Ghosh et al. demonstrated that curcumin treatment improves renal function in animal models with chronic renal failure by antagonizing the effect of TNF-α in peroxisome proliferator-activated receptor gamma (PPARγ) (Ghosh et al. 2009). It also blocked transactivation of NF-κB (Ghosh et al. 2009).
Effect of curcumin on JAK/STAT signaling pathway
The JAK/STAT signaling pathway is one of the most important pathways that regulate inflammation in immune cells by transducing the signal of types 1 and 2 cytokine receptors in response to various pro-inflammatory cytokines (Leonard and O’Shea 1998; O’shea et al. 2013). This pathway includes the four known Janus kinases (JAK1-3 and TYK2), which are associated with the aforementioned receptors, and seven STATs (STAT1-4, 5a, 5b, and 6) (Leonard and O’Shea 1998; O’shea et al. 2013).
In innate immunity, these intracellular molecules mediate signaling cascades induced by type I and type II interferon (i.e., IFN-α/β and IFN-γ). They can effectively induce the activation, maturation, and function of DCs and macrophages (Schindler et al. 2007). In acquired immunity, JAK/STAT signaling regulates the activation and differentiation of different subtype of T cells including TH1 (JAK2, TYK2, STAT1, and STAT4), TH2 (JAK1, JAK3, and STAT6), and TH17 (STAT3) from naïve CD4+ T cells (Leonard and O’Shea 1998; O’shea et al. 2013; Tamiya et al. 2011). Despite the physiologic roles played by JAK/STAT signaling, this pathway is also involved in the pathogenesis of several inflammatory diseases such as RA, IBD, MS, T1DM, SLE, and periodontitis, hence could be considered as a valuable target for the regulation of inflammation (Coskun et al. 2013; Haftcheshmeh et al. 2018; O’shea and Plenge 2012; O’Shea et al. 2015; STAT and EGF 2005).
The inhibitory action of curcumin on JAK/STAT signaling pathway has been confirmed in a study conducted by Kim et al. where it was shown that curcumin suppresses phosphorylation of JAK1, JAK2, and their downstream molecules such as STAT1 and STAT3 in IFN-γ, gangliosides, or LPS-activated microglial cells. As a result, the expression of several pro-inflammatory mediators including inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2), MCP-1 and ICAM-1 were impaired in activated microglial cells (Kim et al. 2003). In this regard, the activation of Src homology 2 domain-containing protein tyrosine phosphatases (SHP)-2, a key negative regulator of JAK activity is one of the several molecular mechanisms by which curcumin mediates the suppression of JAK activation (Kim et al. 2003). Oncostatin M (OSM) is an important member of IL-6 cytokine superfamily that is involved in the pathogenesis of several inflammatory diseases, such as RA, by inducing several matrix metalloproteinases (MMPs). In line with previous findings, it has been reported that curcumin treatment suppressed the OSM-induced phosphorylation and DNA binding activity of STAT1 (but not JAK1, JAK2, and JAK3) in bovine and human primary articular chondrocyte (Li et al. 2001). By its inhibitory action on STAT1, curcumin suppresses the OSM-induced production of MMP1, MMP3, and MMP13 in chondrocytes (Li et al. 2001). Another in vitro study assessing the mechanisms underlying curcumin-regulated JAK/STAT signaling showed that curcumin potently inhibits the expression of LPS-induced IL-6, TNF-α, and COX-2 in macrophage cell line RAW264.7 via its modulatory effect on suppressor of cytokine signaling (SOCS)1 and SOCS3 (Guimarães et al. 2013). SOCS proteins negatively regulate the overactivation of the JAK/STAT signaling in responses to inflammatory cytokines through interaction with both JAKs and STATs (Endo et al. 1997; Starr et al. 1997). This evidence provides a novel molecular mechanism by which curcumin regulates the JAK–STAT-mediated inflammatory responses in macrophages. Another in vitro study suggested that curcumin reduced the expression of several inflammatory mediators including ICAM-1, MCP-1, and IL-8 at both mRNA and protein levels by suppressing the STAT3-phosphorylation in TNF-α-stimulated HUVECs (Kim et al. 2007).
In experimental allergic encephalomyelitis (EAE), characterized by the predominance of autoreactive TH1 and TH17 cell responses, curcumin blocks the IL-12-induced phosphorylation of JAK2, TYK2, and their downstream molecules, i.e., STAT3 and SATA4 in T cells (Natarajan and Bright 2002). Curcumin also inhibits the production of IL-12 by macrophages and DCs (Kang et al. 1999a, b; Natarajan and Bright 2002). With regard to the essential role of IL-12 in the differentiation of TH1 cells (Zhu et al. 2010), curcumin can strongly suppress the proliferation and differentiation of autoreactive TH1 cells in several autoimmune diseases such as MS via inhibition of IL-12 production and its signaling cascade. Similar to effects on TH1 cells, curcumin also effectively suppresses proliferation and differentiation of autoreactive TH17 cells, another important subtype of T CD4+ cells involved in the pathogenesis of EAE (Xie et al. 2009). This is mediated by both suppressing IL-6, IL-21, and IL-17 production, and by inhibiting STAT3-phosphorylation and RAR-related orphan receptor gamma (RORγt) activation in response to the aforementioned cytokines (Xie et al. 2009). It is interesting to note that IL-6 and IL-21 are required for the differentiation of TH17 cells from naïve CD4+ T cells by activating STAT3 signaling and its downstream transcription factor of RORγt (Wei et al. 2007; Zhou et al. 2007). Curcumin treatment attenuated CNS inflammation, demyelination, and severity of clinical paralysis in animal models of EAE owing to its modulatory effects on JAK/STAT signaling (Natarajan and Bright 2002; Xie et al. 2009). This evidence is further supported by other studies which showed curcumin could exert its beneficial anti-inflammatory effects in an animal model of colitis and intestinal inflammation by inhibiting the phosphorylation of JAK2, STAT3, and STAT6 (Liu et al. 2013; Zhang et al. 2016; Zhao et al. 2016). This is followed by downregulated protein expression of TNF-α, IL-1β, IFN-γ, IL-23, and IL-12p70 and upregulated expression of anti-inflammatory cytokines including IL-4, IL-10, and IL-13 and transforming growth factor β (TGF-β) (Liu et al. 2013; Zhang et al. 2016; Zhao et al. 2016). In addition, curcumin also inhibits the activation of CD4+CD7− T cells by downregulation of the STAT-3 signaling pathway (Haftcheshmeh et al. 2019; Zhang et al. 2010a). CD4+CD7− T cells are a distinct subset of CD4+ T cells which produce TH2-like cytokine profiles including IL-4 and IL-10. They are involved in the pathogenesis of several inflammatory skin diseases (Haftcheshmeh et al. 2019).
DCs are key cells crucial for the initiation of pro-inflammatory responses in autoimmune and inflammatory diseases such as colitis and are one of the main targets of curcumin (Blanco et al. 2008; Hart et al. 2005). It has been documented that curcumin suppresses activation and maturation of DCs in colitis mice by targeting JAK/STAT signaling and also by upregulation of three important negative regulators of this pathway including SOCS 1 and 3 and protein inhibitor of activated STAT3 (PIAS3) (Zhang et al. 2016; Zhao et al. 2016).
Taken together, this growing evidence provides a better understanding of the mechanism of anti-inflammatory action for curcumin via modulating of JAK/STAT inflammatory signaling.
Effect of curcumin on MAPKs signaling pathway
MAPKs are a group of serine-threonine protein kinases that contribute to gene induction, proliferation, cellular differentiation, and inflammatory responses (Dong et al. 2002). There are three main groups of MAPKs which include extracellular receptor-activated kinase (ERK), P38, and C-Jun N-terminal kinase (JNK) (Seger and Krebs 1995). MAPKs play major roles in the production of pro-inflammatory cytokines and can be considered as valuable targets for the treatment of inflammatory diseases (Dong et al. 2002; Johnson and Lapadat 2002).
To study the effect of curcumin on inflammation related to MAPKs signaling pathway, Morgana et al. investigated its effects on LPS-stimulated raw 264.7 murine macrophages and found that curcumin remarkably reduced prostaglandin E2 (PGE2) level and the expression of TNF-α and IL-6 by inhibiting phosphorylation and activation of p38 MAPK (Guimarães et al. 2013). In addition, another in vitro study indicated that pretreatment of murine microglia cell line N9 with curcumin and demethoxycurcumin (DMC) could reduce LPS-induced phosphorylation of p38, JNK, and ERK1/2 MAPKs pathways, resulting in inhibition of the production of ROS by microglial cells (Zhang et al. 2010b). Consistent with previous studies, Kim et al. demonstrated that pretreatment of immature DCs cells with curcumin suppressed the LPS-induced maturation function of DCs by inhibiting phosphorylation of all three main MAPKs (JNK, p38, and ERK) (Kim et al. 2005). Moreover, curcumin effectively inhibited COX-2 expressions (both in mRNA and protein levels) in UVB-irradiated HaCaT cells by an inhibitory action on activation of p38 MAPK and JNK (Cho et al. 2005).
RA is a chronic inflammatory disease characterized by the infiltration of several immune cells such as macrophage, DCs, and T and B lymphocytes in the inflamed joints to produce pro-inflammatory cytokines including IL-1β, IL-6, TNF-α, IFN-γ, IL-17, and IL-12 (Firestein and McInnes 2017). In response to these pro-inflammatory cytokines, resident synovial fibroblast cells also produce large amounts of IL-6, IL-8, COX-2, and MMPs which result in the progressive joint destruction, deformity, and disability (Huber et al. 2006; Meinecke et al. 2005). Treatment of human synovial fibroblast cell line MH7A and fibroblast-like synoviocytes (FLS) of RA patients with curcumin decreased PMA or IL-1β-induced phosphorylation of ERK1/2, but not p38, which led to reduced expression of IL-6 (Kloesch et al. 2013).
Dry eye disorder is a common inflammatory eye disease where hyperosmosis followed by the inflammation of the ocular surface is involved (Stevenson et al. 2012). In addition, high expression of pro-inflammatory cytokines such as IL-1β and IL-6 has been observed in patients with dry eye disorder (Brignole et al. 2000; Calonge et al. 2010). In a study by Min Chen et al., pretreatment of hyperosmotic-stimulated human corneal epithelial cells with curcumin prevented an increase in the IL-1β, IL-6, and TNF-α production. Interestingly, p38 inhibitor (SB 203580), but not JNK inhibitor (600125), has been able to completely inhibit the IL-1β production, suggesting that the potential anti-inflammatory effects of curcumin are mediated by its suppressive effect on the p38 pathway. Importantly, p38 inhibitor also reduced the activation of NF-κB, which proves that activation of NF-κB occurs after the activation of p38 (Chen et al. 2010). These findings provide evidence that curcumin is able to suppress NF-κB signaling cascade both through its direct interaction with NF-κB and by inhibition of its upstream activator (i.e., p38 MAPK).
After brain ischemia, brain microvascular endothelial cells (BMECs), the principal cells in the blood–brain barrier (BBB), can cause inflammation by producing several inflammatory cytokines such as IL-1β (Stanimirovic and Satoh 2000). Hence, preventing inflammatory processes in BMECs can potentially reduce brain damage. In a study by Zhan et al., curcumin was able to significantly reduce the lactate dehydrogenase (LDH) release and IL-1β production in oxygen–glucose deprivation (OGD)-stimulated BMECs via inhibition of p38 and JNK phosphorylation. In line with the Min Chen et al. study, P38 inhibitor (SB203580) suppresses activation of NF-κB, suggesting that curcumin can potentially inhibit these two pathways simultaneously (Dong et al. 2014).
In an animal model of colitis, curcumin treatment effectively reduced both myeloperoxidase (MPO) activity and production of TNF-α, COX-2 and iNOS by suppressing p38 phosphorylation. Moreover, the production of anti-inflammatory cytokine IL-10 was upregulated (Camacho-Barquero et al. 2007). These findings are in accordance with a recent study suggesting that treatment with curcumin of IBD patients with positive colonic mucosal biopsies and colonic myofibroblasts (CMF) resulted in reduced p38 phosphorylation, which was followed by a decrease in the IL-1β and MMP-3 production (Epstein et al. 2010b).
Asthma is a long-term chronic inflammatory disease characterized by the production of pro-inflammatory cytokines such as TNF-α and IL-1β in the airways (Barnes 2008; Bousquet et al. 2000). MAPKs are one of the important factors in the production of these pro-inflammatory proteins; hence, inhibiting this pathway can be a valuable treatment option for this disease (Duan and Wong 2006). In this regard, in a study by Singh et al., in an animal model of chronic asthma, intranasal curcumin was able to inhibit all of the three main pathways of MAPKs (p38, JNK, and ERK) (Chauhan et al. 2018). As a result, the levels of nitrite, COX-2, and reactive oxygen species (ROS) were significantly reduced (Chauhan et al. 2018).
Other targets of curcumin
Curcumin has also shown immunomodulatory effects on different signaling molecules in the immune cells. Yang et al. demonstrated that treatment with curcumin downregulated the expression of glycogen synthase kinase 3 (GSK-3), a negative regulator of Wnt/β-catenin signaling pathway, and upregulated the expression of β-catenin, a chief downstream transcription factor of the canonical Wnt signaling pathway, in LPS-stimulated BMDC (Yang et al. 2017). As a result, Wnt/β-catenin signaling was activated in curcumin-treated BMDC that led to the inhibition of DCs activation and maturation (Yang et al. 2017). In addition, in a mouse model of allergic asthma, administration of curcumin for 9 days attenuated asthma symptoms and inflammatory responses in the airway by activating the Wnt/β-catenin signaling pathway, especially in DCs (Yang et al. 2017).
While investigating the further molecular targets of curcumin and its anti-inflammatory effects, Cheong et al. found that treatment of mouse model of acute asthma with curcumin (200 mg/kg) decreased both mRNA and protein levels of Notch 1 receptor and its downstream transcription factor GATA binding protein 3 (GATA3), a master regulator of TH2 cell differentiation, in lung tissues (Chong et al. 2014). Notch 1–GATA3 signaling pathway plays a crucial role in the pathogenesis of allergic asthma by promoting the differentiation of TH2 cells (Fang et al. 2007; Guo et al. 2009; Hozumi et al. 2008; Park 2010). Therefore, curcumin attenuated the allergic airway inflammation by inhibiting the Notch 1–GATA3 signaling pathway and subsequent suppression of TH2 cells differentiation (Chong et al. 2014; Osborne and Minter 2007). Recently, another in vivo study has shown that curcumin can also inhibit the phosphorylation of transforming growth factor (TGF)-activated kinase 1 (TAK1) in inflamed spinal cord cells which suppresses the production of pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6 in a mouse model of acute spinal injury (Zhang et al. 2017). TAK1 is one of the MAPKKK family members and a major upstream modulator for the activation of NF-κB and P38 in microglial cells (Landström 2010). Therefore, curcumin can effectively suppress activation of these important pro-inflammatory transcription factors not only through direct interaction on NF-κB, P38, but also through their upstream molecules, especially TAK1.
Anti-inflammatory effects of curcumin in clinical trials
Over the past decade, a large number of clinical studies have investigated the anti-inflammatory effects of curcumin in several diseases. In a randomized clinical trial conducted by Alizadeh et al., administration of 80 mg curcumin nanomicelle daily for 10 weeks significantly reduced the plasma levels of inflammatory mediators including TNF-α and C-reactive protein (CRP) in infertile men (Alizadeh et al. 2018). Another randomized clinical trial evaluating the anti-inflammatory effects of curcumin supplementation found that oral administration of 500 mg turmeric (containing 22.1 mg the active ingredient curcumin) for 2 months significantly reduced the serum levels of IL-8, but not TNF-α, in patients with type 2 diabetic nephropathy (Khajehdehi et al. 2011). The anti-inflammatory effects of curcumin were further supported by a randomized clinical trial conducted by Panahi et al., which found that curcumin treatment (1 g/day) effectively reduced the serum levels of TNF-α, IL-6, and MCP-1 in patients with metabolic syndrome (Panahi et al. 2016a). In addition, a decrease in the plasma levels of IL-4 and IL-6 was observed after treatment of patients with knee osteoarthritis with pure curcuminoids (1500 mg/day) for 6 weeks (Rahimnia et al. 2015). Another clinical study found that oral administration of curcuminoids (comprising curcumin, demethoxycurcumin, and bisdemethoxycurcumin) at a daily dose of 1 g for 4 weeks significantly reduced the serum concentration of IL-1β, IL-4, and vascular endothelial growth factor (VEGF), but not TNF-α, IL-6, IL-8, IFN-γ, and MCP-1 in obese individuals (Ganjali et al. 2014). Moreover, by reducing TNF-α, IL-8, IL-6, MCP-1, and hs-CRP, curcumin effectively mediated its anti-inflammatory effects in sulfur mustard-intoxicated patients with chronic pulmonary or cutaneous complications. This disease is characterized by the overproduction of several pro-inflammatory cytokines (Panahi et al. 2012, 2015). In line with the findings of previous studies, anti-inflammatory effects of curcumin were also reported in a clinical study where it has been shown that administration of curcumin (180 mg/day) for 8 weeks resulted in a reduction of serum levels of pro-inflammatory mediators including TNF-α, IL-8, IL-6, MCP-1, and hs-CRP in patients with solid tumors. As a consequence, systemic inflammation in these patients was suppressed by curcumin supplementation (Panahi et al. 2014). All of the studies reviewed here have demonstrated the anti-inflammatory effects of curcumin in several diseases by its modulatory effects on inflammatory signaling pathway as the main targets of curcumin. Table 1 summarizes the anti-inflammatory effects of curcumin in recently completed clinical trials.
Concluding remarks
There is growing evidence that curcumin, through interaction with a diverse set of cellular and molecular targets, has an anti-inflammatory role and therefore can be considered as a valuable natural compound for managing various inflammatory diseases. Curcumin can inhibit the inflammatory process in different types of immune cells and animal models (Table 2, Fig. 1). Curcumin has been found to suppress several inflammatory cascades in immune cells which result in (1) inhibition of activation, maturation, and cytokine production of two important cells of innate immunity, i.e., macrophages and DCs, and (2) inhibition of activation, proliferation, maturation, and cytokine production of T cell subsets such as TH1, TH2, and TH17. Interestingly, curcumin as a pleiotropic molecule can simultaneously target multiple signaling molecules such as NF-κB, JAKs/STATs, MAPKs and Wnt/β catenin, suggesting its potential as a signaling molecule-targeted therapeutic agent for inflammatory and immune-related diseases.
Change history
19 August 2019
Unfortunately, the 4th author name was incorrectly published in the original article. The complete correct name is given below.
Abbreviations
- HSPs:
-
Heat shock proteins
- LPS:
-
Lipopolysaccharide
- DCs:
-
Dendritic cells
- TNF-α:
-
Tumor necrosis factor-α
- IL:
-
Interleukin
- IFN:
-
Interferon
- NF-κB:
-
Nuclear factor-κB
- JAK/STAT:
-
Janus kinase/signal transducer and activator of transcription
- MAPK:
-
Mitogen-activated protein kinase
- IBD:
-
Inflammatory bowel disease
- RA:
-
Rheumatoid arthritis
- SLE:
-
Systemic lupus erythematosus
- MS:
-
Multiple sclerosis
- T1DM:
-
Type 1 diabetes mellitus
- IκB:
-
Inhibitors of NF-κB
- IKK:
-
IκB kinase
- BMECs:
-
Brain microvascular endothelial cells
- HUVECs:
-
Human umbilical vein endothelial cells
- ICAM-1:
-
Intercellular adhesion molecule 1
- VCAM-1:
-
Vascular cell adhesion molecule 1
- MCP-1:
-
Monocyte chemoattractant protein-1
- PPARγ:
-
Peroxisome proliferator-activated receptor gamma
- iNOS:
-
Inducible nitric oxide synthase
- COX-2:
-
Cyclooxygenase-2
- SHP2:
-
Src homology 2 domain-containing protein tyrosine phosphatase
- OSM:
-
Oncostatin M
- MMP:
-
Matrix metalloproteinase
- EAE:
-
Experimental allergic encephalomyelitis
- RORγt:
-
RAR-related orphan receptor gamma
- TGF-β:
-
Transforming growth factor β
- SOCS:
-
Suppressor of cytokine signaling
- PIAS:
-
Protein inhibitor of activated STAT
- ERK:
-
Extracellular receptor-activated kinase
- JNK:
-
C-Jun N-terminal kinase
- PGE2:
-
Prostaglandin E2
- MPO:
-
Myeloperoxidase
- CMF:
-
Colonic myofibroblasts
- ROS:
-
Reactive oxygen species
- BBB:
-
Blood–brain barrier
- FLS:
-
Fibroblast-like synoviocyte
- LDH:
-
Lactate dehydrogenase
- OGD:
-
Oxygen–glucose deprivation
- GSK3:
-
Glycogen synthase kinase 3
- GATA3:
-
Transcription factor GATA binding protein 3
- TAK1:
-
Transforming growth factor (TGF)-activated kinase 1
- PMA:
-
Phorbol 12-myristate 13-acetate
- DLN:
-
Draining lymph node
- CRP:
-
C-reactive protein
- VEGF:
-
Vascular endothelial growth factor
References
Abdolahi M et al (2017) The synergistic effects of omega-3 fatty acids and nano-curcumin supplementation on tumor necrosis factor (TNF)-alpha gene expression and serum level in migraine patients. Immunogenetics 69:371–378. https://doi.org/10.1007/s00251-017-0992-8
Abdolahi M et al (2018) A novel combination of omega-3 fatty acids and nano-curcumin modulates interleukin-6 gene expression and high sensitivity C-reactive protein serum levels in patients with migraine: a randomized clinical trial study. CNS Neurol Disord Drug Targets 17:430–438. https://doi.org/10.2174/1871527317666180625101643
Abdolahi M et al (2019) The neuromodulatory effects of omega-3 fatty Acids and nano-curcumin on the COX-2/iNOS network in migraines: a clinical trial study from gene expression to clinical symptoms. Endocr Metab Immun Disord Drug Targets. https://doi.org/10.2174/1871530319666190212170140
Abdollahi E, Momtazi AA, Johnston TP, Sahebkar A (2018) Therapeutic effects of curcumin in inflammatory and immune-mediated diseases: a nature-made jack-of-all-trades? J Cell Physiol 233:830–848. https://doi.org/10.1002/jcp.25778
Abou-Raya A, Abou-Raya S (2006) Inflammation: a pivotal link between autoimmune diseases and atherosclerosis. Autoimmun Rev 5:331–337. https://doi.org/10.1016/j.autrev.2005.12.006
Aggarwal BB, Vijayalekshmi RV, Sung B (2009) Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin Cancer Res 15:425–430. https://doi.org/10.1158/1078-0432.ccr-08-0149
Alizadeh F, Javadi M, Karami AA, Gholaminejad F, Kavianpour M, Haghighian HK (2018) Curcumin nanomicelle improves semen parameters, oxidative stress, inflammatory biomarkers, and reproductive hormones in infertile men: a randomized clinical trial. Phytother Res 32:514–521. https://doi.org/10.1002/ptr.5998
Barnes PJ (2008) The cytokine network in asthma and chronic obstructive pulmonary disease. J Clin Investig 118:3546–3556. https://doi.org/10.1172/JCI36130
Barnes PJ, Karin M (1997) Nuclear factor-κB—a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med 336:1066–1071. https://doi.org/10.1056/NEJM199704103361506
Belcaro G et al (2010a) Efficacy and safety of Meriva(R), a curcumin-phosphatidylcholine complex, during extended administration in osteoarthritis patients. Altern Med Rev 15:337–344
Belcaro G et al (2010b) Product-evaluation registry of Meriva(R), a curcumin-phosphatidylcholine complex, for the complementary management of osteoarthritis. Panminerva Med 52:55–62
Bianchi ME (2007) DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol 81:1–5. https://doi.org/10.1189/jlb.0306164
Blanco P, Palucka AK, Pascual V, Banchereau J (2008) Dendritic cells and cytokines in human inflammatory and autoimmune diseases. Cytokine Growth Factor Rev 19:41–52. https://doi.org/10.1016/j.cytogfr.2007.10.004
Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM (2000) Asthma: from bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med 161:1720–1745. https://doi.org/10.1164/ajrccm.161.5.9903102
Brennan P, O’Neill LA (1998) Inhibition of nuclear factor κB by direct modification in whole cells–mechanism of action of nordihydroguaiaretic acid, curcumin and thiol modifiers. Biochem Pharmacol 55:965–973. https://doi.org/10.1016/S0006-2952(97)00535-2
Brignole F, Pisella P-J, Goldschild M, De Saint Jean M, Goguel A, Baudouin C (2000) Flow cytometric analysis of inflammatory markers in conjunctival epithelial cells of patients with dry eyes. Invest Ophthalmol Vis Sci 41:1356–1363. https://doi.org/10.1016/s0002-9394(00)00701-7
Brydges S, Kastner D (2006) The systemic autoinflammatory diseases: inborn errors of the innate immune system. Curr Top Microbiol Immunol 305:127–160
Calonge M, Enríquez-de-Salamanca A, Diebold Y, González-García MJ, Reinoso R, Herreras JM, Corell A (2010) Dry eye disease as an inflammatory disorder. Ocul Immunol Inflamm 18:244–253. https://doi.org/10.3109/09273941003721926
Camacho-Barquero L, Villegas I, Sánchez-Calvo JM, Talero E, Sánchez-Fidalgo S, Motilva V, Alarcón de la Lastra C (2007) Curcumin, a Curcuma longa constituent, acts on MAPK p38 pathway modulating COX-2 and iNOS expression in chronic experimental colitis. Int Immunopharmacol 7:333–342. https://doi.org/10.1016/j.intimp.2006.11.006
Castro CN et al (2014) Curcumin ameliorates autoimmune diabetes. Evidence in accelerated murine models of type 1 diabetes. Clin Exp Immunol 177:149–160. https://doi.org/10.1111/cei.12322
Chauhan PS, Singh D, Dash D, Singh R (2018) Intranasal curcumin regulates chronic asthma in mice by modulating NF-ĸB activation and MAPK signaling. Phytomedicine. https://doi.org/10.1016/j.phymed.2018.06.022
Chen M, Hu D-N, Pan Z, Lu C-W, Xue C-Y, Aass I (2010) Curcumin protects against hyperosmoticity-induced IL-1β elevation in human corneal epithelial cell via MAPK pathways. Exp Eye Res 90:437–443. https://doi.org/10.1016/j.exer.2009.12.004
Cho JW et al (2005) Curcumin inhibits the expression of COX-2 in UVB-irradiated human keratinocytes (HaCaT) by inhibiting activation of AP-1: p38 MAP kinase and JNK as potential upstream targets. Exp Mol Med 37:186–192. https://doi.org/10.1038/emm.2005.25
Cho JW, Lee KS, Kim CW (2007) Curcumin attenuates the expression of IL-1beta, IL-6, and TNF-alpha as well as cyclin E in TNF-alpha-treated HaCaT cells; NF-kappaB and MAPKs as potential upstream targets. Int J Mol Med 19:469–474. https://doi.org/10.3892/ijmm.19.3.469
Chong L et al (2014) Protective effect of curcumin on acute airway inflammation of allergic asthma in mice through Notch1–GATA3 signaling pathway. Inflammation 37:1476–1485. https://doi.org/10.1007/s10753-014-9873-6
Chuengsamarn S, Rattanamongkolgul S, Luechapudiporn R, Phisalaphong C, Jirawatnotai S (2012) Curcumin extract for prevention of type 2 diabetes. Diabetes Care 35:2121–2127. https://doi.org/10.2337/dc12-0116
Coskun M, Salem M, Pedersen J, Nielsen OH (2013) Involvement of JAK/STAT signaling in the pathogenesis of inflammatory bowel disease. Pharmacol Res 76:1–8. https://doi.org/10.1016/j.phrs.2013.06.007
Dinarello CA (2000) Proinflammatory cytokines. Chest 118:503–508. https://doi.org/10.1378/chest.118.2.503
Dong C, Davis RJ, Flavell RA (2002) MAP kinases in the immune response. Annu Rev Immunol 20:55–72. https://doi.org/10.1146/annurev.immunol.20.091301.131133
Dong HJ, Shang CZ, Peng DW, Xu J, Xu PX, Zhan L, Wang P (2014) Curcumin attenuates ischemia-like injury induced IL-1beta elevation in brain microvascular endothelial cells via inhibiting MAPK pathways and nuclear factor-kappaB activation. Neurol Sci 35:1387–1392. https://doi.org/10.1007/s10072-014-1718-4
Duan W, Wong WS (2006) Targeting mitogen-activated protein kinases for asthma. Curr Drug Targets 7:691–698. https://doi.org/10.2174/138945006777435353
Duran-Salgado MB, Rubio-Guerra AF (2014) Diabetic nephropathy and inflammation. World J Diabetes 5:393–398. https://doi.org/10.4239/wjd.v5.i3.393
Endo TA et al (1997) A new protein containing an SH2 domain that inhibits JAK kinases. Nature 387:921. https://doi.org/10.1038/43213
Epstein J, Docena G, MacDonald TT, Sanderson IR (2010a) Curcumin suppresses p38 mitogen-activated protein kinase activation, reduces IL-1beta and matrix metalloproteinase-3 and enhances IL-10 in the mucosa of children and adults with inflammatory bowel disease. Br J Nutr 103:824–832. https://doi.org/10.1017/s0007114509992510
Epstein J, Docena G, MacDonald TT, Sanderson IR (2010b) Curcumin suppresses p38 mitogen-activated protein kinase activation, reduces IL-1β and matrix metalloproteinase-3 and enhances IL-10 in the mucosa of children and adults with inflammatory bowel disease. Br J Nutr 103:824–832. https://doi.org/10.1017/S0007114509992510
Fang TC, Yashiro-Ohtani Y, Del Bianco C, Knoblock DM, Blacklow SC, Pear WS (2007) Notch directly regulates Gata3 expression during T helper 2 cell differentiation. Immunity 27:100–110. https://doi.org/10.1016/j.immuni.2007.04.018
Firestein GS, McInnes IB (2017) Immunopathogenesis of rheumatoid arthritis. Immunity 46:183–196. https://doi.org/10.1016/j.immuni.2017.02.006
Ganjali S et al (2014) Investigation of the effects of curcumin on serum cytokines in obese individuals: a randomized controlled trial. Sci World J. https://doi.org/10.1155/2014/898361
Gao X et al (2004) Immunomodulatory activity of curcumin: suppression of lymphocyte proliferation, development of cell-mediated cytotoxicity, and cytokine production in vitro. Biochem Pharmacol 68:51–61. https://doi.org/10.1016/j.bcp.2004.03.015
Ghosh SS et al (2009) Curcumin ameliorates renal failure in 5/6 nephrectomized rats: role of inflammation. Am J Physiol Renal Physiol 296:F1146–F1157. https://doi.org/10.1152/ajprenal.90732.2008
Gonzales AM, Orlando RA (2008) Curcumin and resveratrol inhibit nuclear factor-κB-mediated cytokine expression in adipocytes. Nutr Metab 5:17. https://doi.org/10.1186/1743-7075-5-17
Gordon S (2002) Pattern recognition receptors: doubling up for the innate immune response. Cell 111:927–930. https://doi.org/10.1016/S0092-8674(02)01201-1
Grivennikov SI, Greten FR, Karin M (2010) Immunity, inflammation, and cancer. Cell 140:883–899. https://doi.org/10.1016/j.cell.2010.01.025
Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T (2005) Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care 28:164–176. https://doi.org/10.2337/diacare.28.1.164
Guimarães MR, Leite FRM, Spolidorio LC, Kirkwood KL, Rossa C Jr (2013) Curcumin abrogates LPS-induced pro-inflammatory cytokines in RAW 264.7 macrophages. Evidence for novel mechanisms involving SOCS-1,-3 and p38 MAPK. Arch Oral Biol 58:1309–1317. https://doi.org/10.1016/j.archoralbio.2013.07.005
Guo X-j, Zhou M, Ren L-p, Yang M, Huang S-g, Xu W-g (2009) Small interfering RNA-mediated knockdown of Notch1 in lung. Chin Med J 122:2647–2651. https://doi.org/10.3760/cma.j.issn.0366-6999.2009.21.023
Haftcheshmeh SM, Mohammadi A, Soltani A, Momtazi-Borojeni AA, Sattari M (2018) Evaluation of STAT1 and Wnt5a gene expression in gingival tissues of patients with periodontal disease. J Cell Biochem. https://doi.org/10.1002/jcb.27487
Haftcheshmeh SM, Tajbakhsh A, Kazemi M, Esmaeili SA, Mardani F, Fazeli M, Sahebkar A (2019) The clinical importance of CD4(+) CD7(–) in human diseases. J Cell Physiol 234:1179–1189. https://doi.org/10.1002/jcp.27099
Han S-S, Keum Y-S, Seo H-J, Surh Y-J (2002) Curcumin suppresses activation of NF-κB and AP-1 induced by phorbol ester in cultured human promyelocytic leukemia cells. BMB Rep 35:337–342. https://doi.org/10.5483/BMBRep.2002.35.3.337
Hanada T, Yoshimura A (2002) Regulation of cytokine signaling and inflammation. Cytokine Growth Factor Rev 13:413–421. https://doi.org/10.1016/S1359-6101(02)00026-6
Hart AL et al (2005) Characteristics of intestinal dendritic cells in inflammatory bowel diseases. Gastroenterology 129:50–65. https://doi.org/10.1053/j.gastro.2005.05.013
Holt PR, Katz S, Kirshoff R (2005) Curcumin therapy in inflammatory bowel disease: a pilot study. Dig Dis Sci 50:2191–2193. https://doi.org/10.1007/s10620-005-3032-8
Hozumi K et al (2008) Notch signaling is necessary for GATA3 function in the initiation of T cell development. Eur J Immunol 38:977–985. https://doi.org/10.1002/eji.200737688
Huber L, Distler O, Tarner I, Gay R, Gay S, Pap T (2006) Synovial fibroblasts: key players in rheumatoid arthritis. Rheumatology 45:669–675. https://doi.org/10.1093/rheumatology/kel065
Jalili-Nik M, Soltani A, Moussavi S, Ghayour-Mobarhan M, Ferns GA, Hassanian SM, Avan A (2018) Current status and future prospective of curcumin as a potential therapeutic agent in the treatment of colorectal cancer. J Cell Physiol 233:6337–6345. https://doi.org/10.1002/jcp.26368
Jin CY, Lee JD, Park C, Choi YH, Kim GY (2007) Curcumin attenuates the release of pro-inflammatory cytokines in lipopolysaccharide-stimulated BV2 microglia. Acta Pharmacol Sin 28:1645–1651. https://doi.org/10.1111/j.1745-7254.2007.00651.x
Johnson GL, Lapadat R (2002) Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 298:1911–1912. https://doi.org/10.1126/science.1072682
Jurenka JS (2009) Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev 14:141–153
Kang B, Song Y, Kim KM, Choe Y, Hwang S, Kim TS (1999a) Curcumin inhibits Th1 cytokine profile in CD4 + T cells by suppressing interleukin-12 production in macrophages. Br J Pharmacol 128:380–384. https://doi.org/10.1038/sj.bjp.0702803
Kang BY, Chung SW, Chung W-J, Im S-Y, Hwang SY, Kim TS (1999b) Inhibition of interleukin-12 production in lipopolysaccharide-activated macrophages by curcumin. Eur J Pharmacol 384:191–195
Kang BY, Song YJ, Kim KM, Choe YK, Hwang SY, Kim TS (1999c) Curcumin inhibits Th1 cytokine profile in CD4 + T cells by suppressing interleukin-12 production in macrophages. Br J Pharmacol 128:380–384. https://doi.org/10.1038/sj.bjp.0702803
Keyel PA (2014) How is inflammation initiated? Individual influences of IL-1, IL-18 and HMGB1. Cytokine 69:136–145. https://doi.org/10.1016/j.cyto.2014.03.007
Khajehdehi P, Pakfetrat M, Javidnia K, Azad F, Malekmakan L, Nasab MH, Dehghanzadeh G (2011) Oral supplementation of turmeric attenuates proteinuria, transforming growth factor-β and interleukin-8 levels in patients with overt type 2 diabetic nephropathy: a randomized, double-blind and placebo-controlled study. Scand J Urol Nephrol 45:365–370. https://doi.org/10.3109/00365599.2011.585622
Kim HY, Park EJ, E-h Joe, Jou I (2003) Curcumin suppresses Janus kinase-STAT inflammatory signaling through activation of Src homology 2 domain-containing tyrosine phosphatase 2 in brain microglia. J Immunol 171:6072–6079. https://doi.org/10.4049/jimmunol.171.11.6072
Kim G-Y et al (2005) Curcumin inhibits immunostimulatory function of dendritic cells: MAPKs and translocation of NF-κB as potential targets. J Immunol 174:8116–8124. https://doi.org/10.4049/jimmunol.174.12.8116
Kim YS et al (2007) Curcumin attenuates inflammatory responses of TNF-α-stimulated human endothelial cells. J Cardiovasc Pharmacol 50:41–49. https://doi.org/10.1097/FJC.0b013e31805559b9
Kloesch B, Becker T, Dietersdorfer E, Kiener H, Steiner G (2013) Anti-inflammatory and apoptotic effects of the polyphenol curcumin on human fibroblast-like synoviocytes. Int Immunopharmacol 15:400–405. https://doi.org/10.1016/j.intimp.2013.01.003
Kong R, Kang OH, Seo YS, Zhou T, Kim SA, Shin DW, Kwon DY (2018) MAPKs and NF-κB pathway inhibitory effect of bisdemethoxycurcumin on phorbol 12-myristate13-acetate and A23187-induced inflammation in human mast cells. Mol Med Rep 17:630–635. https://doi.org/10.3892/mmr.2017.7852
Kumar A, Dhawan S, Hardegen NJ, Aggarwal BB (1998) Curcumin (Diferuloylmethane) inhibition of tumor necrosis factor (TNF)-mediated adhesion of monocytes to endothelial cells by suppression of cell surface expression of adhesion molecules and of nuclear factor-κB activation. Biochem Pharmacol 55:775–783. https://doi.org/10.1016/S0006-2952(97)00557-1
Kunnumakkara AB, Guha S, Krishnan S, Diagaradjane P, Gelovani J, Aggarwal BB (2007) Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Can Res 67:3853–3861. https://doi.org/10.1158/0008-5472.CAN-06-4257
Kyriakis JM, Avruch J (1996) Sounding the alarm: protein kinase cascades activated by stress and inflammation. J Biol Chem 271:24313–24316. https://doi.org/10.1074/jbc.271.40.24313
Landström M (2010) The TAK1–TRAF6 signalling pathway. Int J Biochem Cell Biol 42:585–589. https://doi.org/10.1016/j.biocel.2009.12.023
Lawrence T (2009) The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb Perspect Biol 1:a001651. https://doi.org/10.1101/cshperspect.a001651
Lee KH et al (2012) BDMC33, a curcumin derivative suppresses inflammatory responses in macrophage-like cellular system: role of inhibition in NF-kappaB and MAPK signaling pathways. Int J Mol Sci 13:2985–3008. https://doi.org/10.3390/ijms13032985
Leonard WJ, O’Shea JJ (1998) Jaks and STATs: biological implications. Annu Rev Immunol 16:293–322. https://doi.org/10.1146/annurev.immunol.16.1.293
Li WQ, Dehnade F, Zafarullah M (2001) Oncostatin M-induced matrix metalloproteinase and tissue inhibitor of metalloproteinase-3 genes expression in chondrocytes requires Janus kinase/STAT signaling pathway. J Immunol 166:3491–3498. https://doi.org/10.4049/jimmunol.166.5.3491
Liu L et al (2013) Curcumin ameliorates dextran sulfate sodium-induced experimental colitis by blocking STAT3 signaling pathway. Int Immunopharmacol 17:314–320. https://doi.org/10.1016/j.intimp.2013.06.020
Liu JL et al (2017) Curcumin inhibits MCF-7 cells by modulating the NF-kappaB signaling pathway. Oncol Lett 14:5581–5584. https://doi.org/10.3892/ol.2017.6860
MacDonald TT, Monteleone I, Fantini MC, Monteleone G (2011) Regulation of homeostasis and inflammation in the intestine. Gastroenterology 140:1768–1775. https://doi.org/10.1053/j.gastro.2011.02.047
Martinon F, Tschopp J (2004) Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell 117:561–574. https://doi.org/10.1016/j.cell.2004.05.004
Medzhitov R (2008) Origin and physiological roles of inflammation. Nature 454:428. https://doi.org/10.1038/nature07201
Medzhitov R (2010) Inflammation 2010: new adventures of an old flame. Cell 140:771–776. https://doi.org/10.1016/j.cell.2010.03.006
Meinecke I, Rutkauskaite E, Gay S, Pap T (2005) The role of synovial fibroblasts in mediating joint destruction in rheumatoid arthritis. Curr Pharm Des 11:563–568. https://doi.org/10.2174/1381612053381945
Mohammadi A et al (2017) Modulatory effects of curcumin on apoptosis and cytotoxicity-related molecules in HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) patients. Biomed Pharmacother 85:457–462. https://doi.org/10.1016/j.biopha.2016.11.050
Mohammadi A, Blesso CN, Barreto GE, Banach M, Majeed M, Sahebkar A (2018a) Macrophage plasticity, polarization and function in response to curcumin, a diet-derived polyphenol, as an immunomodulatory agent. J Nutr Biochem 66:1–16. https://doi.org/10.1016/j.jnutbio.2018.12.005
Mohammadi A, Sharifi A, Pourpaknia R, Mohammadian S, Sahebkar A (2018b) Manipulating macrophage polarization and function using classical HDAC inhibitors: implications for autoimmunity and inflammation. Crit Rev Oncol Hematol 128:1–18. https://doi.org/10.1016/j.critrevonc.2018.05.009
Momtazi AA, Sahebkar A (2016) Difluorinated curcumin: a promising curcumin analogue with improved anti-tumor activity and pharmacokinetic profile. Curr Pharm Des 22:4386–4397. https://doi.org/10.2174/1381612822666160527113501
Momtazi AA, Shahabipour F, Khatibi S, Johnston TP, Pirro M, Sahebkar A (2016) Curcumin as a MicroRNA regulator in cancer: a review. Rev Physiol Biochem Pharmacol 171:1–38. https://doi.org/10.1007/112_2016_3
Momtazi-Borojeni AA, Haftcheshmeh SM, Esmaeili S-A, Johnston TP, Abdollahi E, Sahebkar A (2017) Curcumin: a natural modulator of immune cells in systemic lupus erythematosus. Autoimmun Rev. https://doi.org/10.1016/j.autrev.2017.11.016
Moreillon JJ et al (2013) The use of an anti-inflammatory supplement in patients with chronic kidney disease. J Complement Integr Med. https://doi.org/10.1515/jcim-2012-0011
Moreno JA et al (2018) Targeting inflammation in diabetic nephropathy: a tale of hope. Expert Opin Investig Drugs 27:917–930. https://doi.org/10.1080/13543784.2018.1538352
Natarajan C, Bright JJ (2002) Curcumin inhibits experimental allergic encephalomyelitis by blocking IL-12 signaling through Janus kinase-STAT pathway in T lymphocytes. J Immunol 168:6506–6513. https://doi.org/10.4049/jimmunol.168.12.6506
Osborne BA, Minter LM (2007) Notch signalling during peripheral T-cell activation and differentiation. Nat Rev Immunol 7:64. https://doi.org/10.1038/nri1998
O’shea JJ, Plenge R (2012) JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity 36:542–550. https://doi.org/10.1016/j.immuni.2012.03.014
O’shea JJ, Holland SM, Staudt LM (2013) JAKs and STATs in immunity, immunodeficiency, and cancer. N Engl J Med 368:161–170. https://doi.org/10.1056/NEJMra1202117
O’Shea JJ, Schwartz DM, Villarino AV, Gadina M, McInnes IB, Laurence A (2015) The JAK-STAT pathway: impact on human disease and therapeutic intervention. Ann Rev Med 66:311–328. https://doi.org/10.1146/annurev-med-051113-024537
Palm NW, Medzhitov R (2009) Pattern recognition receptors and control of adaptive immunity. Immunol Rev 227:221–233. https://doi.org/10.1111/j.1600-065X.2008.00731.x
Pan Y et al (2013) Targeting JNK by a new curcumin analog to inhibit NF-kB-mediated expression of cell adhesion molecules attenuates renal macrophage infiltration and injury in diabetic mice. PLoS One 8:e79084. https://doi.org/10.1371/journal.pone.0079084
Panahi Y, Sahebkar A, Parvin S, Saadat A (2012) A randomized controlled trial on the anti-inflammatory effects of curcumin in patients with chronic sulphur mustard-induced cutaneous complications. Ann Clin Biochem 49:580–588. https://doi.org/10.1258/acb.2012.012040
Panahi Y, Saadat A, Beiraghdar F, Sahebkar A (2014) Adjuvant therapy with bioavailability-boosted curcuminoids suppresses systemic inflammation and improves quality of life in patients with solid tumors: a randomized double-blind placebo-controlled trial. Phytother Res 28:1461–1467. https://doi.org/10.1002/ptr.5149
Panahi Y, Ghanei M, Bashiri S, Hajihashemi A, Sahebkar A (2015) Short-term curcuminoid supplementation for chronic pulmonary complications due to sulfur mustard intoxication: positive results of a randomized double-blind placebo-controlled trial. Drug Res 65:567–573. https://doi.org/10.1055/s-0034-1389986
Panahi Y, Hosseini MS, Khalili N, Naimi E, Simental-Mendía LE, Majeed M, Sahebkar A (2016a) Effects of curcumin on serum cytokine concentrations in subjects with metabolic syndrome: a post hoc analysis of a randomized controlled trial. Biomed Pharm 82:578–582. https://doi.org/10.1016/j.biopha.2016.05.037
Panahi Y, Kianpour P, Mohtashami R, Jafari R, Simental-Mendiá LE, Sahebkar A (2016b) Curcumin lowers serum lipids and uric acid in subjects with nonalcoholic fatty liver disease: a randomized controlled trial. J Cardiovasc Pharmacol 68:223–229. https://doi.org/10.1097/FJC.0000000000000406
Panahi Y, Khalili N, Sahebi E, Namazi S, Karimian MS, Majeed M, Sahebkar A (2017a) Antioxidant effects of curcuminoids in patients with type 2 diabetes mellitus: a randomized controlled trial. Inflammopharmacology 25:25–31. https://doi.org/10.1007/s10787-016-0301-4
Panahi Y, Kianpour P, Mohtashami R, Jafari R, Simental-Mendía LE, Sahebkar A (2017b) Efficacy and safety of phytosomal curcumin in non-alcoholic fatty liver disease: a randomized controlled trial. Drug Res 67:244–251. https://doi.org/10.1055/s-0043-100019
Park C-S (2010) Eosinophilic bronchitis, eosinophilia associated genetic variants, and notch signaling in asthma. Allergy Asthma Immunol Res 2:188–194. https://doi.org/10.4168/aair.2010.2.3.188
Rahimnia A-R, Panahi Y, Alishiri G, Sharafi M, Sahebkar A (2015) Impact of supplementation with curcuminoids on systemic inflammation in patients with knee osteoarthritis: findings from a randomized double-blind placebo-controlled trial. Drug Res 65:521–525. https://doi.org/10.1055/s-0034-1384536
Sahebkar A (2013) Why it is necessary to translate curcumin into clinical practice for the prevention and treatment of metabolic syndrome? Biofactors 39:197–208. https://doi.org/10.1002/biof.1062
Sahebkar A, Cicero AFG, Simental-Mendía LE, Aggarwal BB, Gupta SC (2016) Curcumin downregulates human tumor necrosis factor-α levels: a systematic review and meta-analysis of randomized controlled trials. Pharmacol Res 107:234–242. https://doi.org/10.1016/j.phrs.2016.03.026
Samadian F et al (2017) Evaluation of curcumin’s effect on inflammation in hemodialysis patients. Clin Nutr ESPEN 22:19–23. https://doi.org/10.1016/j.clnesp.2017.09.006
Schindler C, Levy DE, Decker T (2007) JAK-STAT signaling: from interferons to cytokines. J Biol Chem 282:20059–20063. https://doi.org/10.1074/jbc.R700016200
Seger R, Krebs EG (1995) The MAPK signaling cascade. FASEB J 9:726–735
Sen R, Baltimore D (1986) Inducibility of κ immunoglobulin enhancer-binding protein NF-κB by a posttranslational mechanism. Cell 47:921–928. https://doi.org/10.1016/0092-8674(86)90807-X
Sha WC, Liou H-C, Tuomanen EI, Baltimore D (1995) Targeted disruption of the p50 subunit of NF-κB leads to multifocal defects in immune responses. Cell 80:321–330. https://doi.org/10.1016/0092-8674(95)90415-8
Shakibaei M, John T, Schulze-Tanzil G, Lehmann I, Mobasheri A (2007) Suppression of NF-kappaB activation by curcumin leads to inhibition of expression of cyclo-oxygenase-2 and matrix metalloproteinase-9 in human articular chondrocytes: implications for the treatment of osteoarthritis. Biochem Pharmacol 73:1434–1445. https://doi.org/10.1016/j.bcp.2007.01.005
Shelmadine BD et al (2017) A pilot study to examine the effects of an anti-inflammatory supplement on eicosanoid derivatives in patients with chronic kidney disease. J Altern Complement Med 23:632–638. https://doi.org/10.1089/acm.2016.0007(New York, NY)
Soetikno V et al (2011) Curcumin ameliorates macrophage infiltration by inhibiting NF-κB activation and proinflammatory cytokines in streptozotocin induced-diabetic nephropathy. Nutr Metab 8:35. https://doi.org/10.1186/1743-7075-8-35
Soltani A, Salmaninejad A, Jalili-Nik M, Soleimani A, Javid H, Hashemy SI, Sahebkar A (2019) 5′-Adenosine monophosphate-activated protein kinase: a potential target for disease prevention by curcumin. J Cell Physiol 234:2241–2251. https://doi.org/10.1002/jcp.27192
Soveyd N, Abdolahi M, Djalali M, Hatami M, Tafakhori A, Sarraf P, Honarvar NM (2018) The combined effects of omega -3 fatty acids and nano-curcumin supplementation on intercellular adhesion molecule-1 (ICAM-1) gene expression and serum levels in migraine patients. CNS Neurol Disord Drug Targets 16:1120–1126. https://doi.org/10.2174/1871527317666171213154749
Stanimirovic D, Satoh K (2000) Inflammatory mediators of cerebral endothelium: a role in ischemic brain inflammation. Brain Pathol 10:113–126. https://doi.org/10.1111/j.1750-3639.2000.tb00248.x
Starr R et al (1997) A family of cytokine-inducible inhibitors of signalling. Nature 387:917. https://doi.org/10.1038/43206
Stat CJK, Egf P (2005) The Jak-STAT pathway in rheumatoid arthritis. J Rheumatol 32:1650–1653
Stevenson W, Chauhan SK, Dana R (2012) Dry eye disease: an immune-mediated ocular surface disorder. Arch Ophthalmol 130:90–100. https://doi.org/10.1001/archophthalmol.2011.364(Chicago, Ill : 1960)
Suresh S, Sankar P, Telang AG, Kesavan M, Sarkar SN (2018) Nanocurcumin ameliorates Staphylococcus aureus-induced mastitis in mouse by suppressing NF-κB signaling and inflammation. Int Immunopharmacol 65:408–412. https://doi.org/10.1016/j.intimp.2018.10.034
Takeuchi O, Akira S (2010) Pattern recognition receptors and inflammation. Cell 140:805–820. https://doi.org/10.1016/j.cell.2010.01.022
Tamiya T, Kashiwagi I, Takahashi R, Yasukawa H, Yoshimura A (2011) Suppressors of cytokine signaling (SOCS) proteins and JAK/STAT pathways: regulation of T-cell inflammation by SOCS1 and SOCS3. Arterioscler Thromb Vasc Biol 31:980–985. https://doi.org/10.1161/ATVBAHA.110.207464
Teymouri M, Pirro M, Johnston TP, Sahebkar A (2017) Curcumin as a multifaceted compound against human papilloma virus infection and cervical cancers: a review of chemistry, cellular, molecular, and preclinical features. Biofactors 43:331–346. https://doi.org/10.1002/biof.1344
Usharani P, Mateen AA, Naidu MU, Raju YS, Chandra N (2008) Effect of NCB-02, atorvastatin and placebo on endothelial function, oxidative stress and inflammatory markers in patients with type 2 diabetes mellitus: a randomized, parallel-group, placebo-controlled, 8-week study. Drugs R&D 9:243–250. https://doi.org/10.2165/00126839-200809040-00004
Wei L, Laurence A, Elias KM, O’Shea JJ (2007) IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. J Biol Chem 282:34605–34610. https://doi.org/10.1074/jbc.M705100200
Xiao C, Ghosh S (2005) NF-kappaB, an evolutionarily conserved mediator of immune and inflammatory responses. Adv Exp Med Biol 560:41–45. https://doi.org/10.1007/0-387-24180-9_5
Xie L, Li X-K, Funeshima-Fuji N, Kimura H, Matsumoto Y, Isaka Y, Takahara S (2009) Amelioration of experimental autoimmune encephalomyelitis by curcumin treatment through inhibition of IL-17 production. Int Immunopharmacol 9:575–581. https://doi.org/10.1016/j.intimp.2009.01.025
Xu Y, Liu L (2017) Curcumin alleviates macrophage activation and lung inflammation induced by influenza virus infection through inhibiting the NF-kappaB signaling pathway. Influenza Other Respir Viruses 11:457–463. https://doi.org/10.1111/irv.12459
Yang X et al (2017) Curcumin reduces lung inflammation via Wnt/β-catenin signaling in mouse model of asthma. J Asthma 54:335–340. https://doi.org/10.1080/02770903.2016.1218018
Yekollu SK, Thomas R, O’Sullivan B (2011) Targeting curcusomes to inflammatory dendritic cells inhibits NF-kappaB and improves insulin resistance in obese mice. Diabetes 60:2928–2938. https://doi.org/10.2337/db11-0275
Zhang C, Li B, Zhang X, Hazarika P, Aggarwal BB, Duvic M (2010a) Curcumin selectively induces apoptosis in cutaneous T-cell lymphoma cell lines and patients’ PBMCs: potential role for STAT-3 and NF-kappaB signaling. Journal Investig Dermatol 130:2110–2119. https://doi.org/10.1038/jid.2010.86
Zhang L et al (2010b) Demethoxycurcumin, a natural derivative of curcumin attenuates LPS-induced pro-inflammatory responses through down-regulation of intracellular ROS-related MAPK/NF-κB signaling pathways in N9 microglia induced by lipopolysaccharide. Int Immunopharmacol 10:331–338. https://doi.org/10.1016/j.intimp.2009.12.004
Zhang X, Wu J, Ye B, Wang Q, Xie X, Shen H (2016) Protective effect of curcumin on TNBS-induced intestinal inflammation is mediated through the JAK/STAT pathway. BMC Complement Altern Med 16:299. https://doi.org/10.1186/s12906-016-1273-z
Zhang N et al (2017) Effect of curcumin on acute spinal cord injury in mice via inhibition of inflammation and TAK1 pathway. Pharmacol Rep 69:1001–1006. https://doi.org/10.1016/j.pharep.2017.02.012
Zhao H-M et al (2016) Curcumin suppressed activation of dendritic cells via JAK/STAT/SOCS signal in mice with experimental colitis. Front Pharmacol 7:455. https://doi.org/10.3389/fphar.2016.00455
Zhou L et al (2007) IL-6 programs T H-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol 8:967. https://doi.org/10.1038/ni1488
Zhu J, Yamane H, Paul WE (2010) Differentiation of effector CD4 T cell populations. Annu Rev Immunol 28:445–489. https://doi.org/10.1146/annurev-immunol-030409-101212
Funding
None.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest in this study.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised to update the 5th author name.
Rights and permissions
About this article
Cite this article
Kahkhaie, K.R., Mirhosseini, A., Aliabadi, A. et al. Curcumin: a modulator of inflammatory signaling pathways in the immune system. Inflammopharmacol 27, 885–900 (2019). https://doi.org/10.1007/s10787-019-00607-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-019-00607-3