Abstract
We evaluated the effect of pyrrolidine dithiocarbamate (PDTC) in superoxide anion-induced inflammatory pain. Male Swiss mice were treated with PDTC and stimulated with an intraplantar or intraperitoneal injection of potassium superoxide, a superoxide anion donor. Subcutaneous PDTC treatment attenuated mechanical hyperalgesia, thermal hyperalgesia, paw oedema and leukocyte recruitment (neutrophils and macrophages). Intraplantar injection of superoxide anion activated NF-κB and increased cytokine production (IL-1β, TNF-α and IL-10) and oxidative stress (nitrite and lipid peroxidation levels) at the primary inflammatory foci and in the spinal cord (L4–L6). PDTC treatment inhibited superoxide anion-induced NF-κB activation, cytokine production and oxidative stress in the paw and spinal cord. Furthermore, intrathecal administration of PDTC successfully inhibited superoxide anion-induced mechanical hyperalgesia, thermal hyperalgesia and inflammatory response in peripheral foci (paw). These results suggest that peripheral stimulus with superoxide anion activates the local and spinal cord oxidative- and NF-κB-dependent inflammatory nociceptive mechanisms. PDTC targets these events, therefore, inhibiting superoxide anion-induced inflammatory pain in mice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Under physiological conditions, cells produce low rates of superoxide anion and by endogenous antioxidant mechanisms control its deleterious effects. During inflammation, however, there is excessive production of inflammatory mediators, including superoxide anion, tumour necrosis factor alpha (TNF-α) and interleukin-1 beta (IL-1β) (Salvemini et al. 2002; Anrather et al. 2006). These cytokines activate and recruit neutrophils and monocytes, which in turn further produce large amounts of superoxide anion and cytokines (Salvemini et al. 2002; Anrather et al. 2006). Excessive production of superoxide anion triggers maladaptive signalling pathways that contribute to enhance its own production inducing inflammation and pain (Wang et al. 2004; Yoshino et al. 2011; Maioli et al. 2015). The emerging literature suggests that superoxide anion and its downstream effector peroxynitrite are critical mediators implicated in neuropathic and inflammatory pain as well as opioid-induced hyperalgesia models (Doyle et al. 2010, 2012; Janes et al. 2012; Little et al. 2013). The physiopathological mechanisms of these models depend on peripheral and spinal cord synthesis of inflammatory mediators (Ndengele et al. 2008; Janes et al. 2012; Maioli et al. 2015). Intraplantar injection of superoxide anion induces nuclear factor kappa B (NF-κB) transcription factor activation in the paw skin. NF-κB is a key redox-sensitive component of inflammation and pain (Ndengele et al. 2008). Other redox-sensitive signalling pathways triggered by superoxide anion and peroxynitrite in inflammatory pain include mitogen-activated protein kinases (MAPKs) p38 and ERK1/2. MAPKs together with the NF-κB can modulate the production of numerous pro-inflammatory mediators such as cytokines, COX-related products and free radicals (Ndengele et al. 2008; Salvemini et al. 2011). However, it is not known whether inhibiting NF-κB activation represents a strategy to inhibit superoxide anion-induced pain and inflammation. Importantly, it remains to be investigated whether peripheral superoxide anion-triggered pain depends on the spinal cord activation of NF-κB.
Pyrrolidine dithiocarbamate (PDTC) is a molecule that presents potent NF-κB inhibition alongside antioxidant properties (Schreck et al. 1992; Cuzzocrea et al. 2002; Cheng et al. 2006; Ivan et al. 2014). Therefore, we investigated the effects of treating mice with PDTC in a model of inflammatory pain induced by potassium superoxide (KO2), a superoxide anion donor. The present results reveal that peripheral stimulus with superoxide anion activates peripheral and spinal cord mechanisms of nociceptive sensitisation that are related to NF-κB activation, cytokine production and oxidative stress. In agreement with that, PDTC treatment efficiently inhibits inflammatory pain by targeting superoxide anion-induced effects in the paw skin and spinal cord.
Methods
Animals
Male Swiss mice weighing 25 ± 5 g from Londrina State University (Paraná State, Brazil) were used in this study. All behavioural testing was performed between 9 a.m. and 5 p.m. in a temperature-controlled (21 ± 1 °C) room. Animals’ care and handling procedures were in accordance with the International Association for the Study of Pain (IASP) guidelines and received the approval of the Ethics Committee for Animal Research of Londrina State University (process number 71.2012.68). All efforts were made to minimise the number of animals used and their suffering.
Drugs and reagents
KO2 was purchased from Alfa Aesar (Ward Hill, MA, USA). Pyrrolidine dithiocarbamate (PDTC) was purchased from Sigma Chemical Co. (St. Louis, MO, USA).
Experimental protocols
Subcutaneous (s.c.) back skin injection of PDTC (3–100 mg/kg, 100 µL) or vehicle (sterile saline, 100 µL) was given 1 h before KO2 injection in all experiments. Intraplantar (i.pl.) injection of 30 µg KO2 (diluted in 20 µL of sterile saline) was administered to induce paw inflammation and hyperalgesia (Figs. 1a–c and 2a–c]). Each figure derives from a different experimental group, except Figs. 1a–c and 2a–c. In this case, the same mice were used to measure mechanical and thermal hyperalgesia, and oedema; after 7 h, animals were terminally anaesthetised and plantar tissue collected for MPO and NAG analysis. In a different experimental group, intraperitoneal (i.p.) injection of 30 µg KO2 (diluted in 100 µL of sterile saline) was used to evaluate leukocyte recruitment at 6 h (Fig. 2d; total and differential cell counting) (Maioli et al. 2015). The dose of 100 mg/kg of PDTC and subcutaneous treatment was selected to determine NF-κB activation in the paw skin and spinal cord (Fig. 3), cytokine production (Fig. 4) and oxidative stress (Fig. 5). Figure 3a, b is derived from experiments using a different set of mice than from Fig. 3c, d. The data on paw skin and spinal cord in Fig. 4 were derived from the same mice. The data on paw skin and spinal cord in Fig. 5 were derived from the same mice. Another experiment using intrathecal (i.t.) administration of PDTC (30–300 µg, 5 µL) was conducted to evaluate its effect on mechanical hyperalgesia, thermal hyperalgesia, paw oedema and paw skin MPO and NAG activity (Fig. 6). All results in Fig. 6 were derived from the same mice. Times, doses and routes of treatment were based on previous studies of our laboratory (Ivan et al. 2014; Maioli et al. 2015; Zarpelon et al. 2016). The investigators were blinded to the treatments.
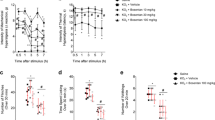
Effects of pyrrolidine dithiocarbamate (PDTC) on inflammatory pain induced by superoxide anion. Mice were treated with PDTC (3–100 mg/kg, 100 µL, s.c.) or vehicle (saline, 100 µL, s.c.) 60 min before the potassium superoxide (KO2) (30 µg, 20 µL) or saline (20 µL) i.pl. injection. Nociceptive thresholds for mechanical (a) and thermal (b) stimuli were evaluated. The results are expressed as mean ± SEM, n = 6 mice per group per experiment, representative of two independent experiments [*p < 0.05 vs. saline control; # p < 0.05 vs. KO2 control (black bars); **p < 0.05 vs. PDTC 3 mg/kg group; ## p < 0.05 vs. PDTC 10 mg/kg group; ***p < 0.05 vs. PDTC 30 mg/kg group, (two-way ANOVA followed by Tukey’s post hoc)]
Effects of PDTC on superoxide anion-induced paw oedema and leukocyte recruitment. Mice were treated with PDTC (3–100 mg/kg, 100 µL, s.c.) or vehicle (saline, 100 µL, s.c.) 60 min before the potassium superoxide (KO2) (30 µg, 20 µL) or saline (20 µL) i.pl. injection (a, b, c), or KO2 (30 μg, 100 μL) or saline (100 μL) i.p. injection (d). Paw oedema (a) was assessed using a calliper, and myeloperoxidase (MPO) activity (b) and N-acetyl-β-d-glucosaminidase (NAG) activity (c) were assessed in paw skin samples. Total and differential cell counts (d) were determined in the peritoneal cavity 6 h after stimulus. The results are expressed as mean ± SEM, n = 6 mice per group per experiment, representative of two independent experiments [*p < 0.05 vs. saline control; # p < 0.05 vs. KO2 control (black bars), (ANOVA followed by Tukey’s post hoc)]
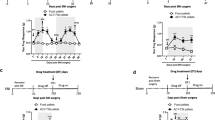
Peripheral stimulus with superoxide anion induces local and spinal cord activation of the NF-κB pathway. Mice were treated with PDTC (100 mg/kg, 100 µL, s.c.) or vehicle (saline, 100 µL, s.c.) 60 min before the potassium superoxide (KO2, 30 µg, 20 µL) or saline (20 µL) i.pl. injection. Tissue samples from the paw skin (a–c) and spinal cord (d) were collected at indicated time points (a, b) or 3 h (c, d) after stimulus. Results are expressed as mean ± SEM, n = 6 mice per group per experiment, representative of two independent experiments [*p < 0.05 vs. saline control; # p < 0.05 vs. KO2 0.5 and 1 h control groups (non-treated); **p < 0.05 vs. KO2 control (at the same interval), (ANOVA followed by Tukey’s post hoc)]
Effect of PDTC in peripheral superoxide anion-induced local and spinal cord cytokine production. Mice were treated with PDTC (100 mg/kg, 100 µL, s.c.) or vehicle (saline, 100 µL, s.c.) 60 min before the potassium superoxide (KO2, 30 µg, 20 µL) or saline (20 µL) i.pl. injection. Tissue samples from the paw skin and spinal cord were collected 3 h after stimulus, and TNF-α (a), IL-1β (b) and IL-10 (c) levels were measured by ELISA. Results are expressed as mean ± SEM, n = 6 mice per group per experiment, representative of two independent experiments [*p < 0.05 vs. saline control; # p < 0.05 vs. KO2 control (black bars), (ANOVA followed by Tukey’s post hoc)]
Effect of PDTC in superoxide anion-induced local and spinal cord oxidative stress. Mice were treated with PDTC (100 mg/kg, 100 µL, s.c.) or vehicle (saline, 100 µL, s.c.) 60 min before the potassium superoxide (KO2, 30 µg, 20 µL, i.pl.) or saline (20 µL, i.pl.) injection. Tissue samples from the paw skin and spinal cord were collected 3 h after stimulus to determine nitrite production (a) and TBARS levels (b). Results are expressed as mean ± SEM, n = 6 mice per group per experiment, representative of two independent experiments [*p < 0.05 vs. saline control; # p < 0.05 vs. KO2 control (black bars), (ANOVA followed by Tukey’s post hoc)]
Effect of PDTC intrathecal (i.t.) treatment in superoxide anion-induced peripheral inflammatory pain. Mice received i.t. treatment with PDTC (30–300 µg, 5 µL) or vehicle (saline, 5 µL) 60 min before the potassium superoxide (KO2, 30 µg, 20 µL) or saline (20 µL) i.pl. injection. Nociceptive thresholds for mechanical (a) and thermal (b) stimuli and paw oedema (c) were evaluated. Samples of paw skin were collected 7 h after the stimulus for MPO and NAG activities determination (d). Results are expressed as mean ± SEM, n = 6 mice per group per experiment, representative of two independent experiments [*p < 0.05 vs. saline control, # p < 0.05 vs. KO2 control, **p < 0.05 vs. KO2 control and KO2 + PDTC 30 µg, (ANOVA followed by Tukey’s post hoc)]
Mechanical hyperalgesia
Mechanical hyperalgesia was evaluated before and 0.5–7 h after KO2 stimulus (Maioli et al. 2015). The test consisted of evoking a hind paw flexion reflex with a hand-held force transducer (electronic anaesthesiometer; Insight, Ribeirão Preto, SP, Brazil) adapted with a 0.5 mm2 polypropylene tip. The detailed methodology has been described previously (Cunha et al. 2004). The results are expressed by delta (∆) withdrawal threshold (in g), calculated by subtracting the mean measurements at the indicated time points after stimulus from the zero-time mean measurements.
Thermal hyperalgesia
Thermal hyperalgesia was evaluated before and 0.5–7 h after i.pl. KO2 stimulus. The test was performed as reported previously (Maioli et al. 2015). In brief, mice were placed in a hot plate apparatus (EFF 361, Insight, Ribeirão Preto, SP, Brazil) maintained at 55 °C. The reaction time was registered when the animal jumped or licked the paw. A maximum latency (cutoff) was set at 20 s to avoid tissue damage (Pinho-Ribeiro et al. 2015).
Paw oedema
The paw oedema was measured 0.5–7 h after i.pl stimulus using a calliper (Digmatic Caliper, Mitutoyo Corporation, Kanagawa, Japan) (Maioli et al. 2015). Paw thickness was expressed as the difference (Δ mm) between the values obtained just before (basal) and after stimulus injection.
MPO and NAG activities
Mice were terminally anaesthetised 7 h after i.pl. stimulus and paw skin samples were collected for myeloperoxidase (MPO) and N-acetyl-β-d-glucosaminidase (NAG) activity colorimetric assays to evaluate the neutrophil and macrophage recruitment as described previously (Hohmann et al. 2013).
Leukocyte recruitment in the peritoneal cavity
Mice were terminally anaesthetised 6 h after i.p. stimulus (Maioli et al. 2015), and the peritoneal cells were harvested with 1.5 mL of PBS. Total cell counts were obtained using the peritoneal wash in a Neubauer chamber and differential cell counts were performed using Panoptic staining kit (Laborclin, Pinhais, PR, Brazil) and light microscope (400× magnification, Olympus Optical Co., Hamburg, Germany) (Verri et al. 2007).
Western blotting analysis
Mice were terminally anaesthetised 0.5, 1 or 3 h after i.pl. stimulus and samples from paw skin were collected. Western blotting was performed as described previously (Zarpelon et al. 2016) using primary antibodies anti-IκBα (1:1000) or anti-β-actin as control (1:1000) (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Image J software (NIH, Bethesda, MD, USA) was used to measure the optical density of the bands.
Enzyme-linked immunosorbent assays (ELISA)
Mice were terminally anaesthetised 3 h after i.pl. stimulus and samples from paw skin and spinal cord were collected. ELISA was performed to evaluate p65 activation (PathScan® Total NF-κB p65 and Phospho-NF-κB p65 Ser536 ELISA kits, Cell Signaling Technology, Danvers, MA, USA) and cytokine production (IL-1β, TNF-α, and IL-10—Ready-SET-Go! kits, eBioscience, San Diego, CA, USA) following the manufacturer’s instructions (Fattori et al. 2015). The results are expressed as picograms of cytokine per milligram of tissue.
Nitrite production
Mice were terminally anaesthetised 3 h after i.pl. stimulus and samples from paw skin and spinal cord were collected, homogenised in 500 µL of saline and nitrite concentration was determined by the Griess reaction as an indicator of nitric oxide production (Ruiz-Miyazawa et al. 2015). Briefly, 100 µL of the homogenate was incubated with 100 µL of Griess reagent for 5 min at 25 °C, and nitrite concentration was determined by measuring the optical density at 550 nm (Multiskan GO, Thermo Scientific, Vantaa, Finland) in reference to a standard curve of NaNO2 solution. The results are expressed as micromoles of nitrite per milligram of tissue.
Lipid peroxidation assay
Mice were terminally anaesthetised 3 h after i.pl. stimulus and samples from paw skin and spinal cord were collected and homogenised in 500 µL of ice-cold KCl buffer (1.15 % w/v) to be processed for thiobarbituric acid reactive substances (TBARS) levels, which evaluates lipid peroxidation as described previously (Ruiz-Miyazawa et al. 2015). Malondialdehyde (MDA) levels, an intermediate product of lipid peroxidation, was determined by the difference between absorbance at 535 and 572 nm (Multiskan GO, Thermo Scientific, Vantaa, Finland). The results are expressed as nanomoles of MDA per milligram of tissue.
Statistical analysis
The results are presented as mean ± SEM of six mice per group per experiment and are representative of two independent experiments. Two-way ANOVA was used when the parameters were measured at different time intervals. One-way ANOVA followed by Tukey’s post hoc was performed for single time point parameters. p < 0.05 was considered to be statistically significant.
Results
PDTC inhibits superoxide anion-induced mechanical and thermal hyperalgesia
Mice were treated with PDTC (3–100 mg/kg, s.c.), a widely used antioxidant and NF-κB inhibitor, 1 h before i.pl. injection of superoxide anion (Fig. 1a, b). Superoxide anion reduced the nociceptive thresholds to mechanical (Fig. 1a) and thermal (Fig. 1b) stimuli, while PDTC inhibited these effects in a dose-dependent manner. The most effective dose tested was 100 mg/kg of PDTC.
PDTC inhibits superoxide anion-induced inflammation
We next evaluated whether the effects of PDTC could be related to inhibition of paw oedema and leukocyte recruitment, due to the role of these events in enhancing and sustaining inflammatory pain. Superoxide anion induced paw oedema at 0.5–7 h (Fig. 2a), and increased neutrophil (MPO activity, Fig. 2b) and macrophage (NAG activity, Fig. 2c) recruitment in the paw skin at 7 h. PDTC treatment at the dose of 100 mg/kg inhibited these effects (Fig. 2a–c). Considering the results of Figs. 1 and 2a–c, the dose of 100 mg/kg of PDTC was selected for the following experiments. Corroborating the results obtained in MPO and NAG assays in paw skin samples (Fig. 2b, c), PDTC inhibited superoxide-induced recruitment of total leukocytes, neutrophils and mononuclear (Fig. 2d) cells in the peritoneal cavity.
Peripheral superoxide anion stimulus activates NF-κB and increases cytokine production and oxidative stress locally and in the spinal cord in a PDTC-sensitive manner
First, we evaluated IκBα (cytoplasm NF-κB inhibitor) levels in the paw skin at varied time points after superoxide anion stimulus and the effects of PDTC treatment as well. Superoxide anion induced IκBα degradation 0.5–3 h after stimulus presenting more pronounced degradation at 3 h (Fig. 3a, b), while PDTC (100 mg/kg) treatment inhibited IκBα degradation at 1 and 3 h after stimulus (Fig. 3a, b). These results suggest that PDTC acts by inhibiting NF-κB activation and the downstream pro-inflammatory signalling, and 3 h after superoxide stimulus is an adequate time point for such studies. Therefore, we next investigated the effects of PDTC treatment in samples collected from the paw skin and spinal cord (L4–L6) 3 h after stimulus. The spinal cord lumbar segment L4–L6 receives the afferent nociceptive neurons from the paw, which justifies its importance for the present experimental condition. Superoxide anion increased p65 (NF-κB subunit) phosphorylation in the paw skin (Fig. 3c) and in the spinal cord (Fig. 3d), and PDTC treatment inhibited the NF-κB activation at both sites (Fig. 3c, d). The superoxide anion also increased the production of IL-1β, TNF-α and IL-10 in paw skin and in the spinal cord (Fig. 4a–c), and PDTC was capable of inhibiting their production at both sites (Fig. 4a–c). Furthermore, superoxide anion stimulus leads to increased oxidative stress in the paw skin (Fig. 5a, b) and spinal cord (Fig. 5c, d) as observed by increased nitrite production and lipid peroxidation levels in both sites, while PDTC was effective in inhibiting these effects.
Intrathecal treatment with PDTC inhibits mechanical hyperalgesia, thermal hyperalgesia, paw oedema and MPO activity, but not NAG activity in the paw skin
Mice were treated with PDTC (30–300 µg, i.t.) 1 h before i.pl. stimulus with superoxide anion. We observed that i.t. treatment with PDTC inhibited in a dose-dependent manner the KO2-induced mechanical hyperalgesia (Fig. 6a), thermal hyperalgesia (Fig. 6b), paw oedema (Fig. 6c) and MPO activity, but not NAG activity (Fig. 6d).
Discussion
We have demonstrated previously that intraplantar injection of superoxide anion induces inflammatory pain by mechanisms including expression and activity of cyclooxygenase-2 (COX-2) and prepro-endothelin-1 as well as TNF-α acting on TNFR1 (tumour necrosis factor receptor 1) (Maioli et al. 2015; Serafim et al. 2015; Yamacita-Borin et al. 2015). These results suggest the possible involvement of transcription factors such as NF-κB, which regulates the production of these molecules in inflammatory pain (Maioli et al. 2015; Serafim et al. 2015; Yamacita-Borin et al. 2015). More recently, we described that curcumin, a compound known to inhibit NF-κB among other targets, inhibits inflammatory pain induced by superoxide anion (Fattori et al. 2015). These data are in line with the studies from other groups describing the key role of superoxide anion in pain and NF-κB activation during inflammation (Ndengele et al. 2005, 2008). Herein, we demonstrated that PDTC reduced superoxide anion-induced pain, suggesting that NF-κB activation is crucial for superoxide anion to induce local and spinal cord inflammatory response. Furthermore, PDTC inhibited superoxide anion-induced cytokine production and oxidative stress in the paw skin and at the spinal cord, therefore, revealing a mechanism by which superoxide anion sensitises nociceptive fibres and, consequently, induces inflammatory pain.
Superoxide anion and its derivatives such as peroxynitrite activate and sensitise nociceptive neurons (Lundberg et al. 2008; Ndengele et al. 2008; Chuang and Lin 2009). Spinal cord-derived superoxide anion and peroxynitrite are considered important molecules in the pathophysiology of hyperalgesia, including opioid-induced hyperalgesia and chronic neuropathic pain (Doyle et al. 2010, 2012; Little et al. 2013). Indeed, superoxide anion and its derivatives can induce inflammatory and neuropathic pain (Doyle et al. 2009; Little et al. 2012a, b). Corroborating the studies presented above, we observed increased levels of nitrite in the paw skin and spinal cord after superoxide anion stimulus. NO reacts with superoxide anion generating peroxynitrite, a molecule closely linked to inflammatory hyperalgesia (Ndengele et al. 2008; Little et al. 2012a, b). Thus, it is possible that in addition to the superoxide anion, its downstream effector peroxynitrite can account for the potassium superoxide-induced inflammatory pain. However, it is also noteworthy that there is evidence that superoxide anion induces hyperalgesia independently of peroxynitrite (Kim et al. 2008, 2009).
Reactive oxygen species (ROS) are also required for PKAc-mediated phosphorylation of NF-κB p65 subunit, which allows recruitment of the co-activator CREB-binding protein and increases the expression of, for instance, IL-8 (Jamaluddin et al. 2007). Furthermore, ROS induce IKK-dependent phosphorylation and acetylation of histone in the promoter region of NF-kB-related pro-inflammatory genes in lung epithelial cells and macrophages (Yang et al. 2008). PDTC does not directly inhibit IκB phosphorylation or proteasome function (Hayakawa et al. 2003; Qiu et al. 2013), but rather inhibits ubiquitin-ligase enzymes, therefore inhibiting the signalling required for IκB degradation via proteasome (Hayakawa et al. 2003). Possible mechanisms by which PDTC inhibits NF-κB activation also include the induction of extracellular Zn2+ translocation to intracellular space (Kim et al. 1999a; Lee et al. 2008), a mechanism known to inhibit NF-κB activation (Kim et al. 1999b; Bruck et al. 2002). PDTC inhibits oxidative stress in vivo and in vitro by increasing the antioxidant capacity, diminishing GSH depletion (Ivan et al. 2014) and reducing total ROS, NOX-2 and NOX-4 mRNA expression (Yu et al. 2015). Thus, considering the role of ROS in NF-κB activation, the antioxidant effect of PDTC might be a contributing mechanism to its effect over NF-κB activation. Therefore, multiple mechanisms of PDTC can contribute to inhibit NF-κB activation.
Additionally, the superoxide anion induces oedema and promotes the recruitment of leukocytes (Gao et al. 2008; Li et al. 2011; Kvietys and Granger 2012; Maioli et al. 2015). Once activated, granulocytes such as neutrophils as well as M1 macrophages represent important sources of pro-inflammatory cytokines and superoxide anion, which contribute to increase inflammation and pain (Anrather et al. 2006; Mantovani et al. 2011; Hohmann et al. 2013; Naito et al. 2014). We demonstrated that PDTC reduced the superoxide anion-induced recruitment of these cells, which could contribute to reduce the sensitisation and activation of nociceptive neurons by further producing TNF-α, IL-1β and the superoxide anion itself (Ndengele et al. 2005; Anrather et al. 2006; Cunha et al. 2008; Guerrero et al. 2008). On the other hand, peripheral activation of first-order nociceptive fibres leads to the transmission of nociceptive impulses into the dorsal horn of the spinal cord. The interactions between neurons and glial cells account for the central sensitisation. In this sense, primary nociceptive neurons release the chemokine fractalkine (CX3CL1) in the dorsal root ganglia and spinal cord upon depolarisation. CX3CL1 binds to CX3CR1 receptor in microglia, therefore, stimulating these cells to produce cytokines and other pro-nociceptive mediators (Basbaum et al. 2009; Gao and Ji 2010; Souza et al. 2013). There is a close relationship between glia and neurons during increased excitability and pain perception process in response to peripheral injuries. For instance, astrocytes and neurons in the rostral ventromedial medulla (RVM) produce superoxide anion derivatives in a model of inflammatory hyperalgesia (Little et al. 2012a, b). Glial cells continuously inspect neuronal activity and are activated by increased nociceptive transmission (Wieseler-Frank et al. 2004), releasing the NF-κB-related cytokines TNF-α and IL-1β (Bressan et al. 2012; Gruber-Schoffnegger et al. 2013), and thus contributing to spinal sensitisation. Corroborating this mechanism, we demonstrated that peripheral stimulus with superoxide anion leads to spinal activation of NF-κB and production of TNF-α and IL-1β. Furthermore, inhibiting TNF-α with etanercept and TNFR1 deficiency reduces superoxide anion-induced hyperalgesia and inflammation (Yamacita-Borin et al. 2015). NF-κB activation also induces the production of IL-10, an anti-inflammatory cytokine that is co-produced with pro-inflammatory cytokines to limit inflammation (Verri et al. 2006; Borghi et al. 2015). In this line, we demonstrated that PDTC inhibited superoxide anion-induced paw skin and spinal cord production of IL-10. These data may seem to be contradictory since previous studies demonstrated the participation of IL-10 in curbing the production of superoxide anion and its downstream effectors and consequently oxidative stress (Little et al. 2013; Doyle et al. 2012; Naito et al. 2014). Nevertheless, PDTC inhibited IL-10 production in models of sepsis (Németh et al. 1998; Kotake et al. 2003). It is possible that as PDTC inhibited superoxide anion-triggered signalling pathways and pro-inflammatory cytokine production, the IL-10 production to limit inflammation and pain is not necessary (Borghi et al. 2015). This evidence suggests that inducing IL-10 production is not a major analgesic and anti-inflammatory mechanism of PDTC in superoxide anion-induced inflammation.
Superoxide anion induced oxidative stress in the site of its injection and also in the spinal cord as observed by increased nitrite production and lipid peroxidation. Treatment with PDTC reduced both parameters, which could be a result of its antioxidant activity and/or a consequence of inhibiting NF-κB signalling (Ivan et al. 2014; Yu et al. 2015). In addition to inhibiting NF-κB activation, PDTC can also induce Nrf2 (nuclear factor erythroid 2-related factor 2) expression. For instance, PDTC activates the mitogen-activated protein kinases (MAPKs) ERK (extracellular regulated kinase) and p38 (Zipper and Mulcahy 2000) to induce Nrf2 translocation to the nucleus of HepG2 cells (Zipper and Mulcahy 2003). As a result, the upregulation of the gamma-glutamylcysteine synthetase subunits genes occurs, which catalyses the rate-limiting step of the glutathione synthesis (Wild et al. 1999). Importantly, PDTC induces Nrf2 expression in astrocytes by mechanisms involving Keap1 and glycogen synthase kinase 3β. On the other hand, PDTC did not induce Nrf2 expression in neurons (Liddell et al. 2016). Considering the role of Nrf2 in the upregulating endogenous antioxidant mechanisms such as glutathione system (Turpaev 2013), it is possible that induction of Nrf2 accounts for PDTC antioxidant effects.
We observed that i.t. treatment with PDTC reduced mechanical hyperalgesia, thermal hyperalgesia, oedema and MPO activity, but not NAG activity in the paw skin after superoxide anion peripheral stimulus. Therefore, peripheral superoxide anion-induced inflammation (paw oedema and neutrophil recruitment [MPO activity]) and pain depend on spinal cord NF-κB activation, which participates in a neuronal retrograde enhancement of inflammation in the paw. This phenomenon has been described as dorsal root ganglia reflex (Rees et al. 1994; Sluka et al. 1995) and teleantagonism (Funez et al. 2008). Furthermore, this spinal cord NF-κB-dependent dorsal root ganglia reflex was selective to cells presenting MPO activity without affecting cells presenting NAG activity. It is possible that superoxide anion induces the activation of tissue resident cells including macrophages (NAG expressing cells), which further produce inflammatory mediators that will in turn activate nociceptors. The peripheral activation of nociceptors will trigger a NF-κB-dependent dorsal root ganglia reflex that contributes to regulating the recruitment of MPO-expressing cells, which are mainly neutrophils at 6–7 h after KO2 injection. On the other hand, NAG-expressing cells are activated before and contribute to dorsal root ganglia reflex development, but are not directly modulated by dorsal root ganglia reflex at 7 h after KO2 injection. However, further mechanistic insight is necessary to determine how NF-κB-dependent dorsal root ganglia reflex modulates peripheral inflammatory paw oedema and MPO activity without affecting NAG activity.
Taken together, our results demonstrated the role of NF-κB activation in peripheral and spinal cord nociceptive sensitisation after peripheral superoxide anion stimulus, revealing the role of this inflammatory cascade during superoxide anion-induced inflammatory pain. Moreover, the NF-κB inhibitor PDTC was capable of inhibiting the pathophysiological alterations induced by superoxide anion, proving to be an interesting compound for the control of inflammatory and/or painful conditions related to the superoxide anion.
Conclusions
The present study demonstrates that pyrrolidine dithiocarbamate (PDTC) inhibited the superoxide anion donor (potassium superoxide)-induced mechanical hyperalgesia, thermal hyperalgesia, paw oedema and recruitment of leukocytes. PDTC also inhibited superoxide anion-induced cytokine production and oxidative stress in the paw skin and spinal cord. The mechanism of PDTC depends, at least in part, on inhibiting NF-κB activation. These data contribute to advancing the knowledge on the mechanism by which superoxide anion modulates inflammatory pain and also reveal that PDTC is an effective compound to control inflammation and hyperalgesia related to increased cytokine production and oxidative stress induced by the superoxide anion. Finally, targeting spinal cord NF-κB with PDTC diminishes peripheral superoxide anion-induced inflammation, possibly by inhibiting dorsal root ganglia reflex.
References
Anrather J, Racchumi G, Iadecola C (2006) NF-kappaB regulates phagocytic NADPH oxidase by inducing the expression of gp91phox. J Biol Chem 281:5657–5667
Basbaum AI, Bautista DM, Scherrer G, Julius D (2009) Cellular and molecular mechanism of pain. Cell 139:267–284
Borghi SM, Pinho-Ribeiro FA, Zarpleon AC (2015) Interleukin-10 limits intense acute swimming-induced muscle mechanical hyperalgesia in mice. Exp Physiol 100:531–544
Bruck R, Aeed H, Schey R et al. (2002) Pyrrolidine dithiocarbamate protects against thioacetamide-induced fulminant hepatic failure in rats. J Hepatol 36:370–377
Bressan E, Peres KC, Tonussi CR (2012) Evidence that LPS-reactive arthritis in rats depends on the glial activity and the fractalkine-TNF-alpha signaling in the spinal cord. Neuropharmacology 62:947–958
Cheng G, Whitehead SN, Hachinskl V et al (2006) Effects of pyrrolidine dithiocarbamate on beta-amyloid (25-35)-induced inflammatory responses and memory deficits in the rats. Neurobiol Dis 23:140–151
Chuang HH, Lin S (2009) Oxidative challenges sensitize the capsaicin receptor by covalent cysteine modification. Proc Natl Acad Sci USA 106:20097–20102
Cunha TM, Verri WA Jr, Vivancos GG et al (2004) An electronic pressure-meter nociception paw test for mice. Braz J Med Biol Res 37:401–407
Cunha TM, Verri WA Jr, Schivo IR et al (2008) Role of neutrophils in the development of mechanical inflammatory hypernociception. J Leukoc Biol 83:824–832
Cuzzocrea S, Chatterjee PK, Mazon E et al (2002) Pyrrolidine dithiocarbamate attenuates the development of acute and chronic inflammation. Br J Pharmacol 135:496–510
Doyle T, Bryant L, Batinic-Haberle I et al (2009) Supraspinal inactivation of mitochondrial superoxide dismutate is a source of peroxynitrite in the development of morphine antinociceptive tolerance. Neurosci 164:702–710
Doyle T, Bryant L, Muscoli C et al (2010) Spinal NADPH oxidase is a source of superoxide in the development of morphine-induced hyperalgesia and antinociceptive tolerance. Neurosci Lett 483:85–89
Doyle T, Bryant L, Muscoli C et al (2012) Targeting the overproduction of peroxynitrite for the prevention and reversal of paclitaxel-induced neuropathic pain. J Neurosci 32:6149–6160
Fattori V, Pinho-Ribeiro FA, Borghi SM et al (2015) Curcumin inhibits superoxide anion-induced pain-like behavior and leukocyte recruitment by increasing Nrf2 expression and reducing NF-kappaB activation. Inflamm Res 64:993–1003
Funez MI, Ferrari LF, Duarte DB et al (2008) Teleantagonism: a pharmacodynamic property of the primary nociceptive neuron. Proc Natl Acad Sci USA 105:19038–19043
Gao YJ, Ji RR (2010) Chemokines, neuronal-glial interactions and central processing of neuropathic pain. Pharmacol Ther 126:56–68
Gao X, Zhang H, Belmadani S et al (2008) Role of TNF-alpha-induced reactive oxygen species in endothelial dysfunction during reperfusion injury. Am J Physiol Heart Circ Physiol 295:H2242–H2249
Gruber-Schoffnegger D, Drdla-Schutting R, Honigsperger C et al (2013) Induction of thermal hyperalgesia and synaptic long-term potentiation in the spinal cord lamina I by TNF-alpha and IL-1beta is mediated by glial cells. J Neurosci 33:6540–6551
Guerrero AT, Verri WA Jr, Cunha TM et al (2008) Involvement of LTB4 in zymosan-induced joint nociceotion in mice: participation of neutrophils and PGE2. J Leukoc Biol 83:122–130
Hayakawa M, Miyashita H, Sakamoto I et al (2003) Evidence that reactive oxygen species do not mediate NF-kappaB activation. EMBO J 22:3356–3366
Hohmann MS, Cardoso RD, Pinho-Ribeiro FA et al (2013) 5-lipoxygenase deficiency reduces acetaminophen-induced hepatotoxicity and lethality. Biomed Res Int 2013:1–13
Ivan AL, Campanini MZ, Martinez RM et al (2014) Pyrrolidine dithiocarbamate inhibits UVB-induced skin inflammation and oxidative stress in hairless mice and exhibits antioxidant activity in vitro. J Photochem Photobiol B 138:124–133
Jamaluddin M, Wang S, Boldogh I, Tian B, Brasier AR (2007) TNF-alpha-induced NF-kappaB/RelA Ser(276) phosphorylation and enhanceosome formation is mediated by an ROS-dependent PKAc pathway. Cell Signal 19:1419–1433
Janes K, Neumann WL, Salvemini D (2012) Anti-superoxide and anti-peroxynitrite strategies in pain suppression. Biochim Biophys Acta 1822:815–821
Kim CH, Kim JH, Hsu CY, Ahn YS (1999a) Zinc is required in pyrrolidine dithiocarbamate inhibition of NF-kappaB activation. FEBS Lett 449:28–32
Kim CH, Kim JH, Moon SJ et al (1999b) Pyrithione, a zinc ionophore, inhibits NF-kappaB activation. Biochem Biophys Res Commun 259:505–509
Kim HY, Chung JM, Chung K (2008) Increased production of mitochondrial superoxide in the spinal cord induces pain behavior in mice: the effect of mitochondrial electron transport complex inhibitors. Neurosci Lett 447:87–91
Kim HY, Wang J, Lu Y et al (2009) Superoxide signalling in pain is independent of nitric oxide signalling. NeuroReport 20:1424–1428
Kotake Y, Moore DR, Vasquez-Walden A et al (2003) Antioxidant amplifies antibiotic protection in the cecal ligation and puncture model of microbial sepsis through interleukin-10 production. Shock 19:252–256
Kvietys PR, Granger DN (2012) Role of reactive oxygen and nitrogen species in the vascular responses to inflammation. Free Radic Biol Med 52:556–592
Lee CH, Kim SH, Lee SM (2008) Effect of pyrrolidine dithiocarbamate on hepatic vascular stress gene expression during ischemia and reperfusion. Eur J Pharmacol 595:100–107
Li H, Han M, Guo L et al (2011) Oxidative stress, endothelial dysfunction and inflammatory response in rat heart to NO(2) inhalation exposure. Chemosphere 82:1589–1596
Liddell JR, Lehtonen S, Duncan C et al (2016) Pyrrolidine dithiocarbamate activates the Nrf2 pathway in astrocytes. J Neuroinflammation 13:49
Little JW, Chen Z, Doyle T et al (2012a) Supraspinal peroxynitrite modulates pain signaling by suppressing the endogenous opioid pathway. J Neurosci 32:10797–10808
Little JW, Doyle T, Salvemini D (2012b) Reactive nitroxidative species and nociceptive processing: determining the roles for nitric oxide, superoxide, and peroxynitrite in pain. Amino Acids 42:75–94
Little JW, Cuzzocrea S, Bryant L et al (2013) Spinal mitochondrial-derived peroxynitrite enhances neuroimmune activation during morphine hyperalgesia and antinociceptive tolerance. Pain 154:978–986
Lundberg JO, Weitzberg E, Gladwin MT (2008) The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov 7:156–167
Maioli NA, Zarpelon AC, Mizokami SS et al (2015) The superoxide anion donor, potassium superoxide, induces pain and inflammation in mice through production of reactive oxygen species and cyclooxygenase-2. Braz J Med Biol Res 48:321–331
Mantovani A, Cassatella MA, Constantini C et al (2011) Neutrophil in the activation and regulation of innate and adaptative immunity. Nat Rev Immunol 11:519–531
Naito Y, Takagi T, Higashimura Y (2014) Heme oxygenase-1 and anti-inflammatory M2 macrophages. Arch Biochem Biophys 15:83–88
Ndengele MM, Muscoli C, Wang ZQ et al (2005) Superoxide potentiates NF-kappaB activation and modulates endotoxin-induced cytokine production in alveolar macrophages. Shock 23:186–193
Ndengele MM, Cuzzocrea S, Esposito E et al (2008) Cyclooxygenases 1 and 2 contribute to peroxynitrite-mediated inflammatory pain hypersensitivity. FASEB J 22:3154–3164
Németh ZH, Haskó G, Vizi ES et al (1998) Pyrrolidine dithiocarbamate augments IL-10, inhibits TNF-α, MIP-1alpha, IL-12, and nitric oxide production and protects from the lethal effect of endotoxin. Shock 10:49–53
Pinho-Ribeiro FA, Hohmann MS, Borghi SM et al (2015) Protective effects of the flavonoid hesperidin methyl chalcone in inflammation and pain in mice: role of TRPV1, oxidative stress, cytokines and NF-kappaB. Chem Biol Interact 228:88–99
Qiu M, Chen Y, Cheng L et al (2013) Pyrrolidine dithiocarbamate inhibits herpes simplex virus 1 and 2 replication, and its activity may be mediated through dysregulation of the ubiquitin-proteasome system. J Virol 87:8675–8686
Rees H, Sluka KA, Westlund KN et al (1994) Do dorsal root reflexes augment peripheral inflammation? NeuroReport 21:821–824
Ruiz-Miyazawa KW, Pinho-Ribeiro FA, Zarpelon AC et al (2015) Vinpocetine reduces lipopolysaccharide-induced inflammatory pain and neutrophil recruitment in mice by targeting oxidative stress, cytokines and NF-κB. Chem Biol Interact 237:9–17
Salvemini D, Riley DP, Cuzzocrea S (2002) SOD mimetics are coming of age. Nat Rev Drug Discov 1:367–374
Salvemini D, Little JW, Doyle T et al (2011) Roles of reactive oxygen and nitrogen species in pain. Free Rad Biol Med 51:951–966
Schreck R, Meier B, Mannel DN et al (1992) Dithiocarbamates as potent inhibitors of nuclear factor κB activation in intact cells. J Exp Med 175:1181–1194
Serafim KG, Navarro SA, Zarpelon AC et al (2015) Bosentan, a mixed endothelin receptor antagonist, inhibits superoxide anion-induced pain and inflammation in mice. Naunyn Schmiedebergs Arch Pharmacol 388:1211–1221
Sluka KA, Rees H, Westlund KN et al (1995) Fiber types contributing to dorsal root reflexes induced by joint inflammation in cats and monkeys. J Neurophysiol 74:981–989
Souza GR, Talbot J, Lotufo CM et al (2013) Fractalkine mediates inflammatory pain through activation of satellite glial cells. Proc Natl Acad Sci USA 110:11193–11198
Turpaev KT (2013) Keap1-Nrf2 signaling pathway: mechanisms of regulation and role in protection of cells against toxicity caused by xenobiotics and electrophiles. Biochemistry (Mosc). 78:111–126
Verri WA Jr, Cunha TM, Parada CA et al (2006) Hypernociceptive role of cytokines and chemokines: targets for analgesic drug development? Pharmacol Ther 112:116–138
Verri WA Jr, Cunha TM, Ferreira SH et al (2007) IL-15 mediates antigen-induced neutrophil migration by triggering IL-18 production. Eur J Immunol 12:3373–3380
Wang ZQ, Porreca F, Cuzzocrea S et al (2004) A newly identified role for superoxide in inflammatory pain. J Pharmacol Exp Ther 309:869–878
Wieseler-Frank J, Maier SF, Watkins LR (2004) Glial activation and pathological pain. Neurochem Int 45:389–395
Wild AC, Moinova HR, Mulcahy RT (1999) Regulation of gamma-glutamylcysteine synthetase subunit gene expression by the transcription factor Nrf2. J Biol Chem 274:33627–33636
Yamacita-Borin FY, Zarpelon AC, Pinho-Ribeiro FA et al (2015) Superoxide anion-induced pain and inflammation depends on TNFalpha/TNFR1 signaling in mice. Neurosci Lett 605:53–58
Yang SR, Valvo S, Yao H et al (2008) IKK alpha causes chromatin modification on pro-inflammatory genes by cigarette smoke in mouse lung. Am J Respir Cell Mol Biol 38:689–698
Yoshino Y, Yamamoto S, Kohsaka S et al (2011) Superoxide anion contributes to the induction of tumor necrosis factor alpha (TNFalpha) through activation of the MKK3/6-p38 MAPK cascade in rat microglia. Brain Res 1422:1–12
Yu XJ, Zhang DM, Jia LL et al (2015) Kang, Inhibition of NF-kappaB activity in the hypothalamic paraventricular nucleus attenuates hypertension and cardiac hypertrophy by modulating cytokines and attenuating oxidative stress. Toxicol Appl Pharmacol 284:315–322
Zarpelon AC, Rodrigues FC, Lopes AH et al (2016) Spinal cord oligodendrocyte-derived alarmin IL-33 mediates neurophatic pain. FASEB J 30:54–65
Zipper LM, Mulcahy RT (2000) Inhibition of ERK and p38 MAP kinases inhibits binding of Nrf2 and induction of GCS genes. Biochem Biophys Res Commun. 278:484–492
Zipper LM, Mulcahy RT (2003) Erk activation is required for Nrf2 nuclear localization during pyrrolidine dithiocarbamate induction of glutamate cysteine ligase modulatory gene expression in HepG2 cells. Toxicol Sci 73:124–134
Acknowledgments
Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), São Paulo Research Foundation (FAPESP) under grant agreements number 2011/19670-0 (Thematic project) and 2013/08216-2 (Center for Research in Inflammatory Disease-CRID), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Ministério da Ciência, Tecnologia e Inovação (MCTI), Secretaria da Ciência, Tecnologia e Ensino Superior (SETI), Fundação Araucária and Parana State Government grants supported this study (Brazil). A.C.Z. received a post-doc fellowship from CAPES/Fundação Araucária, S.M.B. received a post-doc fellowship from CAPES, and L.S.F. received a post-doc fellowship from CNPq. It is noteworthy to mention that this manuscript has been read and approved by all the authors, which contributed substantially to the work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Pinho-Ribeiro, F.A., Fattori, V., Zarpelon, A.C. et al. Pyrrolidine dithiocarbamate inhibits superoxide anion-induced pain and inflammation in the paw skin and spinal cord by targeting NF-κB and oxidative stress. Inflammopharmacol 24, 97–107 (2016). https://doi.org/10.1007/s10787-016-0266-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-016-0266-3