Abstract
Achillea biebersteinii is a perennial aromatic herb that grows in the Mediterranean area. The leaves of this plant are used in foods as bittering and appetizing agents. In folk medicine, it is used for the treatment of stomachache and abdominal pain. In this study, the analgesic effect of A. biebersteinii methanolic flower extract was tested in three pain models, namely: writhing, tail-flick and paw-licking (formalin) tests. A. biebersteinii extract inhibited abdominal cramps produced by acetic acid. The effect of A. biebersteinii was better than that of 70 mg/kg indomethacin. In tail flick, A. biebersteinii extract increased latency at 30 min and was as effective as 100 mg/kg diclofenac sodium. In formalin test, A. biebersteinii extracts decreased paw-licking and flinching response in early and late phases. Atropine blocked the action of A. biebersteinii extract (300 mg/kg) in the late phase of formalin test as well as in writhing and tail-flick tests. GC–MS analysis revealed that ascaridole and iso-ascaridole were the main constituents of A. biebersteinii flower extract. In conclusion, this study shows for the first time that the antinociceptive effect of A. biebersteinii is mediated by the cholinergic receptor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction

Achillea was named after Achilles who used yarrow to heal the wounds of the soldiers in the Trojan War of the Iliad (Saeidnia et al. 2011). Achillea species are distributed in the Mediterranean area (Al-Jaber et al. 2014). Achillea biebersteinii Afan. (Asteraceae) is a non-woody perennial, aromatic, herbaceous plant, 20–50 cm long. The plant usually grows in patches. The leaves of this plant are up to 10 cm long, dissected to a feathery structure into narrow segments. Heads appear as dense flat topped inflorescence yellow aggregation (Al-Eisawi 1998).
Achillea biebersteinii Afan has many biological activities including wound healing (Akkol et al. 2011), anti-ulcer (Abd-Alla et al. 2016), anti-bacterial (Hammad et al. 2013), anti-oxidant (Mirahmadi and Norouzi 2017; Mazandarani et al. 2015), anti-gout (Hudaib et al. 2011), anti-cancer (Ghavami et al. 2010), anti-epimastigote (Saeidnia et al. 2005), anti-cholinesterase (Sevindik et al. 2015), anti-inflammatory (Mohamed et al. 2016), hypoglycemic (Ahmadi et al. 2017), neuroprotective (Alikhanzade et al. 2014) and anti-platelet effects (Al-Jaber et al. 2014), In addition, it is effective in treating endometriosis (Demirel et al. 2014).
Many Achillea species were reported to have antinociceptive effects such as A. nobilis subsp. neilreichii (Karabay-Yavasoglu et al. 2007), Achillea umbellate (Radulovic et al. 2012), A. wilhelmsii, A. Phrygia, A. vermicularis, A. setacea(Kupeli et al. 2007) and A. aleppica DC. subsp. aleppica (Iscan et al. 2006). In folk medicine, A. biebersteinii is taken as tea for abdominal pain and stomachache (Erbay et al. 2017). Also, a combination of this plant and mint leaves was used in bioactive formulations for the same purpose (Zengin et al. 2017). To the best of our knowledge, there is no previous comprehensive study that has evaluated the effectiveness of A. biebersteinii in reducing pain and its mechanism of action. The present study investigates the antinociceptive effect of flower extract of this plant and the possible mechanism involved in its action.
Methods
Plant collection, identification and extraction
Achillea biebersteinii flowers were collected from Al-Bukaan (Salt region, Jordan) during May 2015 and authenticated by Prof. Barakat Abu-Irmaileh, Faculty of Agriculture at the University of Jordan.
A flower extract of A. biebersteinii was prepared by soaking coarsely grinded, dried flowers in 96% methanol. The solvent was evaporated in a rotary evaporator using reduced pressure at 45 °C as a maximum temperature. Then, it was kept at − 20 °C. The extract was dissolved in sterile distilled water immediately before use.
Drugs
Methanol was obtained from Tedia (USA). Na2SO4, Alkane standard (C8–C30) and atropine were purchased from Sigma-Aldrich (USA). Indomethacin was obtained from DAD (Jordan) and diclofenac sodium was from Diclopan (Taiwan). All drugs were prepared immediately before use by being dissolved in sterile normal saline and administered intraperitoneally (i.p).
Experimental animals
Female BALB/c mice (20–25 g) were obtained from Al-Ahliyya Amman University. Experimental animals were maintained at 23 ± 2 °C, 12 h light/12 h dark cycle. Food pellets and water were available ad libitum. At least 2 h before the experiment, the animals were kept in the laboratory for adaptation. Each mouse was only used once. In all experiments, mice were treated i.p with vehicle (control), standard drug 300 mg/kg, 400 mg/kg or 500 mg/kg A. biebersteinii extract 30 min prior to test performance. All followed procedures comply with The Jordanian Animal Welfare By-Law No. (11) of the year 2010 and the International Association for the Study of Pain (IASP) Guidelines for the Use of Animals in Research and were approved on 5 July 2015 by the ethical committee for research on animals at Al-Ahliyya Amman University (No. AAU-2015,7/5).
Writhing test
Writhing test was performed by injecting 1% acetic acid i.p. The animals were immediately placed in transparent cages. Mouse behavior was video recorded. The number of writhes was counted for 20 min starting after 10 min from acetic acid injection. Percentage inhibition was calculated using the formula:
Animals were randomly divided into six groups each consisting of eight mice. Indomethacin (70 mg/kg) was used as a standard drug in the writhing test. Atropine (5 mg/kg), a cholinergic antagonist, was administered 15 min before the plant extract.
Tail-flick test
The tail-flick test was assessed by immersing the tail in a water bath at 55 ± 1 °C. Each group consisted of 14 mice. The time from immersing the tail till producing the first flick was recorded. A cutoff time was considered 10 s as in Abul-Husn et al. (2007). Diclofenac sodium (100 mg/kg) was used as a standard drug in the tail-flick test. Atropine (5 mg/kg) was administered 15 min before the plant extract.
Paw-licking test (formalin test)
The formalin test was carried out by injecting 20 µl of 2.5% formalin intraplantarly. The total time spent in licking, shaking, lifting and flinching of the injected paw was recorded in phase I (0–5 min) and phase II (25–30 min) after formalin injection. The percentage inhibition was calculated using the formula:
Animals were randomly divided into six groups, each consisting of nine mice. Indomethacin (50 mg/kg) was used as a standard drug in the paw-licking test. Atropine (5 mg/kg) was administered 15 min before the plant extract.
Gas chromatography–mass spectrometric (GC–MS) analysis
The methanolic extract of A. biebersteinii was analyzed using a Varian CP-3800 GC/MS/MS-220 (Saturn, the Netherlands) system, equipped with a DB-5 GC capillary column (95% dimethyl polysiloxane, 5% diphenyl, 30 m × 0.25 mm i.d., 0.25 μm film 1 thicknesses). Using an automatic injector in the split mode, an aliquot of 1 μL of methanolic extract was injected into the GC. The column temperature was kept constant (isothermal) at 60 °C for 1 min, ramped to a final temperature of 246 °C at 3 °C min−1 and held isothermal constantly for a further 3 min. The ionization voltage was 70 eV, while the ionization source was 180 °C. Helium, 1.0 mL min−1, was used as a carrier gas. Quantitative analysis was performed on Thermos Focus GC equipped with a split–splitless injector (split ratio 1:50), a flame ionization detector, and an optima-5 fused silica capillary column (5% diphenyl, 95% dimethyl polysiloxane, 30 m × 0.25 mm, 0.25 film thickness) and under the same conditions described for the GC/MS analysis part. Identification of the chemical constituents in the analyzed methanolic extract was performed by built-in libraries including Notational Institution of Standards and Technology Co. and Wiley Registry of Mass Spectral Data (USA) and by comparing their calculated retention indices relative to (C8–C30) n-alkanes with literature values measured on columns of identical polarity and/or co-injection of pure authentic compounds.
Statistical analysis
The statistical significance of differences between groups was assessed by one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test using GraphPad prism version 6 for all measured parameters. A p value less than 5% was considered significant.
Results
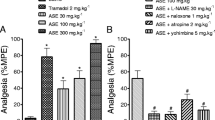
In writhing test, 300, 400 and 500 mg/kg A. biebersteinii methanolic extracts inhibited abdominal cramps by 77.6%, 63.4%, and 55.0%, respectively. The lowest dose (300 mg/kg) of the extract was more efficient than 70 mg/kg indomethacin (59.4% inhibition) in reducing abdominal cramps. Also, the lowest dose (300 mg/kg) had more effect than the highest dose (500 mg/kg). Atropine blocked the action of A. biebersteinii (300 mg/kg) partially and significantly (Fig. 1).
In the tail-flick test, A. biebersteinii extracts (300 and 400 mg/kg) increased latency at 30 min; but not after 1.5 h of treatment. Both doses were not statistically different from 100 mg/kg diclofenac sodium. Atropine blocked the action of A. biebersteinii (300 mg/kg) significantly (Fig. 2).
In paw-licking test (formalin test), A. biebersteinii extracts decreased flinching and paw licking in early and late phases of the test. The percentage inhibition of paw licking in the early phase was 50.2, 49.6, 45.1 and 41.0% for 300, 400 and 500 mg/kg A. biebersteinii extract and 50 mg/kg indomethacin, respectively (Fig. 3). In the late phase of formalin test, the percentage inhibition of paw licking was 68.1, 68.1, 46.8 and 55.9% for 300, 400 and 500 mg/kg A. biebersteinii extract and 50 mg/kg indomethacin, respectively (Fig. 4). The cholinergic receptor antagonist atropine reversed the antinociceptive action of this plant in the late phase only (Figs. 3, 4).
GC–MS analysis
Twenty-four compounds were identified in the methanolic extract of A. biebersteinii flowers collected from Jordan (Table 1). Ascaridole 43.22% and iso-ascaridole 37.87% were the main constituents.
Discussion
The use of plants is gaining renewed interest as potential sources of lead compounds in the search for new medications to treat different diseases or as sources for new drugs. In our work, A. biebersteinii flower extracts reduced the number of writhes produced by 1% acetic acid significantly. The percentage inhibition of 300 mg/kg A. biebersteinii extract was (77.6% inhibition) better than that of 70 mg/kg indomethacin (59.4% inhibition). Similar results were obtained by Pires et al. (2009) in which the hydroalcoholic extracts of A. millefolium aerial parts (500 and 1000 mg/kg) decreased acetic acid-induced abdominal contractions by 65 and 23%, respectively. Polar extract of A. fragrantissima (produced by 400 mg/kg) was more effective, with 55% inhibition, than non-polar extract in reducing abdominal cramps (Abdel-Rahman et al. 2015). In p-benzoquinone writhing test, the ethanol extracts of A. wilhelmsii, A. setacea, A. phrygia and A. vermicularis showed antinociceptive activity at 500 mg/kg dose (Kupeli et al. 2007). Also, the essential oils of different species of Achillea such as A.aleppica DC. subsp. aleppica (Iscan et al. 2006) and A. umbellata (Radulovic et al. 2012) exhibited antinociceptive activity in writhing tests. However, these essential oils were less potent than 200 mg/kg acetylsalicylic acid.
In our study, 300 and 400 mg/kg doses of A. biebersteinii methanolic extracts increased latency of tail-flick test after 30 min, but not after 1.5 h. Similar results were obtained by the aqueous and methanolic extracts of A. ageratum that exhibited analgesic properties (Garcia et al. 1997). This effect was more potent with the methanolic extract in the tail-flick assay where the estimated ED50 was 187.62 ± 37.96 (Garcia et al. 1997). On the other hand, the ethanolic extract of A. nobilis subsp. neilreichii (Kerner) Formanek (400 mg/kg) had no significant activity in the tail-flick test (Karabay-Yavasoglu et al. 2007).
Formalin test is sensitive to various classes of analgesic drugs. Two distinct phases of high licking activity can be observed. Phase I (neurogenic pain) starts within seconds after injecting formalin in the paw. This early phase is due to direct chemical activation of nociceptive primary afferent fibers. The late phase or phase II lasts from 20 to 30 min after the injection of formalin. Phase II is also known as inflammatory pain and reflects ongoing activity in primary afferents and central sensitization of spinal cord circuits secondary to the inputs that occurred during phase I. Opioids inhibit both phases of the formalin test equally, while many non-steroidal anti-inflammatory drugs and corticosteroids inhibit only the second phase (Abotsi et al. 2016). In the present work, all tested doses of A. biebersteinii extract decreased licking time significantly in both the early (neurogenic) and late (inflammatory) phases of formalin test. A. nobilis subsp. neilreichii flower heads extract exhibited an antinociceptive effect during the late phase of the formalin test (100, 200 and 400 mg/kg, i.p.), but not during the early phase (Karabay-Yavasoglu et al. 2007). The hydroalcohol extracts of A. millefolium (500 and 1000 mg/kg) aerial parts without flowers did not exhibit any effect on the early or late responses in formalin test (Pires et al. 2009).
In our work, the action of A. biebersteinii extract in writhing test, tail-flick test and the late phase of formalin test was mediated by interaction with cholinergic receptor. This was evident by abolishing its effect when atropine, a non-specific muscarinic antagonist, was added. To our best knowledge, our study represents the first report on antinociceptive activity of A. biebersteinii and its interaction with cholinergic receptor.
In the writhing test, tail-flick test and phase II of paw-licking test, the activity of the lower dose (300 mg/kg) was more than the effect in higher doses (400 or 500 mg/kg). Plant extracts contain many phytochemicals having complex interactions such as synergistic, additive or antagonistic interactions. At high concentrations, some components of the extract may bind nonspecifically to receptors thereby decreasing the interaction of the active compound with the receptor involved in analgesic action. A similar trend was observed by Pires et al. (2009), in which the antinociceptive effect of A. millefolium high dose (1000 mg/kg) was less than that of the lower dose (500 mg/kg) in the writhing test.
In vitro, anti-cholinesterase activity of A. biebersteinii was reported (Sevindik et al. 2015). The inhibition of cholinesterase enzyme results in accumulation of acetylcholine which may exert an antinociceptive action. This analgesic mechanism of action was reported for several cholinesterase inhibitors such as physostigmine (Mojtahedin et al. 2009), amphetamine and its derivatives (Rezin et al. 2012), khat (Afify et al. 2017) and PhKv toxin (Rigo et al. 2017). However, the side effects of anti-cholinesterase agents may limit their usefulness as analgesic agents (Lauretti 2015). Further studies are needed to explore whether A. biebersteinii exerts anti-cholinesterase activity in vivo and if this mechanism contributes to the antinociceptive activity of this plant.
In our study, GC–MS analysis revealed that ascaridole (43.22%) and iso-ascaridole (37.87%) were the major constituents of A. biebersteinii flower extract. Our results agree with the findings of Hamad (2012) who reported that the major constituents in A. biebersteinii collected from Jordan were ascaridole (61.39%), trans-dihydro-H-terpinyl acetate (14.26%), p-cymene (8.1%) and H–terpinene (4.88%). Similarly, Bader et al. (2003) reported that cis-ascaridole, p-cymene, carvenone oxide and camphor were the major compounds in the oil of the flowering aerial parts of A. biebersteinii collected from Jordan. Using HPLC–MS, Hammad et al. (2013) found that quercetin 3-β-d-glucoside and ferulic acid were the major constituents in aqueous and hydro-ethanolic extract of Jordanian A. biebersteinii. Differences in extract preparation and analysis methods account for differences in the findings between Hammad et al. (2013) and the present study.
In folk medicine, A. biebersteinii is taken orally as herbal tea (Erbay et al. 2017). In our study, methanol was used for extraction of A. biebersteinii flowers by the cold method. Similar to the aqueous extract, the methanolic extract contains hydrophilic compounds. However, differences in composition between hot aqueous and alcoholic extracts are expected to exist. According to Hammad et al. (2013), the aqueous extract contained less phenolics compared to 70% ethanolic extract. Another difference between the present study and the ethnobotanical use of this plant is the route of administration. This study utilized i.p route which has differences in the pharmacokinetics of the tested extract from the oral route. Orally administered compounds are subjected to chemical modifications by gut bacteria. In addition, their absorption varies according to their chemical nature. Therefore, clinical studies are needed to test the pain-relieving efficiency of A. biebersteinii in humans.
The antinociceptive activity of A. biebersteinii can be attributed to several active constituents isolated from this plant such as its main constituent, the monoterpene ascaridole (Mohammadhosseini et al. 2017). In fact, ascaridole exhibited antagonistic effect on the N-methyl-d-aspartate (NMDA) receptor that is responsible for central sensitization and chronic pain (Calado et al. 2015). Other constituents in A. biebersteinii flower extract have been well documented for analgesic effects like eugenol (Park et al. 2011), p-cymene (Bonjardim et al. 2012) and carvacrol (Melo et al. 2012). Further work is needed to study the interaction of A. biebersteinii flower extract with NMDA receptors and to investigate the antinociceptive effect and mechanism of action of the essential oil of A. biebersteinii.
Conclusion
The results of our study indicate the involvement of cholinergic receptor in the antinociceptive action of A. biebersteinii. Further studies are needed to clarify the subtypes of muscarinic receptors (M1–M5) involved in the antinociceptive activity of this plant.
References
Abd-Alla HI, Shalaby NMM, Hamed MA, El-Rigal NS, Al-Ghamdi SN, Bouajila J (2016) Phytochemical composition, protective and therapeutic effect on gastric ulcer and a-amylase inhibitory activity of Achillea biebersteinii Afan. Arch Pharmacol Res 39(1):10–20
Abdel-Rahman RF, Alqasoumi SIB, El-Desoky AH, Solimand GA, Paré PW, Hegazy MEF (2015) Evaluation of the anti-inflammatory, analgesic and anti-ulcerogenic potentials of Achillea fragrantissima (Forssk.). South Afr J Bot 98:122–127
Abotsi WKM, Lamptey SB, Afrane S, Boakye-Gyasi E, Umoh RU, Woode E (2016) An evaluation of the anti-inflammatory, antipyretic and analgesic effects of hydroethanol leaf extract of Albizia zygia in animal models. Pharm Biol 55:338–348
Abul-Husn NS, Sutak M, Milne B, Jhamandas K (2007) Augmentation of spinal morphine analgesia and inhibition of tolerance by low doses of mu- and delta-opioid receptor antagonists. Br J Pharmacol 151(6):877–887
Afify EA, Alkreathy HM, Ali AS, Alfaifi HA, Khan LM (2017) Characterization of the antinociceptive mechanisms of khat extract (Catha edulis) in Mice. Front Neurol 8:69
Ahmadi K, Wassim A, Ream N (2017) Evaluation of hypoglycemic effect of Achillea biebersteinii Afan., growing in Syria, in induced diabetic rats. Int J Pharm Phytochem Res 9(2):215–222
Akkol EK, Koca U, Pesin I, Yilmazer D (2011) Evaluation of the wound healing potential of Achillea biebersteinii Afan (Asteraceae) by in vivo excision and incision models. Evid Based Complement Altern Med. https://doi.org/10.1093/ecam/nep039
Al-Eisawi DM (1998) Field guide to wild flowers of Jordan and neighbouring countries, 1st edn. Jordan Press Foundation “Al Rai”, Amman
Alikhanzade M, Tehranipour M, Khayatzade J (2014) The neuroprotective effect of alcoholic and aqueous extracts of Achillea biebersteinii leaves on spinal motoneurons denegation after sciatic nerve compression in rats. Adv Herb Med 1(1):8–16
Al-Jaber HI, Hammad HM, Al-Qudah MA, Abaza IF, Al-humaidi JYG, Abu-Zarga MH, Afifi FU (2014) Volatile oil composition and antiplatelet activity of Jordanian Achillea biebersteinii collected at different growth stages. J Essent Oil Bear Plants 17(4):584–598
Bader A, Flamini G, Cioni PL, Morelli I (2003) Essential oil composition of Achillea santolina L. and Achillea biebersteinii Afan. collected in Jordan. Flavour Fragr J 18(1):36–38
Bonjardim LR, Cunha ES, Guimarães AG, Santana MF, Oliveira MGB, Serafini MR, Araújo AAS, Antoniolli ÂR, Cavalcanti SCH, Santos MRV, Quintans-Júnior LJ (2012) Evaluation of the anti-inflammatory and antinociceptive properties of p-cymene in mice. Zeitschrift Fur Naturforschung C 67:15–21
Calado GP, Lopes AJ, Costa Junior LM, Lima F, Silva LA, Pereira WS, Amaral FM, Garcia JB, Cartágenes Mdo S, Nascimento FR (2015) Chenopodium ambrosioides L. reduces synovial inflammation and pain in experimental osteoarthritis. PLoS ONE 10(11):e0141886
Demirel MA, Suntar I, Ilhan M, Keles H, Akkol EK (2014) Experimental endometriosis remission in rats treated with Achillea biebersteinii Afan. histopathological evaluation and determination of cytokine levels. Eur J Obstet Gynecol Reprod Biol 175:172–177
Erbay MŞ, Anıl S, Melikoğlu G (2017) Plants used as painkiller in folk medicine in turkey-i stomachache. Marmara Pharm J 21(4):741–755
Garcia MD, Puerta R, Martinez S, Saenz MT (1997) Analgesic, antipyretic and antiinflammatory effects of Achillea ageratum. Phytother Res 11:376–379
Ghavami G, Sardari S, Shokrgozar MA (2010) Anticancerous potentials of Achillea species against selected cell lines. J Med Plants Res 4(22):2411–2417
Hamad YS (2012) Chemical composition and antimicrobial activity of the essential oils hydrodistilled from five Achillea species growing in Jordan. Msc. Thesis, The University of Jordan
Hammad HM, Albu C, Matar SA, Litescu SC, Al Jaber HI, Abualraghib AS, Afif FU (2013) Biological activities of the hydro-alcoholic and aqueous extracts of Achillea biebersteinii Afan. (Asteraceae) grown in Jordan. Afr J Pharm Pharmacol 7(25):1686–1694
Hudaib MM, Tawaha KA, Mohammad MK, Assaf AM, Issa AY, Alali FQ, Aburjai TA, Bustanji YK (2011) Xanthine oxidase inhibitory activity of the methanolic extracts of selected Jordanian medicinal plants. Pharm Mag 7:320–324
Iscan G, Kirimer N, Kurkcuoglu M, Arabaci T, Kupeli E, Baser KH (2006) Biological activity and composition of the essential oils of Achillea schischkinii Sosn. and Achillea aleppica Dc. subsp. aleppica. J Agric Food Chem 54:170–173
Karabay-Yavasoglu U, Karamenderes C, Baykan S, Apaydın S (2007) Antinociceptive and anti-inflammatory activities and acute toxicity of Achillea nobilis subsp. neilreichii extract in mice and rats. Pharm Biol 45:162–168
Kupeli E, Orhan I, Kusmenoglu S, Yesilada E (2007) Evaluation of anti-inflammatory and antinociceptive activity of five Anatolian Achillea species. Turk J Pharm Sci 4(2):89–99
Lauretti GR (2015) The evolution of spinal/epidural neostigmine in clinical application: thoughts after two decades. Saudi J Anest 9:71–81
Mazandarani M, Osia N, Ghafourian M (2015) Antioxidant activity and ethnopharmacological survey of Achillea biebersteinii Afan. in the treatment of dysmenorrhoea in traditional medicine of Golestan Province. Iran. Int J Women’s Health Reprod Sci 3(2):107–110
Melo FHC, Rios ERV, Rocha NFM, Citó MCO, Fernandes ML, de Sousa DP, de Vasconcelos SMM, de Sousa FCF (2012) Antinociceptive activity of carvacrol (5-isopropyl-2-methylphenol) in mice. J Pharm Pharmacol 64:1722–1729
Mirahmadi SF, Norouzi R (2017) Chemical composition, phenolic content, free radical scavenging and antifungal activities of Achillea biebersteinii. Food Biosci 18:53–59
Mohamed AE-HH, Mohamed NS, Hamed AR, Hegazy M-EF (2016) Anti-inflammatory activity of highly oxygenated terpenoids from Achillea biebersteinii Afan. Zeitschrift für Naturforschung C 71:429–432
Mohammadhosseini M, Sarker SD, Akbarzadeh A (2017) Chemical composition of the essential oils and extracts of Achillea species and their biological activities: a review. J Ethnopharmacol 199:257–315
Mojtahedin A, Tamaddonfard E, Zanbouri A (2009) Role of central muscarinic cholinergic receptors in the formalin-induced pain in rats. Indian J Pharmacol 41:144–147
Park S-H, Sim Y-B, Lee J-K, Kim S-M, Kang Y-J, Jung J-S, Suh H-W (2011) The analgesic effects and mechanisms of orally administered eugenol. Arch Pharm Res 34:501–507
Pires JM, Mendes FR, Negri G, Duarte-Almeida JM, Carlini EA (2009) Antinociceptive peripheral effect of Achillea millefolium L. and Artemisia vulgaris L.: both plants known popularly by brand names of analgesic drugs. Phytother Res 23(2):212–219
Radulovic NS, Dekic MS, Randelovic PJ, Stojanovic NM, Zarubica AR, Stojanovic-Radic ZZ (2012) Toxic essential oils: anxiolytic, antinociceptive and antimicrobial properties of the yarrow Achillea umbellata Sibth. et Sm. (Asteraceae) volatiles. Food Chem Toxicol 50(6):2016–2026
Rezin GT, Scaini G, Ferreira GK, Cardoso MR, Gonçalves CL, Constantino LS, Deroza PF, Ghedim FV, Valvassori SS, Resende WR, Quevedo J, Zugno AI, Streck EL (2012) Inhibition of acetylcholinesterase activity in brain and behavioral analysis in adult rats after chronic administration of fenproporex. Metab Brain Dis 27:453–458
Rigo FK, Rossato MF, Trevisan G, De Prá SD, Ineu RP, Duarte MB, de Castro Junior CJ, Ferreira J, Gomez MV (2017) PhKv a toxin isolated from the spider venom induces antinociception by inhibition of cholinesterase activating cholinergic system. Scand J Pain 17:203–210
Saeidnia S, Gohari AR, Kiuchi F, Honda G (2005) In vitro anti-epimastigote activity of some Iranian medicinal plants. Iran J Pharm Res 2:101–103
Saeidnia S, Gohari AR, Mokhber-Dezfuli N, Kiuchi F (2011) A review on phytochemistry and medicinal properties of the genus Achillea. DARU J Pharm Sci 19:173–186
Sevindik HG, Güvenalp Z, Yerdelen KÖ, Yuca H, Demirezer LÖ (2015) Research on drug candidate anticholinesterase molecules from Achillea biebersteinii Afan using by molecular docking and in vitro methods. Med Chem Res 24:3794–3802
Zengin G, Aktumsek A, Ceylan R, Uysal S, Mocan A, Guler GO, Mahomoodally F, Glamocilija J, Ciric A, Sokovic M (2017) Shedding the light on biological and chemical fingerprints of three Achillea species (A. biebersteinii, A. millefolium and A. teretifolia. Food Func 8(3):1152–1165
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Rights and permissions
About this article
Cite this article
Jaffal, S.M., Abbas, M.A. Antinociceptive action of Achillea biebersteinii methanolic flower extract is mediated by interaction with cholinergic receptor in mouse pain models. Inflammopharmacol 27, 961–968 (2019). https://doi.org/10.1007/s10787-018-0524-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-018-0524-7