Abstract
The aim of the present study was to investigate the effect of MMR vaccine on inflammation which was induced by complete Freund’s adjuvant (CFA) in male Sprague–Dawley rats. Rats were randomly divided into the control, CFA, MMR and CFA + MMR groups. Inflammatory symptoms such as paw oedema was measured in CFA-injected rats’ paw. Body weight changes and alterations in some haematological parameters and oxidative stress markers following CFA injection were checked. In CFA-inflammed rats, there was a significant increase in rat paw thickness and decrease in body weight increment. MMR exhibited a significant anti-inflammatory effect as manifested by reduction in paw thickness and normal gain in body weight when administered 1 week prior to induction of inflammation. The altered haematological parameters (TLC) and oxidative stress markers (MDA, GSH, SOD) in the inflammed rats were significantly brought back to near normal by MMR treatment. In conclusion, MMR vaccine showed a reduction in rat paw thickness and it could significantly normalize the haematological and biochemical abnormalities in CFA-induced inflammatory pain model in rats. Our data suggested that MMR could be a potential protective agent against certain types of inflammatory pain. Further histopathological and radiological studies are required to confirm the possibility of developing novel therapeutic vaccines against some forms of arthritis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
MMR vaccine is a combination vaccine containing three attenuated live virus vaccines––measles, mumps and rubella. This combination vaccine was first licensed in USA in 1971 (Puvvada et al. 1993).
It has been reported that “one vaccine decreases cell-mediated immunity by 50%, two vaccines by 70% and all triple vaccines such as MMR markedly impair cell-mediated immunity” (Beckenhauer and Gill 1983).
Immune system is involved in the etiology and pathophysiologic mechanisms of inflammation. Inflammatory pain mechanisms feature in many chronic pain states, including osteoarthritis (OA) and rheumatoid arthritis (RA). Rheumatoid arthritis is a chronic, autoimmune, destructive inflammatory disease characterized by inflammation of the synovial tissue, cartilage loss, bone erosion and eventual joint destruction (Lawrence et al. 1998; Tastekin et al. 2007).
Many research efforts have focused on oxidative stress in RA (Ramprasath et al. 2005; Mythilypriya et al. 2007; Tastekin et al. 2007). There have been claims that pro-oxidant/antioxidant balance is interrupted by some cellular reactions or an insufficiency in antioxidant defense system.
The chronic vascular inflammation associated with RA results in the recruitment and accumulation of numerous cell types (i.e. activated macrophages, neutrophils, mast cells, and lymphocytes) into the synovium. Under defined microenvironmental conditions, these cell types can be induced to generate reactive oxygen species (ROS) (Tak et al. 1995). Also in many joint diseases, proinflammatory factors such as cytokines and prostaglandins are released at sites of inflammation, together with ROS (Henrotin et al. 2003).
Since a cure for RA with unknown etiology is not available yet, there is still an urgent need for a therapeutic agent capable of preventing disease progression or reverting joint destruction. This study was undertaken to evaluate the possible modulatory effect of MMR vaccine in a rat model of inflammation (as a feature of arthritis) using parameters of inflammation and oxidative stress.
Materials and methods
Experimental animals
Twenty-four adult male Spraque–Dawley rats, weighing between 150 and 180 g were used. Animals were purchased from Urology and Nephrology Center, Mansoura University, Egypt and were kept at constant environmental and nutritional conditions throughout the experimental period at a temperature of 25 ± 2°C with a 12 h on/off light schedule. Standard food and water were allowed to animals throughout the experiment. The animal experiments described in this study comply with the ethical principles and guidelines for the care and use of laboratory animals adopted by the “Research Ethics Committee” of Faculty of Pharmacy, Mansoura University, Egypt in accordance with “Principles of Laboratory Animal Care” (NIH publication No. 85-23, revised 1985). The study was approved by the head of Pharmacology and Toxicology department, Faculty of Pharmacy, Mansoura University, Egypt.
Drugs and chemicals
MMR (measles, mumps, rubella) vaccine (PRIORIX®), white to slightly pink pellet for reconstitution with 5 ml sterile water for injection, was purchased from GlaxoSmithKline Biologicals (SARue de l’Institut 89/1330 Rixensart, Belgium). Each ml of Complete Freund’s adjuvant (CFA), contains 1 mg of Mycobacterium tuberculosis (H37Ra, ATCC 25177) heat killed and dried, 0.85 ml paraffin oil and 0.15 mannide monoleate, Ellman’s reagent, pyrogallol, reduced glutathione, 1,1′,3,3′-tetramethoxypropane and tris(hydroxymethyl)aminomethane were purchased from Sigma Aldrich Chemical Co (St. Louis, MO, USA). n-Butanol was purchased from El-Nasr Chemical Co. (Abou-Zaabal, Cairo, Egypt). Thiobarbituric acid (TBA) was purchased from Fluka (Chemie, Switzerland). Trichloroacetic acid (TCA) was purchased from Winlab (Leicestershire, UK).
Buffers Phosphate buffer 0.1 M (pH 8): Anhydrous K2HPO4 (14.98 g) was dissolved in 600 ml distilled water and the pH was adjusted by addition of suitable volume of KH2PO4 (13.6 g dissolved in 1,000 ml distilled water).
Experimental protocol
Inflammatory pain rat model was induced by an injection of CFA into the plantar surface of right hind paw. The animals were allocated into four groups each consisting of six rats. Group 1 served as control and received paraffin oil (0.2 ml) SC in right hind paw, and equivalent volume of normal saline (0.2 ml) SC under the skin. Group 2 received single injection of CFA (0.2 ml, SC) (Broderson 1989). Group 3 received MMR vaccine (0.2 ml) SC under the skin. Group 4 received single injection of MMR vaccine (0.2 ml) SC 1 week before CFA injection.
Assessment of inflammation
The severity of inflammation was assessed using paw thickness method which was measured using caliber on day 0 before inoculation and days 3, 7 and 14 after inoculation (Tastekin et al. 2007). The weight of animals was also recorded on the same days.
Biochemical measurements
Blood was collected in tubes containing EDTA from the retro-orbital venous plexus under light ether anesthesia by heparinized capillary tubes on days 3, 7 and 14 after injecting CFA and/or MMR. Blood samples were centrifuged at 4,000 rpm for 15 min to separate plasma for analysis.
The following parameters were estimated in plasma. Thiobarbituric acid reactive substance was measured as malondialdehyde (MDA), the end product of lipid peroxidation, according to the method of Ohkawa et al. (1979). The absorbance was determined at 532 nm spectrophotometrically. In TCA-deproteinized plasma, non-protein sulfhydryl (NPSH) compound was determined as reduced glutathione (GSH) by the method of Ellman (1959) which was based on the reaction of GSH with Ellman’s reagent [5,5′-dithio-bis(2-nitrobenzoic acid)] to give a compound that absorbs at 412 nm. Superoxide dismutase activity was measured by the method of Marklund and Marklund (1974), the degree of inhibition of the auto-oxidation of pyrogallol at an alkaline pH by SOD was used as a measure of the enzyme activity.
Total leukocyte count (TLC)
TLC (lymphocytes, neutrophils, monocytes, eosinophils and basophils) was estimated in one drop of blood smear on a slide using Leishmann stain under the light microscope using a haemocytometer.
Statistical analysis
Results are expressed as mean ± SEM. Statistical analysis was performed by the aid of Instat-2 computer program (GraphPad Software Inc. V2.04, San Diego, CA, USA) using one-way analysis of variance (ANOVA) followed by Tukey–Kramer multiple comparisons test. Significance was accepted when p < 0.05.
Results
Effect of MMR vaccine on body weight and paw thickness in CFA-inflamed rats
Injection of CFA in rats led to significant decrease in body weight (Table 1) and increase in paw thickness (Table 2) on days 3, 7, and 14 in comparison with healthy control (HC) group.
Administration of MMR vaccine 1 week before induction of inflammation significantly increased the body weight at third and seventh days in comparison with CFA group while a significant decrease in paw thickness was observed on all experimental days.
Effect of MMR vaccine on TLC in CFA-inflamed rats
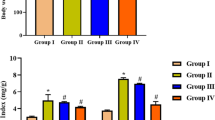
Injection of CFA in rats led to a significant increase in TLC on days 3, 7 and 14 compared to HC group (Fig. 1).
Effect of MMR vaccine on total leukocyte count (TLC) in CFA-inflamed rats. Data are expressed as mean ± SEM, n = 6. Mean values were compared using one-way ANOVA followed by Tukey–Kramer multiple comparisons test (p < 0.05). Plasma was collected on days 3, 7 and 14 after injecting vehicle, CFA, MMR or MMR-CFA. *Significantly different from healthy control (HC) group. $Significantly different from CFA group
Administration of MMR vaccine 1 week before induction of inflammation significantly decreased TLC in all experimental days compared to CFA group.
Effect of MMR vaccine on plasma MDA, GSH levels and SOD activity in CFA-inflamed rats
Injection of CFA in rats led to a significant increase in plasma MDA level (Fig. 2) on days 3, 7 and 14 while a significant decrease in plasma GSH level (Fig. 3) on days 7 and 14 compared to HC group.
Effect of MMR vaccine on plasma MDA level in CFA-inflamed rats. Data are expressed as mean ± SEM, n = 6. Mean values were compared using one-way ANOVA followed by Tukey–Kramer multiple comparisons test (p < 0.05). Plasma was collected on days 3, 7 and 14 after injecting vehicle, CFA, MMR or MMR-CFA. *Significantly different from healthy control (HC) group. $Significantly different from CFA group
Effect of MMR vaccine on plasma GSH level in CFA-inflamed rats. Data are expressed as mean ± SEM, n = 6. Mean values were compared using one-way ANOVA followed by Tukey–Kramer multiple comparisons test (p < 0.05). Plasma was collected on days 3, 7 and 14 after injecting vehicle, CFA, MMR or MMR-CFA. *Significantly different from healthy control (HC) group. $Significantly different from CFA group
Administration of MMR vaccine 1 week before induction of inflammation significantly decreased plasma MDA level during all experimental days compared to CFA group. A significant increase in plasma GSH level was observed on days 7 and 14 in comparison with CFA group.
Injection of CFA in rats led to a significant decrease in plasma SOD activity on days 3, 7 and 14 in comparison with HC group (Fig. 4).
Effect of MMR vaccine on plasma SOD activity in CFA-inflamed rats. Data are expressed as mean ± SEM, n = 6. Mean values were compared using one-way ANOVA followed by Tukey–Kramer multiple comparisons test (p < 0.05). Plasma was collected on days 3, 7 and 14 after injecting vehicle, CFA, MMR or MMR-CFA. *Significantly different from healthy control (HC) group. $Significantly different from CFA group
In MMR-CFA group, a significant increase in plasma SOD activity was observed during the specified days in comparison with CFA group.
Discussion
In the present study, CFA was used in rats and the effect of protective dose of MMR vaccine was studied.
The CFA is an effective means of potentiating cellular and humoral antibody response to injected immunogens. Adjuvant activity is a result of sustained release of antigen from the oily deposit and stimulation of a local innate immune response resulting in enhanced adaptive immunity. The essential component of this response is an intense inflammatory reaction, resulting from an influx of leukocytes and their interaction with antigen, may cause local inflammation and granulomatous reactions at the site of injection (Broderson 1989).
In the present study, the body weight of control rats increased steadily during the experimental period, while the inflamed rats showed significantly lowered body weight from day 3 after CFA injection; this observation agreed with the report of Chen et al. (2009). This may be attributed to the inflammatory effect of CFA, swelling and oedema in the injected paw that may lead to reduction in the ability of rats’ intake of food due to pain.
The thickness of the paw injected with CFA has been widely used for studying the extent of inflammation and the therapeutic efficacy of the drug. Inhibition of paw oedema in adjuvant arthritic rats is a hallmark for anti-inflammatory drug action (Ramprasath et al. 2005).
In the present study, a remarkable swelling, redness, increase in paw thickness developed in the paw injected with CFA and reached maximum intensity on day 3, probably because of the local inflammatory effect of CFA which increases blood supply, accumulation of neutrophils and leukocyte resulting in swelling and oedema in the injected paw. This inflammatory reaction subsides slightly during the next 7–14 days. These results are supported by the study of Jung et al. (2005) and Tastekin et al. (2007).
Administration of MMR before CFA-induced inflammation significantly decreased swelling, redness and paw thickness compared to the inflamed group; this observation could be possibly attributed to the presence of attenuated measles virus in MMR vaccine which is able to elevate immunoregulatory interleukin (IL-10), which suppresses macrophage activation and T-cell proliferation (based on a preliminary study using measles vaccine solely). In addition, it is well known that measles virus impairs antigen-presenting cells (Servet-Delprat et al. 2000) and thus reduces secretion of inflammatory markers and inflammatory cytokines resulting in reduction of oedema, inflammation and swelling.
In the present study, total leukocyte count in CFA group showed a significant increase from the third day of CFA injection when compared to control group. Administration of MMR before induction of inflammation resulted in a significant decrease in TLC observed from day 3. This may be due to the impairment of antigen-presenting cells capability of attenuated measles virus in MMR vaccine.
Adjuvant-induced arthritis is thought to occur through cell-mediated autoimmunity by structural mimicry between mycobacteria and cartilage proteoglycans in rats. Thus, activated macrophages and lymphocytes by adjuvant inoculation or their products, monokines, may be involved in abnormal lipid and protein metabolism (Van et al. 1985). Several reports have shown that during inflammatory joint diseases, such as adjuvant-induced arthritis, joint diseases, phagocytes accumulate in the joints and produce superoxide radicals and hydrogen peroxide, which in the presence of traces of iron salts found in synovial fluids, interact to form the highly reactive hydroxyl radical (OH·). Thus, free radicals might contribute in the biochemical changes (Fahim et al. 1995; Chamundeeswari et al. 2004). In order to reduce the damage caused by the ROS, multiple defense systems collectively called antioxidants are present in both human and in animals.
ROS have been proposed to mediate cell damage via a number of independent mechanisms including the initiation of lipid peroxidation, the inactivation of a variety of enzymes and depletion of GSH (Cuzzocrea et al. 2000). Prime targets of ROS attack are the polyunsaturated fatty acids in the membrane lipids causing lipid peroxidation which may lead to disorganization of cell structure and function. Furthermore, decomposition of peroxidized lipids yields a wide variety of end products, including MDA (Lunec et al. 1981; Floyd 1990).
In the present study, significantly elevated levels of MDA in the plasma of CFA-inflamed rats were observed when compared to control group. These observations indicate an increased ROS production, the deactivation of SOD, GSH and thus increased levels of lipid peroxidation. These results are supported by the reports of Chamundeeswari et al. (2004) and Mythilypriya et al. (2007).
Treatment of rats with MMR prior to induction of inflammation showed a significant decrease in MDA level compared to CFA group from day 3, this may be due to the impairment of antigen-presenting cell property of measles in MMR vaccine; thus reducing accumulation of neutrophils and leukocyte cells ROS production and MDA as an end product.
Reduced glutathione, an intra-cellular thiol rich tripeptide, is an important member of the antioxidant team as it has been shown to destroy ROS and other free radicals by enzymatic as well as non-enzymatic mechanisms and thus it plays a major role in the protection of cells and tissue structures (Meister and Anderson 1983; Chamundeeswari et al. 2003).
Superoxide dismutase, an enzymatic antioxidant, catalytically scavenges the superoxide radical by dismutation of O2 to H2O2. The cell is then protected from the potential toxicity of H2O2 by the catalase enzyme and thus provides the first line of defense against free radical damage. The anti-inflammatory activity of SOD has been shown in several animal models of induced inflammation as well as in clinical trials in humans (Mccord et al. 1980; Petrone et al. 1980).
Jaswal et al. (2003) reported that GSH levels of RA patients were significantly lower than that in healthy subjects. The decrease of GSH in RA patients was emphasized for its relation with tissue damage. The work of Vijayalakshmi et al. (1997) is consistent with the present study and showed GSH decline in plasma, liver and kidney of arthritic rats.
In this study, the level of GSH was significantly decreased from the seventh day after CFA injection. MMR administration before induction of inflammation significantly increased GSH levels when compared to CFA group.
A significant decrease in SOD activity was observed from the third day in CFA group when compared to control group, while in the group pre-treated with MMR, a significant increase in SOD activity was observed compared to inflamed group from the third day.
The lower level of antioxidant system after CFA injection may be attributed to phagocytes accumulation and production of ROS. This leads to more consumption of antioxidant system including GSH and SOD and appearance of redox imbalance.
In a previous study, Heijstek et al. (2007) investigated the effect of the MMR vaccination in juvenile idiopathic arthritis (JIA) showing that MMR booster vaccination does not seem to aggravate disease activity in JIA indicating that most patients with JIA can be vaccinated safely with the MMR vaccine. However, our results demonstrate for the first time that MMR vaccine could be helpful in reducing the inflammation associated with certain diseases such as rheumatoid arthritis or osteoarthritis and suggest the use of MMR vaccine as a therapeutic vaccine against some forms of arthritis.
Abbreviations
- CFA:
-
Complete Freund’s adjuvant
- MMR:
-
Measles–Mumps–Rubella
- TLC:
-
Total leukocyte count
- MDA:
-
Malondialdehyde
- GSH:
-
Reduced glutathione
- SOD:
-
Superoxide dismutase
- ROS:
-
Reactive oxygen species
References
Beckenhauer WH, Gill MA (1983) Immunosuppression with combined vaccines. J Am Vet Med Assoc 183:389–390
Broderson JR (1989) A retrospective review of lesions associated with the use of Freund’s adjuvant. Lab Anim Sci 39:400–405
Chamundeeswari D, Vasantha J, Gopalakrishnan S, Sukumar E (2003) Free radical scavenging activity of the alcoholic extract of Trewia polycarpa roots in arthritic rats. J Ethnopharmacol 88:51–56
Chamundeeswari D, Vasantha J, Gopalakrishnan S, Sukumar E (2004) Anti-inflammatory and antinociceptive activities of Trewia polycarpa roots. Fitoterapia 75:740–744
Chen H, Shoumura S, Emura S, Isono H (2009) Tibetan medicated-bath therapy may improve adjuvant arthritis in rat. Evid Based Complement Alternat Med 6:211–217
Cuzzocrea S, McDonald MC, Mota-Filipe H, Mazzon E, Costantino G, Britti D, Mazzullo G, Caputi AP, Thiemermann C (2000) Beneficial effects of tempol, a membrane-permeable radical scavenger, in a rodent model of collagen-induced arthritis. Arthritis Rheum 43:320–328
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Fahim AT, Abd-el Fattah AA, Agha AM, Gad MZ (1995) Effect of pumpkin-seed oil on the level of free radical scavengers induced during adjuvant-arthritis in rats. Pharmacol Res 31:73–79
Floyd RA (1990) Role of oxygen free radicals in carcinogenesis and brain ischemia. FASEB J 4:2587–2597
Heijstek MW, Pileggi GC, Zonneveld-Huijssoon E, Armbrust W, Hoppenreijs EP, Uiterwaal CS, Kuis W, Wulffraat NM (2007) Safety of measles, mumps and rubella vaccination in juvenile idiopathic arthritis. Ann Rheum Dis 66:1384–1387
Henrotin YE, Bruckner P, Pujol JP (2003) The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthr Cartil 11:747–755
Jaswal S, Mehta HC, Sood AK, Kaur J (2003) Antioxidant status in rheumatoid arthritis and role of antioxidant therapy. Clin Chim Acta 338:123–129
Jung HJ, Nam JH, Choi J, Lee KT, Park HJ (2005) Antiinflammatory effects of chiisanoside and chiisanogenin obtained from the leaves of Acanthopanax chiisanensis in the carrageenan- and Freund’s complete adjuvant-induced rats. J Ethnopharmacol 97:359–367
Lawrence RC, Helmick CG, Arnett FC, Deyo RA, Felson DT, Giannini EH, Heyse SP, Hirsch R, Hochberg MC, Hunder GG, Liang MH, Pillemer SR, Steen VD, Wolfe F (1998) Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum 41:778–799
Lunec J, Halloran SP, White AG, Dormandy TL (1981) Free-radical oxidation (peroxidation) products in serum and synovial fluid in rheumatoid arthritis. J Rheumatol 8:233–245
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474
Mccord JM, Wong K, Stokes SH, Petrone WF, English D (1980) Superoxide and inflammation: a mechanism for the anti-inflammatory activity of superoxide dismutase. Acta Physiol Scand Suppl 492:25–30
Meister A, Anderson ME (1983) Glutathione. Annu Rev Biochem 52:711–760
Mythilypriya R, Shanthi P, Sachdanandam P (2007) Restorative and synergistic efficacy of Kalpaamruthaa, a modified siddha preparation, on an altered antioxidant status in adjuvant induced arthritic rat model. Chem Biol Interact 168:193–202
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Petrone WF, English DK, Wong K, Mccord JM (1980) Free radicals and inflammation: superoxide-dependent activation of a neutrophil chemotactic factor in plasma. Proc Natl Acad Sci USA 77:1159–1163
Puvvada L, Silverman B, Bassett C, Chiaramonte LT (1993) Systemic reactions to measles–mumps–rubella vaccine skin testing. Pediatrics 91:835–836
Ramprasath VR, Shanthi P, Sachdanandam P (2005) Evaluation of antioxidant effect of Semecarpus anacardium Linn. nut extract on the components of immune system in adjuvant arthritis. Vascul Pharmacol 42:179–186
Servet-Delprat C, Vidalain PO, Azocar O, Le DF, Fischer A, Rabourdin-Combe C (2000) Consequences of Fas-mediated human dendritic cell apoptosis induced by measles virus. J Virol 74:4387–4393
Tak PP, Thurkow EW, Daha MR, Kluin PM, Smeets TJ, Meinders AE, Breedveld FC (1995) Expression of adhesion molecules in early rheumatoid synovial tissue. Clin Immunol Immunopathol 77:236–242
Tastekin N, Aydogdu N, Dokmeci D, Usta U, Birtane M, Erbas H, Ture M (2007) Protective effects of L-carnitine and alpha-lipoic acid in rats with adjuvant arthritis. Pharmacol Res 56:303–310
Van EW, Holoshitz J, Nevo Z, Frenkel A, Klajman A, Cohen IR (1985) Arthritis induced by a T-lymphocyte clone that responds to Mycobacterium tuberculosis and to cartilage proteoglycans. Proc Natl Acad Sci USA 82:5117–5120
Vijayalakshmi T, Muthulakshmi V, Sachdanandam P (1997) Salubrious effect of Semecarpus anacardium against lipid peroxidative changes in adjuvant arthritis studied in rats. Mol Cell Biochem 175:65–69
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abd El-Rahman, R.S., Suddek, G.M., Gameil, N.M. et al. Protective potential of MMR vaccine against complete Freund’s adjuvant-induced inflammation in rats. Inflammopharmacol 19, 343–348 (2011). https://doi.org/10.1007/s10787-011-0094-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-011-0094-4