Abstract
Sepsis is a systemic response to infection that can result in acute hepatic and splenic damage. Ziziphus spina-christi (L.) is a wild tree used as a medicinal plant by ancient Egyptians. However, little is known about the mechanism underlying its effects on sepsis. The current study investigated the protective effects of a Z. spina-christi leaf extract (ZSCLE) on liver and spleen damage in a male C57BL/6 mouse model of sepsis, induced by cecal ligation and puncture (CLP). Prior to CLP, ZSCLE was administered daily for five consecutive days via oral gavage at doses of 100, 200, or 300 mg/kg. The mice were euthanized 9 h after CLP, and oxidative stress markers were measured (myeloperoxidase, lipid peroxidation, nitric oxide, and reduced glutathione). In addition, we investigated histological changes, anti-oxidant enzyme activities (superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase), cytokine levels, protein expression of nuclear factor-κB and inducible nitric oxide synthase (iNOS), and mRNA levels of mitogen-activated protein kinase (8, 9, and 14), iNOS, tumor necrosis factor-α, and interleukin-1β. Our results indicated that ZSCLE significantly and dose-dependently inhibited sepsis-induced liver and spleen injury. These results suggest that ZSCLE could provide a therapeutic agent for sepsis by inducing anti-inflammatory and anti-oxidant effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sepsis is a life-threatening systemic inflammatory response to pathologic infection and is one of the most frequent reasons for intensive care treatment of hospitalized patients worldwide (Bacanli et al. 2016). Despite research advances, the full pathophysiology of sepsis and its role in the development of multi-organ dysfunction (MOD), and the related septic shock, are poorly understood (Osterbur et al. 2014). However, the progression of inflammation from a local to a systemic response involves activation of circulating leukocytes; these release proinflammatory cytokines such as interleukin (IL)-1, -6, -8, and tumor necrosis factor-alpha (TNF-α) into the blood (Rahim et al. 2017). Increased chemokine production is responsible for attracting leukocytes to the site of inflammation (Shi and Pamer 2011).
There is growing evidence that the oxidant/anti-oxidant imbalance arising from overproduction of free radicals plays a fundamental role in MOD (Zolali et al. 2015). Reactive oxygen species (ROS) are considered to be implicated in the development of sepsis (Zhou et al. 2012). ROS have pro-inflammatory effects that cause endothelial damage, production of chemotactic factors, leucocyte infiltration, cytokine release, mitochondrial dysfunction, lipid peroxidation, and DNA damage (Bacanli et al. 2016); all of these effects participate in free radical overload and oxidative stress (Andrades et al. 2011). Hence, anti-oxidants and alternative medicines can ameliorate sepsis-induced inflammation and tissue injury in two ways: (1) directly, by scavenging free radicals; and (2) indirectly, by enhancing the endogenous anti-oxidant defense system (Zolali et al. 2014). Several studies have confirmed that the administration of a natural anti-oxidant in a septic animal model produced a significantly favorable effect on the manifestations of sepsis (Xiao et al. 2012).
Ziziphus spina-christi (L.) (ZSC) belongs to the Rhamnaceae family and produces small, orange-yellow fruits. This plant grows wild in Egypt (especially in Sinai) and is commonly known as Nabka and Sidr in other Arabic countries (Michel et al. 2011). Phytochemical analysis has confirmed that ZSC contained essential oils such as geranyl acetate, methyl palmitate, and methyl stearate, alkaloids such as spinanine-A, tannins, phytosterols like beta-sitosterol, flavonoids such as quercetin derivatives, and saponins such as betulinic acid and triterpenoid sapogenins (Kadioglu et al. 2016). In traditional folk medicine, ZSC is used for its antibacterial, antiviral, anticathartic, diuretic, hypoglycemic, and tonic activities (Amin and Mahmoud-Ghoneim 2009; Michel et al. 2011; Mubaraki et al. 2017). However, no studies have focused on the beneficial effects of ZSC on sepsis.
Hence, the present study explored the therapeutic potential of ZSC leaf extract (ZSCLE) in a mouse model of sepsis induced by cecal ligation and puncture (CLP). This investigation was conducted to improve the understanding of the mechanisms underlying the use of ZSC as a traditional medicine, as well as to provide further information concerning the anti-inflammatory effects of this plant.
Methods
Chemicals and reagents
High-performance liquid chromatography-grade methanol, bovine serum albumin, and Tris–HCl were obtained from Sigma-Aldrich (St Louis, MO, USA). Nitroblue tetrazolium, phenazine methosulfate, hexadecyltrimethylammonium bromide, and O-dianisidine were obtained from Alfa Aesar (Tewksbury, MA, USA). PCR primers for the indicated genes were synthesized by Jena Bioscience (Jena, Germany). TNF-α and IL-1β enzyme-linked immunosorbent assay kits were obtained from R&D Systems (Minneapolis, MN, USA). Thiobarbituric acid was purchased from Merck (Darmstadt, Germany). Other reagents and dilutions were as follows: anti-nuclear factor-κB (NF-κB; 1:500) and anti-inducible nitric oxide synthase (iNOS; Santa Cruz Biotechnology, Santa Cruz, CA, USA); goat anti-rabbit IgG (1:2000; Life Technologies, Carlsbad, CA, USA); RNeasy Plus Mini kit (Qiagen, Valencia, CA, USA); Power SYBR® Green (Life Technologies); and alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, and lactate dehydrogenase kits (Biodiagnostic, Giza, Egypt).
Experimental animals
Male C57BL/6 mice (25–28 g, 8 weeks old) were obtained from the animal facility of Theodor Bilharz Research Institute (Giza, Egypt) and acclimatized to a standard environment (22 ± 2 °C) and a 12-h light/12-h dark cycle for 1 week; animals were provided with standard rodent chow and water ad libitum. The experimental procedures were approved by the Institutional Animal Ethics Committee at Helwan University (approval No. HU2017/Z/01), in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals, 8th edition.
Plant material and extraction procedure
ZSC leaves were purchased from a local spice dealer in Cairo, Egypt. The identity of this leaf was approved by a taxonomist (Botany Department, Faculty of Science, Helwan University, Egypt). The leaves were washed with water to remove dirt, and air-dried under shade. The dried leaves were crushed to a powder using an electrical miller. The resultant powder was extracted with 70% (w/v) methanol in the dark for 27 h at 25 °C. The collected extract was filtered using Whatman No-1 filter paper. The solvent was then evaporated off in a vacuum rotary evaporator (IKA, Germany). The remaining extract was then frozen at − 80 °C prior to lyophilization. The residue was dissolved in distilled water and stored at − 20 °C in a dark bottle until use. The levels of polyphenols and flavonoids in this ZSCLE were estimated using standard methods (Abdel Moneim 2013). The polyphenol content of ZSCLE ranged from 98.4 to 104.3 mg gallic acid equivalents/g extract, while the total flavonoid content ranged from 38.6 to 45.1 mg quercetin equivalents/g extract.
HPLC analysis
The polyphenolic and flavonoid compounds present in the ZSCLE were detected via a photo diode array (PDA) detector of a high-pressure liquid chromatography (HPLC; Perkin Elmer Series 200 liquid chromatography; PerkinElmer, USA) using the method described by Kadioglu et al. (2016). Briefly, HPLC was carried out with a Phenomenex C-18 column (4.6 mm × 250 mm, 5 μm), volume injection was 25 μL. Mobile phase consists of 2.0% acetic acid (solvent A) and acetonitrile (solvent B). The flow rate was set at 0.80 ml/min throughout the elution. HPLC operation was carried out at ambient temperature.
Protocol design
Sepsis induction in C57BL/6 mice was performed using the CLP procedure, according to Wan et al. (2016), and a small amount of stool was extruded to ensure the patency of the puncture sites. Healthy mice were randomly allocated to one of the following seven groups (14 mice/group): sham-operated and untreated; sham-operated and treated with ZSCLE (300 mg/kg); sepsis-induced; sepsis-induced and treated with ZSCLE (100 mg/kg); sepsis-induced and treated with ZSCLE (200 mg/kg); and sepsis-induced and treated with ZSCLE (300 mg/kg). Sham-operated mice were not subjected to CLP and were anaesthetized only to perform the laparotomy and caecum manipulation procedures. The animals were not fasted either pre- or post-operatively and had free access to water and food. In the ZSCLE-treated groups, the relevant dose was administrated orally (200 µl) for 5 consecutive days before surgery. Liver, spleen, and blood samples of seven mice were collected 9 h after CLP.
Survival study
Survival was monitored at least every 8 h for 3 days after operation. The mean survival times of each study group were analyzed via the Kaplan–Meier survival curve, which was drawn by Prism v.6.
Protein determination
Protein determinations were carried out according to the method of Lowry et al. (1951) using bovine serum albumin as the standard.
Liver function parameters
Serum levels of alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, and lactate dehydrogenase were determined using standard diagnostic kits.
Myeloperoxidase (MPO) activity measurement
MPO activity was measured as a marker of leucocyte migration and aggregation. Liver or spleen tissue specimens were homogenized (1:20, w/v) in ice-cold 50-mM potassium phosphate buffer (pH 6.0) containing hexadecyltrimethylammonium bromide (0.5%). The resultant homogenate was frozen and thawed three times, and then centrifuged at 3000g for 25 min at 4 °C. The activity of MPO in the specimen was measured using the O-dianisidine method (Bradley et al. 1982). The change in absorbance was determined spectrophotometrically at 460 nm. MPO activity data were expressed in units per milligram of protein.
Lipid peroxidation measurement
Malondialdehyde (MDA) levels in the liver and spleen were estimated as a marker of lipid peroxidation (Ohkawa et al. 1979). Briefly, 500 µl of each supernatant was mixed with a reaction mixture containing 0.22% sulfuric acid, 0.67% thiobarbituric acid, and distilled water. The resulting mixture was boiled for 30 min at 95 °C and then cooled at room temperature. Then, the samples were centrifuged at 1000g for 15 min and the absorbance of the supernatant was spectrophotometrically (V-630; Jasco, Japan) measured at 540 nm. Data are expressed as nanomoles MDA per milligram of protein.
Nitrite/nitrate estimation
The level of nitrite/nitrate (nitric oxide; NO) was estimated using a colorimetric method (Grisham et al. 1996). Briefly, 100 μl of each supernatant was reacted with 1000 ml Griess reagent at room temperature for 10 min in the dark. The resultant bright reddish purple azo dye was measured spectrophotometrically at 540 nm. A solution of known sodium nitrite concentration was used as a working standard. These data were expressed as micromoles per milligram of protein.
Estimation of anti-oxidant enzymes
Superoxide dismutase activity was determined in liver and spleen homogenates by monitoring the ability of the enzyme to inhibit the reduction in nitroblue tetrazolium dye by phenazine methosulfate (Nishikimi et al. 1972). Briefly, the sample (0.05 ml) was added to the reaction mixture (1.3 ml) consisting of phosphate buffer (0.1 M; pH 8.5) containing nitroblue tetrazolium (0.3 mM), reduced nicotinamide adenine dinucleotide (0.47 mM), and phenazine methosulfate (93 µM). The increase in absorbance was recorded for 5 min at 560 nm. Superoxide dismutase activity data were expressed as units per milligram of protein.
Catalase activity was measured by determining the rate of H2O2 decomposition at 240 nm (Aebi 1984). The decrease in absorbance was monitored for 180 s and the activity of catalase was defined as μM H2O2 decomposed/s/mg protein. Briefly, 0.1 ml of the homogenate was added to the reaction mixture (0.9 ml) containing phosphate buffer (50 mM, pH 7.0) and H2O2 (30 mM).
The method described by Ursini et al. (1985) was employed to determine the activity of glutathione peroxidase (GPx) indirectly by a coupled reaction with glutathione reductase (GR). GSH is oxidized to GSSG by GPx and recycled to its reduced form by GR. This reaction consumes reduced nicotinamide adenine dinucleotide phosphate (NADPH). The decrease in NADPH level (monitored at 340 nm) is therefore proportional to the GPx activity. Briefly, 0.05 ml of each sample was added to a reaction mixture (1.1 ml) containing 0.05-M Tris–HCl with 5-mM ethylenediaminetetraacetic acid (pH 7.6), GSH (24 µM), and NADPH (4.8 µM). GPx data were expressed as nanomoles NADPH oxidized/min/mg protein. GR activity was assayed by recording the decrease in absorbance at 340 nm that resulted from GR-mediated catalysis of GSSG in the presence of NADPH, which is oxidized to NADP− (Carlberg and Mannervik 1985). Briefly, 0.05 ml of the sample was mixed with a reaction mixture (1.2 ml) of phosphate buffer/ethylenediaminetetraacetic acid (100 mM/2 mM; pH 7.5), GSSG (20 mM), and NADPH (2 mM). The change in absorbance was recorded at 340 nm for 3 min and the GR activity data were expressed as micromoles GSSG reduced/min/mg protein.
TNF-α and IL-1β determinations
The levels of TNF-α and IL-1β in the liver and spleen tissue supernatants were assayed using commercial enzyme-linked immunosorbent assay kits, in accordance with the manufacturer’s instructions.
Histological examination
To assess liver and spleen damage, histological examinations were performed. Briefly, liver and spleen tissues were fixed in buffered 10% formaldehyde and then embedded in paraffin. The embedded tissue samples were sectioned (5 μm) and stained with haematoxylin and eosin to examine general histological features. A semi-quantitative scoring system was used. For liver tissue evaluation, hepatocyte degeneration and portal/lobular inflammation were scored (each 0–3), whereas the spleen sample was scored for the enlargement of B- and T-lymphocyte areas in red and white pulps (0–3) and necrosis/apoptosis (each 0–2). Scoring of each tissue sample represented the mean score of ten different fields. The stained tissue sections were evaluated under a light microscope (Eclipse E200-LED; Nikon, Kawasaki, Japan) at ×400 magnification.
Immunohistochemistry
Liver and spleen tissue blocks were prepared as described in the previous paragraph and then sectioned (4 μm) and blocked with methanol containing H2O2 (0.1%) before incubating overnight at 4 °C with either anti-NF-κB (1:500) or anti-iNOS (1:500). The sections were then washed three times for 5 min with phosphate-buffered saline containing Tween 20 (PBST), and incubated with goat anti-rabbit IgG (1:2000) for 2 h at 25 °C. Next, the sections were washed three times for 5 min with PBST, and then incubated with 3,3′-diaminobenzidine tetrahydrochloride containing H2O2 (0.1%) in Tris–HCl buffer (0.05 M; pH 7.6; Life Technologies) for 5 min at 25 °C. After again washing the sections three times for 5 min with PBST, they were lightly counterstained with haematoxylin and observed using an Eclipse E200-LED (Nikon; magnification ×400).
Gene expression analyses
To assess the gene expression of inflammatory mediators, the mRNA levels of mitogen-activated protein kinase (MAPK) 8, MAPK9, MAPK14, iNOS, IL-1β, and TNF-α were determined by real-time polymerase chain reaction (PCR). Total RNA was extracted from liver or spleen tissues using RNeasy Plus Mini kits. First-strand cDNA synthesis was performed using 100 ng total RNA and a Script™ cDNA synthesis kit (Bio-Rad, CA, USA), according to the manufacturer’s instructions. Quantitative real-time PCR were performed using Power SYBR® Green on an Applied Biosystems 7500 Instrument (Applied Biosystems, USA). Each reaction was performed in duplicate. PCR cycling conditions involved initial denaturation at 95 °C for 12 min, followed by 40 cycles of denaturation at 94 °C for 60 s, annealing at 55 °C for 60 s, and extension at 72 °C for 90 s; a final extension step was included at 72 °C for 10 min. The ΔΔCt method was used to determine the difference in the mean expression levels of the studied genes, normalized to the expression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The primers for MAPK8 were forward 5′-gccagtcaggcgagagattt-3′ and reverse 5′-ggacgcatctatcaccagca-3′. The primers for MAPK9 were forward 5′-gtgacagtaaaagcgatggcc-3′ and reverse 5′-ttgagtctgccacttgcacac 3′. The primers for MAPK14 were 5′-gacctactggagaagatgctcgtt-3′ and reverse 5′-tttcaaaggactggtcataagggt-3′. The primers for iNOS were 5′-gttcctcaggcttgggtctt-3′ and reverse 5′-tgggggaacacagtaatggc-3′. The primers for IL-1β were 5′-gacttcaccatggaacccgt-3′ and reverse 5′-ggagactgcccattctcgac-3′.The primers for TNF-α were 5′-agaactcagcgaggacaccaa-3′ and reverse 5′-gcttggtggtttgctacgac-3′. The primers for GAPDH were 5′-gcatcttcttgtgcagtgcc-3′ and reverse 5′-gatggtgatgggtttcccgt-3′.
Statistical analysis
Data were analyzed by one-way analysis of variance with Duncan’s multiple post-test. The statistical significance of all data was set at p < 0.05. All values were expressed as the mean ± the standard error of the mean (SEM). The statistical analyses were performed using the Statistical Package for the Social Sciences software (SPSS v.20.0).
Results
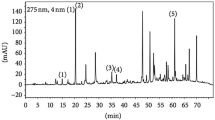
The polyphenolic and flavonoid compounds’ fingerprint for the ZSCLE detected at 280 nm is presented in Fig. 1. The HPLC profile of ZSCLE depicted the presence of seven peaks with retention times ranging from 3.53 min to 91.52 min. Based on the UV–Visible spectral data and their retention time, the ZSCLE have a UV band and λmax at 280 nm, characteristic for phenolic and flavonoid compounds, such as spinosin, catechin, epicatechin, epigallocatechin, gallocatechin, gallic acid, ellagic acid, chlorogenic acid, caffeic acid, rutin, isoquercitrin, quercetin, apigenin, syringic acid and kaempferol.
HPLC profile of Ziziphus spina-christi leaf extract analyzed at 280 nm. The most abundent polyphenolics and flavonids detected were spinosin, catechin, epicatechin, epigallocatechin, gallocatechin, gallic acid, ellagic acid, chlorogenic acid, caffeic acid, rutin, isoquercitrin, quercetin, apigenin, syringic acid and kaempferol. Briefly, HPLC was carried out with a Phenomenex C-18 column (4.6 mm × 250 mm, 5 μm), volume injection was 25 μL. Mobile phase consists of 2.0% acetic acid (solvent A) and acetonitrile (solvent B). The flow rate was set at 0.80 ml/min throughout the elution. HPLC operation was carried out at ambient temperature
The results illustrated in Fig. 2 show that the mean survival time in the presence of sepsis (untreated CLP mice) was 15 h, while, following pretreatment with ZSCLE (100, 200, or 300 mg/kg) s urvival was extended, reaching a maximum or 35 h in mice treated with 300 mg/kg ZSCLE. Thus, ZSCLE produced a significant increase in survival time, as compared with the septic mice. No death was observed in the group of sham-operated mice during the first 48 h.
Administration of ZSCLE alone to the mice did not cause any adverse effect on the liver and spleen weights or liver function parameters (Table 1). However, sepsis caused a significant (p < 0.05) elevation in the spleen weight and in serum levels of alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, and lactate dehydrogenase. As compared to the CLP group, ZSCLE (100 and 200 mg/kg) administration significantly (p < 0.05) attenuated the increase in serum levels of liver function enzymes, although the serum level of aspartate aminotransferase in mice receiving 100 mg/kg ZSCLE + CLP was not significantly different from the level observed in the CLP group. Mice from the 300 mg/kg ZSCLE + CLP group exhibited much greater protection of liver function parameters and spleen weight, as compared to the low- and medium-dose ZSCLE groups.
Histological examination of the liver and spleen showed that ZSCLE pretreatment minimized CLP-induced liver and spleen damage (Fig. 3). These investigations showed that CLP-induced sepsis in mice caused hepatic inflammatory cellular infiltration, hepatic steatosis, and hepatic fibroplasia in the portal tract. In the spleen, the white pulp had started to enlarge and fuse together; this may be due to splenomegaly and an active immune response of spleen macrophages around the white pulp. Further, in some cells necrotic and apoptotic bodies appeared in the red pulp areas. However, these histopathological alterations were ameliorated in a dose-dependent manner in the CLP mice pretreated with ZSCLE (supplementary material).
Ziziphus spina-christi leaf extract (ZSCLE) prevents cecal ligation and puncture (CLP)-induced liver and spleen injury in mice. The livers and spleens of sham-operated and ZSCLE-treated mice showed normal histological architecture. CLP-induced sepsis in mice elicited marked hepatic inflammation, hepatic steatosis, and hepatic portal tract fibroplasia. Enlargement and fusion of some white pulp within the spleen. Whereas, CLP mice pretreated with ZSCLE showed improved hepatic and splenic architecture. Different magnifications were chosen to show either detailed histopathologic alterations or normal tissue architecture over a broader area
Splenic and hepatic MPO activities and MDA levels were significantly (p < 0.05) elevated in mice with CLP-induced sepsis, as compared with the sham mice, and an obvious depletion in GSH was noted in the CLP untreated group (Fig. 4). In sepsis-challenged mice treated with ZSCLE, the splenic and hepatic oxidative markers (MPO and MDA) were reduced and the non-enzymatic anti-oxidant molecule (GSH) was significantly (p < 0.05) increased when compared with the CLP untreated mice.
Effects of Ziziphus spina-christi leaf extract (ZSCLE) administration on the markers of oxidative stress levels in mice with cecal ligation and puncture (CLP)-induced sepsis. Values represent the mean ± SEM (n = 7). aSignificantly different from sham (p < 0.05); bsignificantly different from CLP (p < 0.05). MPO myeloperoxidase, MDA malondialdehyde, GSH glutathione
The potential effects of ZSCLE administration on anti-oxidant enzyme activities in the spleen and liver of sham and CLP-treated mice are illustrated in Fig. 5. In the spleen and liver, superoxide dismutase, catalase, and GR activities were significantly (p < 0.05) suppressed, whereas GPx activities were significantly (p < 0.05) enhanced in the CLP-untreated mice, as compared with the sham mice. Administration of ZSCLE (300 mg/kg) significantly attenuated these CLP-induced changes in the anti-oxidant defense system. ZSCLE (200 mg/kg) pre-administration in CLP-operated mice significantly (p < 0.05) reversed the changes induced in superoxide dismutase, catalase, and GR activities in both liver and spleen, and in hepatic GPx, when compared with the CLP-untreated mice. However, ZSCLE (100 mg/kg) administration failed to reverse these CLP-induced changes in anti-oxidant enzyme activities, as compared to the sham mice.
Effects of Ziziphus spina-christi leaf extract (ZSCLE) administration on the anti-oxidant enzyme activities in C57BL/6 mice with cecal ligation and puncture (CLP)-induced sepsis. Values represent the mean ± SEM (n = 7). aSignificantly different from sham (p < 0.05); bSignificantly different from CLP (p < 0.05). SOD superoxide dismutase, CAT catalase, GPx glutathione peroxidase, GR glutathione reductase
Investigation of NF-kB and iNOS in liver and spleen tissues identified NF-kB expression in cell nuclei in CLP mice, while ZSCLE-treated groups showed more cytoplasmic localization. Furthermore, as shown in Fig. 6, immunohistochemical staining of iNOS showed strong expression in the liver and spleen tissues of septic mice and moderate expression in the ZSCLE-treated groups.
Ziziphus spina-christi leaf extract (ZSCLE) suppressed the protein expression of nuclear factor-κB (NF-κB) and inducible nitric oxide synthase (iNOS) in C57BL/6 mice with cecal ligation and puncture (CLP)-induced sepsis. In mice with CLP-induced sepsis, NF-κB was strongly localized in the nucleus and iNOS was strongly expressed in liver and spleen tissues. NF-κB and iNOS expression were reduced by ZSCLE pretreatment in a dose-dependent manner, and NF-κB was mainly localized within the cytoplasm (magnification ×400)
To clarify the possible mechanism by which ZSCLE attenuates inflammation in sepsis, we evaluated NO, IL-1β, and TNF-α levels, and iNOS mRNA, expression in response to CLP-induced sepsis. Levels of NO in liver and spleen tissues, and the percentage changes (relative to the sham) of IL-1β and TNF-α, are illustrated in Figs. 7 and 8, respectively. NO production, and IL-1β and TNF-α levels, were significantly (p < 0.05) increased in the CLP-untreated mice. ZSCLE (300 mg/kg) pre-administration successfully attenuated the inflammation associated with CLP-induced sepsis in mice, when compared with the sham group. Conversely, ZSCLE (100 mg/kg) failed to attenuate this inflammation. Consistent with the biochemical findings, the quantitative real-time PCR results showed that the mRNA levels of iNOS, IL-1β, and TNF-α in the liver and spleen tissues were up-regulated post-CLP operation in untreated mice; ZSCLE (300 mg/kg) pre-administration attenuated these expression changes. ZSCLE (100 mg/kg) did not prevent CLP-induced up-regulation of iNOS, TNF-α, or IL-1β expression in the liver and spleen.
Effects of Ziziphus spina-christi leaf extract (ZSCLE) administration on nitric oxide (NO) levels and inducible nitric oxide synthase (iNOS) mRNA expression in C57BL/6 mice with cecal ligation and puncture (CLP)-induced sepsis. The NO data are expressed as the mean ± SEM (n = 7). The mRNA levels (mean ± SEM of three assays) were normalized to the level of glyceraldehyde 3-phosphate dehydrogenase mRNA and are shown as the fold induction (in log2 scale) relative to the level observed in controls. aSignificantly different from sham (p < 0.05); bsignificantly different from CLP (p < 0.05)
Effects of Ziziphus spina-christi leaf extract (ZSCLE) administration on inflammatory marker levels in C57BL/6 mice with cecal ligation and puncture (CLP)-induced sepsis. The data for interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) levels are expressed as the mean ± SEM (n = 7). The mRNA levels (mean ± SEM of three assays) were normalized to the level of glyceraldehyde 3-phosphate dehydrogenase mRNA and are shown as the fold induction (in log2 scale) relative to the mRNA level observed in controls. aSignificantly different from sham (p < 0.05); bsignificantly different from CLP (p < 0.05)
To elucidate whether the suppression of inflammatory mediator production by ZSCLE is mediated through the mitogen-activated protein kinase pathway, we evaluated the effect of ZSCLE on CLP-induced expression of MAPK8, -9, and -14 in the liver and spleen via real-time PCR analysis. As shown in Fig. 9, these MAPKs were significantly (p < 0.05) upregulated in the CLP-untreated mice, as compared to the sham-untreated mice. Pretreatment with ZSCLE (300 mg/kg) resulted in a notable attenuation of CLP-mediated up-regulation of these MAPKs, as compared with the CLP-untreated mice. In this context, 200 mg/kg ZSCLE produced a greater effect than 100 mg/kg ZSCLE; however, both of these doses were less effective than 300 mg/kg ZSCLE.
Effects of Ziziphus spina-christi leaf extract (ZSCLE) administration on mitogen-activated protein kinases (MAPK) in C57BL/6 mice with cecal ligation and puncture (CLP)-induced sepsis. The mRNA levels (mean ± SEM of three assays) were normalized to the level of glyceraldehyde 3-phosphate dehydrogenase mRNA and are shown as the fold induction (in log2 scale) relative to the mRNA level observed in controls. aSignificantly different from sham (p < 0.05); bsignificantly different from CLP (p < 0.05)
Discussion
Sepsis causes progressive MOD and, if it progresses to septic shock, a significant drop in blood pressure can lead to death. Treatment with antibiotics alone is not adequate to increase survival rates in septic patients. Anti-oxidants may have the potential to improve the treatment of septic patients (Alici et al. 2015). In the current study, we investigated the potential role of ZSCLE on sepsis in a mouse model. Our findings showed that ZSCLE markedly prevented the liver and spleen injuries, decreased proinflammatory mediators and cytokines, mitigated oxidative stress and boosted the anti-oxidant defense system.
ZSC is a natural product with potent anti-inflammatory, antimicrobial, anti-oxidant, and hepatoprotective properties (Guizani et al. 2013). In the current study, HPLC analysis revealed the presence of many phenolic and flavonoids compounds. Those active compounds have many biological activities: for example, spinosin has neuroprotective activity via the anti-inflammatory effects (Ko et al. 2015), catechin, epicatechin, epigallocatechin and gallocatechin have strong anti-inflammatory and hepatoprotective effects (Al-Olayan et al. 2014), chlorogenic acid can mitigate oxidative and inflammatory stresses (Liang and Kitts 2015), syringic acid is a potential anti-oxidant (Thipparaboina et al. 2016) and quercetin, apigenin, and kaempferol are potent anti-oxidants (Al-Olayan et al. 2014). Recently, molecular docking calculations indicated that some compounds isolated from ZSC interacted with the NF-κB pathway proteins with similar binding energies and docking positions to the known inhibitor, MG-132 (Kadioglu et al. 2016). Furthermore, the anti-oxidant and anti-inflammatory properties of ZSC were demonstrated in an animal model of liver fibrosis with severe carbon tetrachloride-induced inflammation (Amin and Mahmoud-Ghoneim 2009). In this study, we found that ZSCLE produced marked protection against sepsis, as evidenced by an attenuation of liver and spleen damage in mice with CLP-induced sepsis, and an increase in their survival. This protective effect of ZSCLE was mediated by its anti-inflammatory activities on NF-κB and MAPKs.
Oxidative stress activates molecular pathways that drive inflammation, and can directly cause tissue injury. This type of tissue injury is believed to be one of the most important mechanisms underlying the development of MOD in septic shock (Zolali et al. 2015). In addition, altered serum liver function parameters and splenomegaly reflected the pathological changes within the liver and spleen. These pathological changes were significantly attenuated by ZSCLE pretreatment. This multi-organ protective effect of ZSCLE, facilitated by its anti-inflammatory activity, resulted in improved survival.
CLP-induced sepsis is associated with a marked failure of neutrophil recruitment to the infection site and the migration of activated neutrophils to secondary organs, which contribute to MOD (Wang et al. 2016). Hence, we measured the activity of MPO as an important marker of neutrophil migration and aggregation. MPO activities in hepatic and splenic tissues were enhanced in mice with CLP-induced sepsis. MPO stimulates the formation of hypochlorous acid, which is a potent oxidant implicated in bacterial elimination and tissue destruction via necrosis and apoptosis (Kothari et al. 2011). Pretreatment with ZSCLE reduced the level of MPO activity, reflecting the suppression of neutrophil infiltration. Furthermore, the histopathological findings demonstrated that ZSCLE prevented injury to the hepatic and splenic tissues.
In sepsis, TNF-α is the central mediator that regulates subsequent events. Its level is generally elevated in this condition, and this correlates with increased mortality in patients with sepsis. TNF-α activates specific transmembrane TNF receptors, leading to immune cell activation and the release of an array of down-stream inflammatory mediators, which cause oxidative DNA damage. IL-1 is mainly released by stimulated macrophages in timely and functional manners similar to TNF-α. IL-1 acts through two distinct membrane receptors (Schulte et al. 2013). In our study, up-regulation of the production of these cytokines during CLP-induced sepsis in liver and spleen was associated with tissue damage. Pretreatment of mice with ZSCLE was able to prevent these elevations of TNF-α and IL-1β levels in the liver and spleen. Moreover, ZSCLE attenuated the CLP-induced increases in hepatic and splenic TNF-α and IL-1β mRNA expression in these mice. We also suggest that the mechanism underlying these effects may involve an inhibition of inflammatory cells activation by the polyphenols and flavonoids present in ZSCLE, thus inhibiting TNF-α and IL-1β synthesis by preventing the translocation of NF-κB from the cytoplasm into the nucleus. Recently, Kadioglu et al. (2016) demonstrated that ZSC phytochemicals strongly bound to proteins involved in regulating NF-κB (inhibitor of NF-κB kinase and NF-κB essential modulator); these proteins regulate the nuclear translocation and DNA binding of NF-κB. NF-κB plays a central role in signaling and the inflammatory response reaction, and overexpression of nuclear NF-κB is associated with poor clinical outcomes in septic patients (Abraham 2003). Collectively, our findings indicated that the protective effects of ZSCLE on the sepsis-induced liver and spleen injuries might be partially due to an inhibition of NF-κB activity and suppression of pro-inflammatory cytokine production.
MAPKs are key signaling molecules that mediate inflammatory responses by regulating and forwarding extracellular stress signals and coordinate important cellular responses, including secretion of cytokines and inflammation-related factors, cell migration, and apoptosis (Asaduzzaman et al. 2008). Previous reports have indicated that phosphorylation of MAPKs regulated macrophage and T cell responses to traumatic damage. Furthermore, MAPK stimulation by phosphorylation participates in sepsis-induced organ failure (Kim et al. 2016; Yang et al. 2008). MAPK8 is also called c-Jun N-terminal kinase 1 (JNK1). The stimulation of this protein by TNF-α participates in TNF-α-induced apoptosis, which is thought to be related to the cell death pathway mediated by cytochrome c released from the mitochondria. MAPK9 is very close to MAPK8, but this protein blocks the ubiquitination of tumor suppressor p53 and increases the stability of p53 in non-stressed cells, while MAPK14 is known as p38-α and plays an important role in cytokines production by activated microphages. In this study, the mRNA levels of MAPK8, -9, and -14 increased significantly after CLP, while ZSCLE pretreatment attenuated these increases and abolished the accumulation of inflammatory leukocytes in the hepatic and splenic tissues of septic mice. These findings indicate that ZSCLE polyphenols and flavonoids attenuated inflammatory response by down-regulating MAPKs expression.
Within the biochemical results obtained in the current study of sepsis, we found NO overproduction in liver and spleen tissues. NO is capable of reacting with O −2͘ to produce peroxynitrite (Prauchner 2017), a more powerful oxidizing molecule that is capable of inhibiting anti-oxidant enzymes and oxidative phosphorylation, which results in diminished synthesis of ATP. Together, these effects result in the overproduction of ROS, particularly O −2͘ , which sensitizes the cell to hypoxia-induced apoptosis (Brown and Borutaite 2007). NO overproduction in CLP-induced sepsis seemed to result from the over-expression of iNOS (Fig. 7). In sepsis, oxidative stress and ROS activate NF-κB, which increases iNOS expression (Morgan and Liu 2011; Prauchner 2017). Consistent with our observations, Escames et al. (2007) reported that genetic deletion of iNOS in mice restored ATP production and improved survival. In the present study, NO levels and iNOS expression were higher in the CLP group than in the sham mice, and treatment with ZSCLE was able to significantly decrease the NO levels, iNOS protein and mRNA expression in septic mice. These observations indicate that ZSCLE suppresses NO production by down-regulating iNOS expression.
Lipid and protein oxidation, and the overwhelming production of ROS, have been well reported during sepsis; many studies have documented that oxidative stress and MOD were correlated with pathogenesis and mortality in sepsis (Prauchner 2017; Zhong et al. 2016). Oxidative stress markers were reported to be higher in patients with sepsis than in healthy subjects (Jang et al. 2017). In the current study, we observed increased lipid peroxidation, with GSH depletion and inhibition of anti-oxidant enzyme activities. Lipid oxidation damages cell membranes and leads to a loss of plasma membrane integrity, thereby causing cell death. GSH is a major non-enzymatic anti-oxidant molecule that neutralizes ROS and is found both intracellularly and extracellularly within living organisms (Abdel Moneim 2016). In addition, superoxide dismutase plays a major role in scavenging O −2͘ , which can react equivalently with NO and ROS as an anti-oxidant. Catalase degrades H2O2 into H2O and O2 and thus reduces the level of ROS. GPx is known to catalyze lipid hydroperoxide to the corresponding alcohol, and H2O2 to H2O in the presence of GSH, which contribute to protecting the organism from oxidative damage (Zhong et al. 2016). In CLP-treated mice, our results clearly demonstrated that the level of lipid peroxidation was successfully restricted and the content of GSH and the anti-oxidant enzyme activities were significantly increased in a dose-dependent manner, indicating that ZSCLE produced an anti-oxidant effect and thus reduced oxidative damage. In accordance with our findings, Mubaraki et al. (2017) found that ZSC extract treatment markedly reinstated the levels of oxidative markers and enhanced anti-oxidant enzyme activities in mice with cerebral malaria. Amin and Mahmoud-Ghoneim (2009) demonstrated that ZSC effectively protected against carbon tetrachloride-induced liver damage by restoring the normal levels of lipid peroxidation and retaining the activities of endogenous anti-oxidants.
Hence, our study provides evidence for the anti-oxidant and anti-inflammatory effects of ZSCLE in CLP-induced sepsis. The present findings indicate that this protection could be attributed to: (1) ZSCLE-induced inhibition of MAPK-mediated inflammatory responses, thus attenuating liver and spleen injury; (2) ZSCLE-mediated prevention of NF-κB translocation to the nucleus, thus blocking the NF-κB-dependent pathway; and (3) ZSCLE-mediated quenching of free radicals and maintenance or improvement of endogenous anti-oxidant defense systems. However, further rigorous research should be conducted in future to determine which of the many components of the ZSCLE are responsible for its effects on sepsis.
Abbreviations
- CLP:
-
Cecal ligation and puncture
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- GPx:
-
Glutathione peroxidase
- GR:
-
Glutathione reductase
- GSH:
-
Glutathione
- IL-1β:
-
Interleukin-1β
- iNOS:
-
Inducible nitric oxide synthase
- MDA:
-
Malondialdehyde
- MPO:
-
Myeloperoxidase
- MAPK:
-
Mitogen-activated protein kinase
- MOD:
-
Multi-organ dysfunction
- NADPH:
-
Reduced nicotinamide adenine dinucleotide phosphate
- NF-κB:
-
Nuclear factor-κB
- NO:
-
Nitric oxide
- ROS:
-
Reactive oxygen species
- TNF-α:
-
Tumor necrosis factor-α
- ZSC:
-
Ziziphus spina-christi (L.)
- ZSCLE:
-
Ziziphus spina-christi leaf extract
References
Abdel Moneim AE (2013) The neuroprotective effects of purslane (Portulaca oleracea) on rotenone-induced biochemical changes and apoptosis in brain of rat. CNS Neurol Disord Drug Targets 12:830–841 (CNSNDDT-EPUB-53920)
Abdel Moneim AE (2016) Indigofera oblongifolia prevents lead acetate-induced hepatotoxicity, oxidative stress, fibrosis and apoptosis in rats. PLoS ONE 11:e0158965. https://doi.org/10.1371/journal.pone.0158965
Abraham E (2003) Nuclear factor-kappaB and its role in sepsis-associated organ failure. J Infect Dis 187(Suppl 2):S364–S369 (JID21050)
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Alici O, Kavakli HS, Koca C, Altintas ND, Aydin M, Alici S (2015) Value of caffeic acid phenethyl ester pretreatment in experimental sepsis model in rats. Mediat Inflamm 2015:810948. https://doi.org/10.1155/2015/810948
Al-Olayan E, El-Khadragy M, Metwally D, Abdel Moneim A (2014) Protective effects of pomegranate (Punica granatum) juice on testes against carbon tetrachloride intoxication in rats. BMC Complement Altern Med 14:164. https://doi.org/10.1186/1472-6882-14-164
Amin A, Mahmoud-Ghoneim D (2009) Zizyphus spina-christi protects against carbon tetrachloride-induced liver fibrosis in rats. Food Chem Toxicol 47:2111–2119. https://doi.org/10.1016/j.fct.2009.05.038
Andrades M, Ritter C, de Oliveira MR, Streck EL, Fonseca Moreira JC, Dal-Pizzol F (2011) Antioxidant treatment reverses organ failure in rat model of sepsis: role of antioxidant enzymes imbalance, neutrophil infiltration, and oxidative stress. J Surg Res 167:e307–e313 (pii:S0022-4804(09)00425-9)
Asaduzzaman M, Wang Y, Thorlacius H (2008) Critical role of p38 mitogen-activated protein kinase signaling in septic lung injury. Crit Care Med 36:482–488. https://doi.org/10.1097/01.CCM.0B013E31816204FA
Bacanli M, Aydin S, Taner G, Goktas HG, Sahin T, Basaran AA, Basaran N (2016) Does rosmarinic acid treatment have protective role against sepsis-induced oxidative damage in Wistar Albino rats? Hum Exp Toxicol 35:877–886. https://doi.org/10.1177/0960327115607971
Bradley PP, Priebat DA, Christensen RD, Rothstein G (1982) Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol 78:206–209 (S0022-202X(15)46301-8)
Brown GC, Borutaite V (2007) Nitric oxide and mitochondrial respiration in the heart. Cardiovasc Res 75:283–290 (pii:S0008-6363(07)00147-2)
Carlberg I, Mannervik B (1985) Glutathione reductase Methods Enzymol 113:484–490
Escames G, Lopez LC, Ortiz F, Lopez A, Garcia JA, Ros E, Acuna-Castroviejo D (2007) Attenuation of cardiac mitochondrial dysfunction by melatonin in septic mice. FEBS J 274:2135–2147 (EJB5755)
Grisham MB, Johnson GG, Lancaster JR Jr (1996) Quantitation of nitrate and nitrite in extracellular fluids. Methods Enzymol 268:237–246
Guizani N, Waly MI, Singh V, Rahman MS (2013) Nabag (Zizyphus spina-christi) extract prevents aberrant crypt foci development in colons of azoxymethane-treated rats by abrogating oxidative stress and inducing apoptosis. Asian Pac J Cancer Prev 14:5031–5035. https://doi.org/10.7314/APJCP.2013.14.9.5031
Jang JY, Lee SH, Shim H, Lee JG (2017) Serum oxygen radical activity and total antioxidation capacity are related with severities of surgical patient with sepsis: prospective pilot study. J Crit Care 39:131–136 (pii:S0883-9441(16)30731-6)
Kadioglu O et al (2016) Evaluating ancient Egyptian prescriptions today: anti-inflammatory activity of Ziziphus spina-christi. Phytomedicine 23:293–306 (S0944-7113(16)00022-2)
Kim SJ, Baek KS, Park HJ, Jung YH, Lee SM (2016) Compound 9a, a novel synthetic histone deacetylase inhibitor, protects against septic injury in mice by suppressing MAPK signalling. Br J Pharmacol 173:1045–1057. https://doi.org/10.1111/bph.13414
Ko SY et al (2015) Spinosin, a C-glucosylflavone, from Zizyphus jujuba var. spinosa Ameliorates Abeta1-42 oligomer-induced memory impairment in mice. Biomol Ther (Seoul) 23:156–164. https://doi.org/10.4062/biomolther.2014.110
Kothari N et al (2011) Increased myeloperoxidase enzyme activity in plasma is an indicator of inflammation and onset of sepsis. J Crit Care 26(435):e431–e437 (pii:S0883-9441(10)00262-5)
Liang N, Kitts DD (2015) Role of chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients. https://doi.org/10.3390/nu8010016
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Michel CG, Nesseem DI, Ismail MF (2011) Anti-diabetic activity and stability study of the formulated leaf extract of Zizyphus spina-christi (L.) Willd with the influence of seasonal variation. J Ethnopharmacol 133:53–62 (pii:S0378-8741(10)00639-2)
Morgan MJ, Liu ZG (2011) Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res 21:103–115. https://doi.org/10.1038/cr2010.178
Mubaraki MA, Hafiz TA, Al-Quraishy S, Dkhil MA (2017) Oxidative stress and genes regulation of cerebral malaria upon Zizyphus spina-christi treatment in a murine model. Microb Pathog 107:69–74. https://doi.org/10.1016/j.micpath.2017.03.017
Nishikimi M, Appaji N, Yagi K (1972) The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun 46:849–854
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Osterbur K, Mann FA, Kuroki K, DeClue A (2014) Multiple organ dysfunction syndrome in humans and animals. J Vet Intern Med 28:1141–1151. https://doi.org/10.1111/jvim.12364
Prauchner CA (2017) Oxidative stress in sepsis: pathophysiological implications justifying antioxidant co-therapy. Burns 43:471–485 (pii:S0305-4179(16)30400-4)
Rahim I et al (2017) Melatonin administration to wild-type mice and non-treated NLRP3 mutant mice share similar inhibition of the inflammatory response during sepsis. J Pineal Res. https://doi.org/10.1111/jpi.12410
Schulte W, Bernhagen J, Bucala R (2013) Cytokines in sepsis: potent immunoregulators and potential therapeutic targets—an updated view. Mediat Inflamm 2013:165974. https://doi.org/10.1155/2013/165974
Shi C, Pamer EG (2011) Monocyte recruitment during infection and inflammation. Nat Rev Immunol 11:762–774. https://doi.org/10.1038/nri3070
Thipparaboina R, Mittapalli S, Thatikonda S, Nangia A, Naidu VGM, Shastri NR (2016) Syringic acid: structural elucidation and co-crystallization. Cryst Growth Des 16:4679–4687. https://doi.org/10.1021/acs.cgd.6b00750
Ursini F, Maiorino M, Gregolin C (1985) The selenoenzyme phospholipid hydroperoxide glutathione peroxidase. Biochim Biophys Acta 839:62–70
Wan SX, Shi B, Lou XL, Liu JQ, Ma GG, Liang DY, Ma S (2016) Ghrelin protects small intestinal epithelium against sepsis-induced injury by enhancing the autophagy of intestinal epithelial cells. Biomed Pharmacother 83:1315–1320 (pii:S0753-3322(16)30767-3)
Wang Y et al (2016) Berberine in combination with yohimbine attenuates sepsis-induced neutrophil tissue infiltration and multiorgan dysfunction partly via IL-10-mediated inhibition of CCR2 expression in neutrophils. Int Immunopharmacol 35:217–225 (pii:S1567-5769(16)30123-0)
Xiao X, Yang M, Sun D, Sun S (2012) Curcumin protects against sepsis-induced acute lung injury in rats. J Surg Res 176:e31–e39 (pii:S0022-4804(11)01992-5)
Yang M, Wu J, Martin CM, Kvietys PR, Rui T (2008) Important role of p38 MAP kinase/NF-kappaB signaling pathway in the sepsis-induced conversion of cardiac myocytes to a proinflammatory phenotype. Am J Physiol Heart Circ Physiol 294:H994–H1001. https://doi.org/10.1152/ajpheart.01044.2007
Zhong W, Qian K, Xiong J, Ma K, Wang A, Zou Y (2016) Curcumin alleviates lipopolysaccharide induced sepsis and liver failure by suppression of oxidative stress-related inflammation via PI3K/AKT and NF-kappaB related signaling. Biomed Pharmacother 83:302–313 (pii:S0753-3322(16)30602-3)
Zhou J et al (2012) Hydrogen-rich saline reverses oxidative stress, cognitive impairment, and mortality in rats submitted to sepsis by cecal ligation and puncture. J Surg Res 178:390–400 (pii:S0022-4804(12)00062-5)
Zolali E, Hamishehkar H, Maleki-Dizaji N, Majidi Zolbanin N, Ghavimi H, Kouhsoltani M, Asgharian P (2014) Selenium effect on oxidative stress factors in septic rats. Adv Pharm Bull 4:289–293. https://doi.org/10.5681/apb.2014.042
Zolali E, Asgharian P, Hamishehkar H, Kouhsoltani M, Khodaii H (2015) Effects of gamma oryzanol on factors of oxidative stress and sepsis-induced lung injury in experimental animal model. Iran J Basic Med Sci 18:1257–1263. https://doi.org/10.22038/IJBMS.2015.6283
Acknowledgement
The authors would like to express their appreciation to the Deanship of Scientific Research at King Saud University for funding this study through the research group project No. RG-198.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.

10787_2017_439_MOESM1_ESM.jpg
Supplementary Figure: Effects of Ziziphus spina-christi leaf extract (ZSCLE) administration on the scoring index of histopathological evaluation in mice with cecal ligation and puncture (CLP)-induced sepsis. Values represent the mean ± SEM (n = 7). aSignificantly different from sham (p < 0.05); bSignificantly different from CLP (p < 0.05). (JPEG 189 kb)
Rights and permissions
About this article
Cite this article
Dkhil, M.A., Al-Quraishy, S. & Moneim, A.E.A. Ziziphus spina-christi leaf extract pretreatment inhibits liver and spleen injury in a mouse model of sepsis via anti-oxidant and anti-inflammatory effects. Inflammopharmacol 26, 779–791 (2018). https://doi.org/10.1007/s10787-017-0439-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-017-0439-8