Abstract
This study was undertaken to investigate the effect of α-chymotrypsin on methyl nitrosourea (MNU) induced mammary gland carcinoma in albino wistar rats. Animals were randomized into four groups (six animals in each). Group I (sham control 0.9 % normal saline p.o.); Group II (toxic control, MNU 47 mg/kg, i.v.); Group III (α-chymotrypsin, 5 mg/kg, p.o.); Group IV (α-chymotrypsin, 10 mg/kg p.o.). Toxicity was induced by single i.v. injection of MNU followed by α-chymotrypsin supplementation therapy for 100 days. MNU treatment was evident with increased alveolar bud count, differentiation score, upregulated inflammatory enzymes markers (COX, LOX and NO) antioxidative stress markers (TBARs, SOD, catalase and GSH).MNU associated toxicity was also ascertained by PGP 9.5 and NF-κB expression in the mammary gland tissue followed by FAME analysis for fatty acid profiling. α-chymotrypsin afforded significant protection against the deleterious effects of MNU.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
α-Chymotrypsin is one among the chymotrypsin series and is a digestive enzyme component of pancreatic juice. These are primarily associated with proteolysis leading to breakdown proteins and polypeptides (Wilcox 1970). α-Chymotrypsin preferably cleaves peptide amide bond where the carboxyl side of the amide bond is hydrophobic amino acid and is activated in the presence of trypsin (Appel 1986). Clinically, α-chymotrypsin is considered to be safe once taken by oral route to reduce redness and swelling following surgery or even skin burn (Ray 1964). α-Chymotrypsin is also prescribed in liver injuries of burn patients. Despite the consideration that α-chymotrypsin is safe to be used, a very little is known about its efficacy in other limits.
Mammary gland carcinogenesis is the second most prevailing type of cancer in Indian women. One in fifty-eight women are affected by mammary gland cancer in the age group of 30–70 years. Recent clinical and epidemiological studies have also affirmed that high blood level of oestrogen is linked with increased risk of mammary gland cancer in premenopausal and postmenopausal women (Kaaks et al. 2005; Lyytinen et al. 2009; Key et al. 2011; Hormones and BCC Group 2013). Long back in 80’s, the estradiol was also reported to increase the production of 1-α-antichymotrypsin along with inhibitory activity toward α-chymotrypsin. Thereby, suggesting a possible link between estradiol, α-chymotrypsin and cancer progression (Massot et al. 1985). It would be worth to mention that fatty acid synthase (FASN) is an enzyme, over expressed in mammary gland cancer and is reported to observe proteolytic cleavage by α-chymotrypsin. Therefore, we hypothesis that exogenous α-chymotrypsin may exert beneficial effect of either modulating the estradiol or by increasing the proteolytic cleavage of over expressed FASN (Agradi et al. 1976; Esslimani-Sahla et al. 2007).
Henceforth, this study was undertaken to elucidate the role of exogenous possible link methyl nitrosourea (MNU) induced mammary gland carcinoma in female albino rats.
Materials and methods
Drug and chemicals
α-Chymotrypsin (Catalogue no. RM 801) (derived from bovine pancreas non-preactivated) was procured from Himedia Laboratories, Mumbai, India. N-Methyl-N-nitrosourea was obtained from Sigma Aldrich Co. St. Louise Mo 63103 USA. All other chemicals were of analytical grade and procured from Himedia Laboratories Mumbai, India; else otherwise stated in the text.
Experimental protocol
Albino wistar female rats of 100–120 g body weight were used for this study. The rats were procured from the central animal house facility. The animals housed in propylene cages under controlled conditions (23 °C, 12 h light/dark cycle), with a free access to a standard pellet diet and water ad libitum. They were acclimatized for a period of 2 weeks prior to the commencement of the experiment. Animals were randomized and divided into four groups of six animals each. Group I (sham control 0.9 % normal saline p.o.); Group II (toxic control, MNU 47 mg/kg); Group III (α-chymotrypsin, 5 mg/kg p.o.); Group IV (α-chymotrypsin, 10 mg/kg p.o.). Toxicity was induced by single i.v. injection of MNU followed by α-chymotrypsin supplementation therapy for 100 days using the above doses. The blood samples were collected under chloroform anaesthesia through retro-orbital plexus in centrifugation tubes. The blood samples were incubated at 37 °C for 1 h and centrifuged at 10,000 rpm for 15 min to collect serum. The serum samples were stored at −20 oC till further use. Animals were killed on the 107th day and subjected to estimation. The animal experiments were carried out in compliance with the standard ethical guidelines and approved by the institutional animal ethics committee (SDCOP & VS/AH/CPCSEA/01/0036).
Mammary gland whole mount
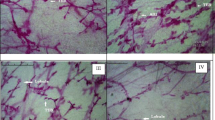
The mammary glands obtained from female rats, stretched onto a slide, placed in a fixative solution and stained with a carmine aluminium solution to prepare whole mounts (De Assis et al. 2010). Whole mounts were examined under the 4× microscope and evaluated to assess the number of TEBs. Also, whole mounts were evaluated for ductal elongation and differentiation. Ductal elongation was measured, using a ruler, as the distance (in cm) from the nipple to the end of the epithelial tree. Mammary gland differentiation was assessed by scoring the number of alveolar buds type 1(AB1) and type 2 (AB2). The score values (0–5) from AB1 and AB2 were added for a final differentiation score (0–10). The average rating values (0–5) from AB1 and AB2 were added to the lobule score values (0–5) for a final differentiation score (0–10).
Biochemical estimation
The mammary gland tissues (10 % w/v) were homogenized in 0.15 M KCl and centrifuged at 10,000 rpm. The supernatants were scrutinized for biochemical parameters, including thiobarbituric acid reactive substances (TBARS), superoxide dismutase (SOD), catalase, glutathione (GSH) and acetylcholinesterase (AchE) using the methods established at our laboratory (Ellman et al. 1961; Mäkelä et al. 2002; Kaye and Jick 2004; Kaithwas and Majumdar 2012).
Serum nitric oxide (NO) levels
Generation of NO in the serum samples were arbitrated by measuring nitrite accumulation, using Griess reagent [1 % sulphanilamide, 0.1 % N-(1-napthyl)- ethylenediamine dihydrochloride in 5 % H3P04]. Equal quantity (500 µl) of serum and Griess reagent were mixed and incubated at 37 °C for 5 min. The test mixture was subsequently read on UV–Visible spectrophotometer (Cary60, Agilent technologies, CA95051, US) at 540 nm (Torres 2003).
Enzymatic activity of COX and LOX
A 10 % tissue homogenate in tris buffer (50 mM) was centrifuged at 5000 rpm for 5 min followed by sonication. The tissue supernatant (10 µl) was incubated for 5 min with tris buffer (160 µl). A 10 µl each of N,N,N’,N’-tetramethyl-p-phenylenediamine (TMPD) reagent and arachidonic acid (AA) solution (prepared in ethanol) were added and read at 630 nm using multiplate reader (ALERE Microplate Reader, AM-2100) at 0 and 30 s interval. AA solution was prepared by mixing 50 µl of 40 mM AA with 50 µl of 0.1 N potassium hydroxide using vortexing, and subsequently 900 µl of double distilled water. TMPD stock solution was prepared by dissolving 0.3 mg in 1 ml of distilled water and subsequent 1:10 dilution was prepared for the assay (Altman and Sudarshan 1975; Schneider and Przewłocki 2005). The assay was performed in the whole serum, and therefore, reflects the nonspecific COX activity.
For LOX assay, 25 µl of AA solution was added to the 475 µl supernatant (as prepared for COX assay) and incubated for 6 min. A 500 µl of ferrithiocyanate (FTC) reagent was added and read at 480 nm using UV–visible spectrophotometer (Cary 60, Agilent Technologies International Private Limited, CA United States) after 5 min. FTC reagent was prepared by mixing the reagent 1 (4.5 mM FeSO4 in 0.2 M HCl) and reagent 2 (3 % NH4SCN methanolic solution) in 1:1 ratio (Schapiro et al. 1970).
Fatty acid methyl ester (FAME) analysis of mammary gland tissue
Mammary gland tissue homogenate (0.5 %) was prepared in the mixture of chloroform: methanol (2:1) using REMI homogenizer followed by sonication at 4 °C for 5 min. The homogenate was subsequently filtered using a Whatman filter and final volume made up by methanol. The filtrate was mixed thoroughly with 0.2 volume of double distilled water for removal of non-lipid contaminants. The mixture was kept for 30 min and centrifuged at 5000 rpm for 5 min; upper phase was removed and lower phase was collected with mammary gland lipids. Methyl esters for the lipid samples were prepared by stirring 0.75 gm of samples with hexane (2 ml) and methanolic KOH (2 N) (0.2 ml) followed by vortexing for 15 min. The phases were allowed to settle down and the upper layer containing the FAME was collected (Gregory and Pfaff 1971).
The FAME samples were filtered using 0.2 µm syringe filters and subjected to the gas chromatographic analysis (Perkin Elmer GC-clarus 480; Column : Elite-5 Length-30 m, Internal diameter–0.25 mm) using Flame ionization detector (250 °C; carrier gas: nitrogen (10 psi); volume of injection 1 µl; Oven temperature: 150 (1 min), ramp 1–5 °C/min to 230 °C (5 min), ramp 2–150 °C/min to 245 for 12 min; internal standard: cetyl alcohol (Kaithwas et al. 2011).
Western blotting
Protein samples were prepared from the mammary gland tissue through acetone precipitation and quantified using the Bradford reagent (Ahmad and Sharma 2009). SDS–PAGE analysis was performed following the principles of Laemmli with slight modifications (Laemmli 1970). Briefly, protein samples were mixed with sample buffer (125mMTris-HCl, pH6.8, 20 %glycerol, 4 % SDS, 0.05 % bromophenol blue, 10 % 2-mecaptoethanol). A 30 µg of protein sample was allowed to resolve through 12 % polyacrylamide gel using SDS–PAGE (GX-SCZ2+, Genetix Biotech Asia Pvt. Ltd, New Delhi). The proteins as resolved through SDS–PAGE were transferred to a PVDF membrane (IPVH 00010 Millipore, Bedford, MA USA) using semidry transfer (GX-ZY3, Genetix Biotech Asia Pvt. Ltd, New Delhi). Subsequently, membrane was blocked with 3 % BSA and 3 % not fat milk in TBST for 2 h and incubated overnight with primary antibody against PGP 9.5 [(MA1-83428) (1:2000 dilution)]; NF-κB p65 (MA5-1616) (1:2000 dilution) and β-actin MA5-15739-HRP (1:300 dilution) (Pierce, Thermo scientific, USA). The membrane was washed with TBST thrice and incubated with HRP conjugated rat anti-mouse secondary antibody (31430, 1:5000 dilutions) (Pierce Thermo Scientific, USA) at room temperature for 2 h. The signals were detected using an enhanced chemiluminescence substrate (Western Bright ECL HRP substrate, Advansta, Melanopark, California, US). The quantification of protein was done through densitometric digital analysis of protein bands using Image J software (Laemmli 1970; Towbin et al. 1979).
Statistical analysis
All data were presented as mean ± SEM and analyzed by one-way ANOVA followed by Bonferroni test and for the possible significance identification between the various groups. *p < 0.05, **p < 0.01, ***p < 0.001 were considered as statistically significant. Statistical analysis was performed using Graph Pad Prism software (5.02).
Results
Treatment with MNU recorded marked increase in the AB/TEB score and α-chymotrypsin afforded a marked protection against the same. As a marker for growth and differentiation of the mammary gland tissue α-chymotrypsin also regulated the differentiation score favourably (Table 1; Fig. 1). Mammary gland tissue was further scrutinized for the oxidative markers and treatment with MNU demonstrated significant increase in TBARS and GSH with nonsignificant negating effects on the enzymatic antioxidant defence. Treatment with α-chymotrypsin (5 mg/kg p.o.) curtailed the increased TBARS levels. However, high dose α-chymotrypsin further increased the TBARS levels. On the contrary, the α-chymotrypsin at both the doses favourably regulated the tissue GSH levels. α-Chymotrypsin failed to provide any significant change in the tissue SOD levels, whereas curtailed the catalase levels at both the doses (Table 2).
The enzymatic activity of inflammatory markers (COX and LOX) was upregulated with MNU treatment. Similar pattern of up-regulation was perceived for the serum NO levels. Treatment with α-chymotrypsin (5 mg/kg p.o.) contemplated with significant restoration of the inflammatory markers and NO levels (Fig. 2). It would be appropriate to mention that high dose of α-chymotrypsin failed to regulate the COX and NO levels, as in case of TBARS.
Effect of MNU and α-chymotrypsin on inflammatory enzymes and nitric oxide level: (values are Mean ± SEM), each group contains six animals and comparisons were made on the basis of the one-way Anova followed by Bonferroni test. All groups were compared to the toxic control group (*p < 0.05, **p < 0.01, ***p < 0.001). [Group I: Control; Group II: MNU (47 mg/kg); Group III: α-chymotrypsin + MNU (5 mg/kg + 47 mg/kg); Group IV: α-chymotrypsin + MNU (10 mg/kg + 47 mg/kg)]
FAME analysis of mammary gland tissue was evident with decrease in the unsaturated fatty acid content with MNU treatment with inflation of the same after α-chymotrypsin treatment (Table 3). Treatment with MNU upregulated the PGP 9.5 (UCHL-1) and NF-κB expression in the mammary gland tissue. Treatment with α-chymotrypsin demonstrated a dose dependent negating effect on the PGP and NF-κB expression (Fig. 3).
Effect of α-chymotrypsin on UCHL-1/PGP 9.5 expression against MNU induces mammary gland carcinogenesis in albino rats: western blot analysis of UCHL-1/PGP 9.5 expression from female rat mammary gland tissue, detected very low expression in sham control. Immunoreactivity was evident as a band of molecular weight of 27 kDa in case of toxic with increasing expression. A significant decrease in UCHL-1/PGP 9.5 expression is noted in low dose of α-chymotrypsin. In high dose, it shows a decrease in immunoreactivity in dose dependent manner. Present experiment also revealed NF-κB P65 activation in MNU treated animals; its immunoreactivity was evident as band of 65 kDa which is decreased in treatment group in a dose dependent manner. Results of β-actin analysis are shown as an internal control
Discussion
This study perceived momentous protection by α-chymotrypsin to combat the deleterious effects of MNU on mammary gland. The favourable regulation by α-chymotrypsin was very well evident after 60 days of treatment. Authors would also like to submit that the chymotrypsin used in the current experiment was derived from the bovine pancreas. However, no immunogenic reaction was recorded in the animals at any of the doses used in the study.
The whole mount preparations are frequently used as a convenient method for the examination of small proliferative lesions as represented through increase in the number of AB/TEBs and lobules; the corresponding structures in the human breast are the terminal ductal lobular units. The AB/TEBs represents the largest bulbous structures located at the distal end of the mammalian epithelial tree. The undifferentiated AB/TEBs are the sites for the malignant transformations and increase in AB/TEB’s number is directly correlated with increased chances of developing malignancy (Russo and Russo 1996). AB/TEBs are potentially used as an early positive marker of anti-angiogenic activity in cancer preventive therapies. The MNU treatment was evident with increase in AB/TEB count and differentiation score, which is in corroboration with the antecedent studies (Kaithwas and Majumdar 2012). Treatment with α- chymotrypsin curtailed the AB/TEB count and differentiation score to sizable amount in dose dependent manner, suggesting positive modulatory effect of α-chymotrypsin against MNU induced mammary gland differentiation.
Chronic inflammation and release of NO by macrophages and epithelial cells resulting in DNA damage and nitrosylation of proteins is a key event in the progression of carcinogenesis (Coussens and Werb 2002; Ellies et al. 2003). Significant increase in the enzymatic activity of COX and LOX enzymes, which was further accomplished by inflated NO levels after the MNU treatment. Dual inhibition of the arachidonic acid pathway, in particular COX-2 and 5-LOX inhibition are nowadays recognized to impart anticancer activity (Matsuyama and Yoshimura 2008). Upsurged enzymatic activity of COX and LOX after the MNU treatment affirms the association between inflammation and carcinogenesis in the current experiment as well. In the same line, NO has been implicated as one of the major marker for tumour progression and was hypothesized that increased NO generation in a cell may select mutant p53 cells and contribute to tumour angiogenesis by upregulating VEGF (Weiming et al. 2002). Inflated NO levels subsequent to MNU treatment has been foreseen as a marker of cancer progression and is in line with various preclinical studies. Treatment with α-chymotrypsin (low dose) regulated the overexpression of inflammatory markers (COX and LOX enzymes) along with NO levels. It would pertinent to mention that α-chymotrypsin (high dose) failed to positively regulate the COX and NO levels, which is not in line with the previous reports and precedent findings from our experiment. Authors would like to mention that such observation is beyond scope of this work and could be matter of future investigation. Efficacy of α-chymotrypsin to regulate the inflammatory markers prompted us further scrutinize the levels of NF-κB, P65 (a group of sequence-specific transcription factor and a key regulator of inflammatory responses) in the mammary gland tissue. Elevated NF-κB DNA binding activity is detected in both mammary carcinoma cell lines and primary human breast cancer tissues. In fact, NF-κB is reported to contribute expansion of breast tumour stem cells and enhancement of vasculogenesis (Loizou et al. 2010), and therefore, has emerged as a viable target for cancer progression. In the same line, we perceived significant up-regulation of the levels of NF-κB in MNU treated animals, which subsided to a noticeable amount by α-chymotrypsin treatment. Prima facie one can commensurate that low dose of α-chymotrypsin can impart noticeable negating effects on the MNU induced carcinogenesis.
Chemical and physical agents can induce cancer through production of reactive oxygen species (ROS). Oxidative damage to cellular macromolecules can arise through overproduction of ROS and faulty antioxidant and/or DNA repair mechanisms. In addition, ROS can stimulate signal transduction pathways and lead to activation of key transcription factors such as Nrf2 and NF-κB (Klaunig et al. 2010). Accordingly, sizable deterioration in the oxidative stress markers is contemplated with cancer progression and the same was evident in this study.
A momentous increase was recorded in the MNU treated animals when accounted for the peroxidative markers of lipid (TBARS) peroxidation and tissue GSH (Kaithwas and Majumdar 2012; Raj et al. 2014). In the same line, the enzymatic antioxidant defence of SOD and catalase were nonsignificantly deteriorated in consequent to MNU administration. The distorted battery of antioxidant defence subsequent to MNU treatment is in line with the previous reports. Treatment with the α-chymotrypsin nudged down the inflated TBARS and GSH levels, suggesting the positive testimony by α-chymotrypsin towards modifying the disease progression. Nonetheless, the SOD and catalase constitute major team of antioxidant defence to counteract the deleterious effects of reactive oxygen species. Treatment with MNU imparted marginal decrease in the enzymatic activity of SOD (nonsignificant) and catalase, suggesting reasonable levels of ROS attack leading to increased utilization. Interestingly, treatment with α-chymotrypsin failed to impart any significant change in the SOD levels with further decrease in the catalase levels. The unchanged SOD levels and diminished activity of catalase could be attributed to the proteolytic proficiency of α-chymotrypsin to selectively hydrolyze peptide bonds on the C-terminal side of tyrosine, phenylalanine, tryptophan, and leucine (Appel 1986). Considering the availability of all the four amino acids (tyrosine, phenylalanine, tryptophan, and leucine) in the C terminus side chain of SOD and catalase, one may postulate that both the enzymes may be preferably cleaved by the α-chymotrypsin, and thereby accounting for the curtailment of catalase levels (Merlino et al. 2014; Loewen et al. 2015). The cholinergic abnormalities are well studied phenomenon in mammary gland carcinogenesis. In fact, AchE inhibitor (eserine) has been reported to promote cell proliferation and tumour formation, thereby suggesting decreased AchE signalling in the mammary gland cancer cells (Olexová et al. 2013). In the same line, we perceived downregulated AchE levels in the mammary gland tissue after the MNU treatment. Concomitant administration of the α-chymotrypsin was accorded with normalized levels of AchE to control at higher dose. With all above, one can derive that α-chymotrypsin can impart a demarcating biochemical effects on the MNU induced carcinogenesis through modifying the inflammatory signalling and regulating the oxidative stress markers.
Alterations in fatty acid metabolism in cancer cells have received less attention but are increasingly being recognized. Manipulating the fatty acid composition through exogenous supplementation has been found to be closely associated with mammary gland cancer risk in rodent models and humans (Sasaki et al. 1993; Fay et al. 1997; Chajès et al. 2008). Plethora of scientific studies is available pointing the positive association between saturated/monounsaturated fatty acid and progression of mammary gland carcinoma, with a negative modulation by polyunsaturated fatty acids (PUFAS) (in particular omega-6 fatty acids). Similar frame work of decreased total PUFAS in the mammary glands of MNU treated animals was perceived in the current experiment. The results from the FAME analysis of the mammary gland tissue could be categorized into two major findings. First, the clinical findings have scrutinized the inverse association between PUFAS content and breast cancer risk, which was very well evident in the current experiment after MNU administration (Zaridze et al. 1990; Vatten et al. 1993). Moreover, the oleic acid concentration in the tissues has been negatively correlated with the tumour progression by number of researchers and the same was well demarcated in the present experiment as well after the MNU administration (Carrillo et al. 2012). Treatment with α-chymotrypsin randomized the overall PUFAS content and oleic acid concentration in the mammary glands of the treated rats. With all above, we would like to commend that α-chymotrypsin can impart favourable effects against the MNU induced mammary gland carcinoma through modifying the oxidative stress and inflammatory markers, which in repercussion regulated the fatty acid profile.
PGP 9.5 (ubiquitin COOH-terminal esterase L1 or UCHL-1) is an ubiquitin COOH-terminal hydrolase, which is widely expressed in different type of cancer cells including breast, colorectal and pancreatic tumours (Brichory et al. 2001; Hurst-Kennedy et al. 2012). Studies also demonstrate that UCHL-1/PGP 9.5 promotes G1/S arrest in breast cancer cells (Zhao et al. 2014). The high expression of UCHL-1/PGP 9.5 in metaplastic carcinomas of the breast, may implicate an association between UCHL-1/PGP 9.5 expression and the epithelial–mesenchymal transition in breast cancer, and therefore, has been imparted the status of oncogenic marker (Tokumaru et al. 2004; Romon et al. 2010). In this particular study, the toxic group showed elevated expression of UCHL-1/PGP 9.5 in comparison with control. The dose dependent decrease in UCHL-1/PGP 9.5 expressions was observed after α-chymotrypsin treatment.
Previous experimental data reveal that the 26S centre of UCHL-1/PGP 9.5 is regulated by 20S immunoproteasome which inhibit degradation of the ubiquitinated proteins (Hegde and Upadhya 2011). The same could be attributed to the 20S immunoproteasome having chymotrypsin-like, trypsin-like, and postglutamyl peptidase activities, which cleaves the hydrophobic, basic, and acidic residues (Cheng 2009; Marques et al. 2009). In MNU-induced mammary gland carcinoma model, the α-chymotrypsin causes a selective inhibition of the chymotrypsin-like subunit 20S of the immunoproteasome. Previous report also suggests that the high expression of 20S immunoproteasome subunits can be related to the considerably higher chymotrypsin-like activity. The same has been linked with faster processing rate of the NF-κB/p50 precursor p105 and IκBα. In line with to above, dose dependent decrease in NF-κB/p65 expressions was observed after α-chymotrypsin treatment.
Henceforth, the increased expression of UCHL-1/PGP 9.5 and subsequent high expression of NF-κB/p65 subunit could be attributed to high expression of 20S immunoproteasome subunits/chymotrypsin-like subunit through faster processing of NF-κB/p50 precursor p105 and IκBα. It would be appropriate to remark that the present finding is endorsed by the previous reports that UCHL-1 not only activate NF-κB/p50 precursor p105 and IκBα subunit but also stabilize the expression of p65 subunit by inhibiting its deacetylation (Groll and Huber 2004; Visekruna et al. 2006; Muchamuel et al. 2009).
With all above, one can conclude that α-chymotrypsin can impart significant protection against the MNU-induced mammary gland carcinogenesis through modifying the pathological, biochemical and inflammatory markers through regulating the 20S immunoproteasome subunits/chymotrypsin-like subunit of UCHL-1/PGP 9.5 leading to faster processing of NF-κB/p50 precursor p105 and IκBα.
Abbreviations
- MNU:
-
N-Methyl-N-nitrosourea
- NO:
-
Nitric oxide
- FAME:
-
Fatty acid methyl ester
- COX:
-
Cyclooxygenase
- LOX:
-
Lipoxygenase
- TBARs:
-
Thiobarbituric acid reactive substances
- SOD:
-
Super oxide dismutase
- CAT:
-
Catalase
- GSH:
-
Glutathione
- TMPD:
-
N,N,N′,N′-tetramethyl-p-phenylenediamine
- AA:
-
Arachidonic acid
- FTC:
-
Ferrithiocynate
- UCHL-1:
-
Ubiquitin carboxy-terminal hydrolase L-1
References
Merlino A, Krauss IR (2014) Structural and denaturation studies of two mutants of a cold adapted superoxide dismutase point to the importance of electrostatic interactions in protein stability. Biochim Biophys Acta (BBA) Proteins Proteom 1844(3):632–640
Agradi E, Libertini L et al (1976) Specific modification of fatty acid synthetase from lactating rat mammary gland by chymotrypsin and trypsin. Biochem biophys Res Comm 68(3):894–900
Ahmad Y, Sharma N (2009) An effective method for the analysis of human plasma proteome using two-dimensional gel electrophoresis. J Proteom Bioinform 2(12):495–499
Altman J, Sudarshan K (1975) Postnatal development of locomotion in the laboratory rat. Anim Behav 23:896–920
Appel W (1986) Chymotrypsin: molecular and catalytic properties. Clin Biochem 19(6):317–322
Brichory F, Beer D et al (2001) Proteomics-based identification of protein gene product 9.5 as a tumor antigen that induces a humoral immune response in lung cancer. Cancer Res 61(21):7908–7912
Carrillo C, Cavia M et al (2012) Antitumor effect of oleic acid; mechanisms of action: a review. Nutri Hosp 27(6):1860–1865
Chajès V, Thiébaut AC et al (2008) Association between serum trans-monounsaturated fatty acids and breast cancer risk in the E3 N-EPIC Study. Am J Epidemiol 167(11):1312–1320
Cheng Y (2009) Toward an atomic model of the 26S proteasome. Curr Opin Struct Biol 19(2):203–208
Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420(6917):860–867
De Assis S, Warri A et al (2010) Changes in mammary gland morphology and breast cancer risk in rats. J Vis Exp 44:e2260–e2260
Ellies LG, Fishman M et al (2003) Mammary tumor latency is increased in mice lacking the inducible nitric oxide synthase. Int J Cancer 106(1):1–7
Ellman GL, Courtney KD et al (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7(2):88–95
Esslimani-Sahla M, Thezenas S et al (2007) Increased expression of fatty acid synthase and progesterone receptor in early steps of human mammary carcinogenesis. Int J Cancer 120(2):224–229
Fay MP, Freedman LS et al (1997) Effect of different types and amounts of fat on the development of mammary tumors in rodents: a review. Cancer Res 57(18):3979–3988
Gregory EH, Pfaff DW (1971) Development of olfactory-guided behavior in infant rats. Physiol Behav 6(5):573–576
Groll M, Huber R (2004) “Inhibitors of the eukaryotic 20S proteasome core particle: a structural approach”. Biochim Biophys Acta (BBA) Mol Cell Res 1695(1):33–44
Hegde AN, Upadhya SC (2011) Role of ubiquitin–proteasome-mediated proteolysis in nervous system disease. Biochim Biophys Acta (BBA) Gene Regul Mech 1809(2):128–140
Hormones E, BCC Group (2013) Sex hormones and risk of breast cancer in premenopausal women: a collaborative reanalysis of individual participant data from seven prospective studies. Lancet Oncol 14(10):1009–1019
Hurst-Kennedy J, Chin LS et al (2012) Ubiquitin C-terminal hydrolase l1 in tumorigenesis. Biochem Res Int 2012(2012):123706. doi:10.1155/2012/123706
Kaaks R, Rinaldi S et al (2005) Postmenopausal serum androgens, oestrogens and breast cancer risk: the European prospective investigation into cancer and nutrition. Endocr Relat Cancer 12(4):1071–1082
Kaithwas G, Mukherjee A et al (2011) Antiinflammatory, analgesic and antipyretic activities of Linum usitatissimum L. (flaxseed/linseed) fixed oil. Indian J Exp Biol 49(12):932–938
Kaithwas G, Majumdar DK (2012) In vitro antioxidant and in vivo antidiabetic, antihyperlipidemic activity of linseed oil against streptozotocin-induced toxicity in albino rats. Eur J Lipid Sci Technol 114(11):1237–1245
Kaye J, Jick H (2004) Epidemiology of lower limb fractures in general practice in the United Kingdom. Inj Prev 10(6):368–374
Key T, Appleby P et al (2011) Circulating sex hormones and breast cancer risk factors in postmenopausal women: reanalysis of 13 studies. Br J Cancer 105(5):709–722
Klaunig JE, Kamendulis LM et al (2010) Oxidative stress and oxidative damage in carcinogenesis. Toxicol Pathol 38(1):96–109
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259):680–685
Loewen PC, Villanueva J et al (2015) Unprecedented access of phenolic substrates to the heme active site of a catalase: Substrate binding and peroxidase-like reactivity of Bacillus pumilus catalase monitored by X-ray crystallography and EPR spectroscopy. Proteins Struct Funct Bioinf 83(5):853–866
Loizou S, Lekakis I et al (2010) β-Sitosterol exhibits anti-inflammatory activity in human aortic endothelial cells. Mol Nutr Food Res 54(4):551–558
Lyytinen H, Pukkala E et al (2009) Breast cancer risk in postmenopausal women using estradiol–progestogen therapy. Obstet Gynecol 113(1):65–73
Mäkelä A, Nuorti JP et al (2002) Neurologic disorders after measles-mumps-rubella vaccination. Pediatrics 110(5):957–963
Marques AJ, Palanimurugan R et al (2009) Catalytic mechanism and assembly of the proteasome. Chem Rev 109(4):1509–1536
Massot O, Baskevitch PP et al (1985) Estradiol increases the production of α 1-antichymotrypsin in MCF 7 and T 47 D human breast cancer cell lines. Mol Cell Endocrinol 42(3):207–214
Matsuyama M, Yoshimura R (2008) “The target of arachidonic acid pathway is a new anticancer strategy for human prostate cancer”. Biol Target Ther 2(4):725
Muchamuel T, Basler M et al (2009) A selective inhibitor of the immunoproteasome subunit LMP7 blocks cytokine production and attenuates progression of experimental arthritis. Nat Med 15(7):781–787
Olexová L, Senko T et al (2013) Habituation of exploratory behaviour in VPA rats: animal model of autism. Interdiscip Toxicol 6(4):222–227
Raj P, Singh M et al (2014) Effect of enteral administration of α-linolenic acid and linoleic acid against methotrexate induced intestinal toxicity in albino rats. RSC Adv 4(104):60397–60403
Ray P (1964) Use of 200 times the recommended dose of alpha-chymotrypsin without complications. Br J Ophthalmol 48(4):230
Romon R, Adriaenssens E et al (2010) Nerve growth factor promotes breast cancer angiogenesis by activating multiple pathways. Mol Cancer 9(1):1
Russo IH, Russo J (1996) Mammary gland neoplasia in long-term rodent studies. Environ Health Perspect 104(9):938
Sasaki S, Horacsek M et al (1993) An ecological study of the relationship between dietary fat intake and breast cancer mortality. Prev Med 22(2):187–202
Schapiro S, Salas M et al (1970) Hormonal effects on ontogeny of swimming ability in the rat: assessment of central nervous system development. Science 168(3927):147–151
Schneider T, Przewłocki R (2005) Behavioral alterations in rats prenatally exposed to valproic acid: animal model of autism. Neuropsychopharmacology 30(1):80–89
Tokumaru Y, Yamashita K et al (2004) Inverse correlation between cyclin A1 hypermethylation and p53 mutation in head and neck cancer identified by reversal of epigenetic silencing. Cancer Res 64(17):5982–5987
Torres AR (2003) Is fever suppression involved in the etiology of autism and neurodevelopmental disorders? BMC Pediatr 3(1):9
Towbin H, Staehelin T et al (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci 76(9):4350–4354
Vatten LJ, Bjerve KS et al (1993) Polyunsaturated fatty acids in serum phospholipids and risk of breast cancer: a case-control study from the Janus serum bank in Norway. Eur J Cancer 29(4):532–538
Visekruna A, Joeris T et al (2006) Proteasome-mediated degradation of IκBα and processing of p105 in Crohn disease and ulcerative colitis. J Clin Investig 116(12):3195–3203
Weiming X, Liu LZ et al (2002) The role of nitric oxide in cancer. Cell Res 12(5):311–320
Wilcox P (1970) [5] Chymotrypsinogens—chymotrypsins. Method Enzymol 19:64–108
Zaridze D, Chevchenko V et al (1990) Fatty acid composition of phospholipids in erythrocyte membranes and risk of breast cancer. Int J Cancer 45(5):807–810
Zhao Q, Yang Y et al (2014) The clinicopathological significance of neurogenesis in breast cancer. BMC Cancer 14(1):1
Acknowledgments
The author would like to thanks University Grants Commission for providing Fellowship to MS, JKR, SG and RKY.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Availability of supporting data
Nil.
Conflict of interest
No conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10787_2016_280_MOESM1_ESM.docx
FAME analysis of mammary gland tissue treated with MNU and α-chymotrypsin: I, Control; II, MNU; III, α-chymotrypsin (5 mg/kg p.o.); IV, α-chymotrypsin (10 mg/kg) (DOCX 315 kb)
Rights and permissions
About this article
Cite this article
Rani, A., Roy, S., Singh, M. et al. α-Chymotrypsin regulates free fatty acids and UCHL-1 to ameliorate N-methyl nitrosourea induced mammary gland carcinoma in albino wistar rats. Inflammopharmacol 24, 277–286 (2016). https://doi.org/10.1007/s10787-016-0280-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-016-0280-5