Abstract
Chronic fatigue is an illness characterised by persistent and relapsing fatigue, often accompanied by numerous neuropsychiatric problems, such as anxiety and depression. The aetiology of chronic fatigue remains unclear so far. However, recent studies suggested the involvement of oxidative stress in this chronic debilitating disease. Alternatively, antioxidants have also been reported to have beneficial effect against chronic fatigue-like conditions. Therefore, present study has been designed to explore the potential role of pioglitazone, caffeic acid and their combination against chronic fatigue-like condition in mice. In the experimental protocol, the mice were put on the running wheel apparatus for 6 min test session daily for 21 days which produced fatigue-like condition. The locomotor activity and anxiety levels were measured on 0, 8th, 15th and 22nd days. The brains were isolated on 22nd day immediately after the behavioural assessments, oxidative damage and mitochondrial enzyme complexes were then estimated subsequently. Three weeks pioglitazone (5 and 10 mg/kg) and caffeic acid (5 and 10 mg/kg) pretreatment significantly attenuated the chronic fatigue-like condition (restored running wheel activity, locomotor activity and reduced anxiety-like behaviour) as compared to that in control (chronic fatigue) animals. Further, pioglitazone (5 and 10 mg/kg) and caffeic acid (5 and 10 mg/kg) drug treatments for 3 weeks significantly attenuated oxidative damage (decreased lipid peroxidation, nitrite concentration, restored reduction in glutathione and catalase levels), altered mitochondrial enzymes complex (I, II and IV) activities and mitochondrial redox activity (MTT assay) when compared with control. Further, combination of lower dose of pioglitazone (5 mg/kg) and caffeic acid (5 mg/kg) showed significant synergism in their protective effect which was significant as compared to their effect per se. The present study highlights the potential role of pioglitazone, caffeic acid and their combination in the pathophysiology of chronic fatigue-like condition in mice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic fatigue is a complex, debilitating disease characterised by extreme fatigue that does not improve with rest. Chronic persistent fatigue is often associated with sleep difficulties, neuropsychiatric problems (anxiety, depression), neuroendocrine abnormalities and various somatic complaints (Afari and Buchwald 2003). It is a disorder of multifactorial aetiology (Afari and Buchwald 2003). Abnormal activation of the pro-inflammatory cytokines and formation of reactive oxygen species explain many manifestations of this disease process (Lorusso et al. 2009). Experimental evidence showed that oxidative stress and more specifically lipid peroxidation contribute to the progression of chronic fatigue (Manuel et al. 2001; Nijs and De Meirleir 2004).
The role of oxidative stress in the pathogenesis of chronic fatigue is an emerging focal area of research (Fulle et al. 2003; Jammes et al. 2005). Recent studies demonstrated that oxidative stress contributes to the pathology and clinical symptoms of fatigue (Manuel et al. 2001; Kennedy et al. 2005; Madrigal et al. 2001). There is no immediate treatment available for the effective management of this disease. However, plenty of evidence suggest the potential beneficial effects of antioxidants in the management of chronic fatigue and related problems (Singh et al. 2002; Singal et al. 2005; Gupta et al. 2009).
Pioglitazone activates peroxisome proliferator-activated receptor gamma (PPAR-γ), a ligand-activated transcription factor, thereby inducing cell differentiation and inhibiting cell growth and angiogenesis. It also modulates the transcription of insulin-responsive genes, inhibits macrophage and monocyte activation, and stimulates adipocyte differentiation. Recent studies have suggested the therapeutic potential of the PPAR-γ agonist in animal models of neurodegenerative diseases (Watson et al. 2005; Kiaei et al. 2005). Some of the PPAR-γ agonists have already been reported to be useful in stress-related disorders (García-Bueno et al. 2005). Previous study suggested that rosiglitazone attenuated stress-induced changes in brain by inhibiting proinflammatory mediators, such as cytokines tumour necrosis factor-α and inducible nitric oxide synthase (NOS-2) (García-Bueno et al. 2005). Pioglitazone has also been reported to show its beneficial effect in memory processing which is attributed to its favourable effect on glucose utilisation and metabolism and antioxidant action (Pathan et al. 2006).
Caffeic acid (3, 4-dihydroxycinnamic acid), is a natural phenolic compound, exerts potent antioxidant, antiinflammatory properties and free-radical scavenging action by acting through lipoxygenase pathways (Sul et al. 2009). Recently neuroprotective effect of caffeic acid has also been suggested (Kalonia et al. 2009a, b). Takeda et al. have also demonstrated that caffeic acid reduced the duration of immobility period in the forced swimming test and altered expression of BDNF mRNA in the frontal cortex, which suggests its antidepressive-like activity (Takeda et al. 2006). Reports from our laboratory also established the antioxidant profile of caffeic acid (Singh et al. 2002). Though both these drugs are already reported to possess antioxidant-like action, however, their exact statuses in different neurodegenerative conditions are still lacking and poorly understood. On the basis of previous reports, present study has been designed to explore the potential action of pioglitazone, caffeic acid and their combination against stress-induced behavioural and biochemical and cellular alterations. Combination of pioglitazone and caffeic acid was tried with a hope that this combination will produce synergistic-like effect and provide more neuroprotective and antioxidant-like effects when compared with their effect per se.
Present study was designed to explore the potential role of pioglitazone, caffeic acid and their combination against chronic fatigue syndrome-induced behavioural, biochemical and mitochondrial alterations in mice.
Materials and methods
Animals
Male albino Laca mice (20–30 g) bred in Central Animal House Facility of the Panjab University, Chandigarh, India were used for the study. The animals were housed in normal temperature and humidity with alternate 12 h light and dark cycle and had free access to standard rodent food pellets and water. They were acclimatised to the laboratory conditions before the experiment. All the experiments were conducted between 09.00 and 17.00. The experimental protocol was approved by the Institutional Animal Ethics Committee and conducted according to the National Science Academy Guidelines for the use and care of animals.
Drugs and treatment schedule
The following drugs were used in the present study. Pioglitazone (Indo-swift laboratory, Chandigarh, India) and caffeic acid (Hi-Media, Mumbai, India) were suspended in 0.5% w/v sodium carboxymethylcellulose (CMC) solution and administered by oral route in a constant volume of 0.5 ml/100 g of body weight. Animals were randomly divided into seven groups; consist of ten animals in each.
Group 1 was designated as naive animals, received vehicle (0.5% w/v CMC); group 2 was treated as control group (exposed to running wheel test session for 6 min daily for 21 days); groups 3 and 4 received pioglitazone (5 and 10 mg/kg p.o.), respectively, followed by running wheel test session for 6 min for 21 days; similarly Groups 5 and 6 received caffeic acid (5 and 10 mg/kg p.o.), respectively, followed by, running wheel test session for 6 min for 21 days. Further, group 7 received combination of pioglitazone (5 mg/kg) with caffeic acid (5 mg/kg) followed by running wheel test session for 6 min for 21 days.
Drugs were administered 1 h prior to the animals being subjected to running wheel test session for 6 min. Doses were selected on the basis of previous reported studies (Kalonia et al. 2009a, b; Sharma et al. 2009; Kumar et al. 2009).
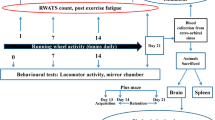
Running wheel activity
The apparatus used in the present study is a modification of the original apparatus proposed by Nomura et al. (1982), Tadokoro et al. (1997), Shimizu et al. (1984). The apparatus consisted of a plastic glass water tank (38 × 30 × 15 cm) with a wheel at a height of 6 cm. The wheel resembles a circular hollow cage with diameter of 28 cm. Water at 15 ± 2°C was put in the tank to a level such that half part of wheel dips in water. One hour after oral administration of drugs, the mice were placed individually in the tank at the base of wheel and removed from water after 6 min. As an attempt to escape cold water, the mice would try to climb on the walls of wheel. However, it would fail to escape due to the rotation of wheel. However, when attempts to escape were finally abandoned the wheel would stop turning. In the mean time, the number of rotations of the wheel was counted with the help of a digital counting device attached to the assembly. The wheel rotations during the complete 6 min test for mice receiving drug treatment were compared with those of control group which had been similarly administered vehicle, an hour prior to the test. After the completion of 6 min session, the mice were dried with cotton and put under a heating lamp to avoid hypothermia. The same procedure was repeated after every 24 h with all the groups, but naive group for 21 days.
The animals were subjected for a session of wheel running activity for 6 min for 21 days produced fatigue-like condition in animals and thus gradually decreased the number of rotations of the wheel.
Behavioural assessments
Measurement of locomotor activity
To detect the association of decreased activity in running wheel apparatus with changes in motor activity, the locomotor activity was recorded for a period of 5 min using actophotometer (IMCORP, Ambala) on 0th, 8th, 15th and 22nd day of running wheel activity. Each animal was observed in a square (30 cm) closed arena equipped with infrared light-sensitive photocells using digital actophotometer and locomotor activity was expressed in terms of total photobeam counts for 5 min per animal. Animals were placed individually in the activity chamber for a 3 min acclimation period before starting actual activity tasks. The apparatus was placed in a darkened, light and sound attenuated and ventilated testing room (Kulkarni 1999; Kumar et al. 2006).
Measurement of anxiety (elevated plus maze test)
The elevated plus maze apparatus was used to evaluate the anxiety. The apparatus consisted of two open arms (16 × 5 cm2) and two enclosed arms (16 × 5 × 12 cm3). The arm extended from a central platform (5 × 5 cm2). The mice were placed individually at the centre of the arms facing either of the open arms. During a 5 min session, the following parameters were noted: (1) preference to open or closed arm (2) number of entries in open and closed arm, (3) time spent in open and closed arm. An anxiogenic response was observed when number of entries and time spent in the closed arm increased (Kulkarni 1999).
Preparation of brain homogenate
On the 22nd day, animals were randomized into two groups, one group was used for the biochemical assays and second group was used for the estimation of mitochondrial enzyme complex activities, immediately after the behavioural quantification. Brains were dissected and rinsed in ice-cold saline. Cerebellum was discarded. For biochemical estimations, a 10% (w/v) tissue homogenates were prepared in 0.1 M phosphate buffer (pH 7.4). The homogenates were centrifuged at 10,000g for 15 min and aliquots of supernatants were separated which were further used for estimating lipid peroxidation, nitrite, reduced glutathione and catalase assay.
Measurement of oxidative stress parameters
Measurement of lipid peroxidation
The quantitative measurement of lipid peroxidation in brain was performed according to the method of Wills 1966. The amount of malondialdehyde, a measure of lipid peroxidation was measured by reaction with thiobarbituric acid at 532 nm using Perkin Elmer Lambda 20 spectrophotometer (Norwalk, CT, USA). The values were calculated using molar extinction coefficient of chromophore (1.56 × 105 M−1 cm−1) and expressed as percentage of vehicle-treated group.
Estimation of nitrite
The accumulation of nitrite in the supernatant, an indicator of the production of nitric oxide (NO), was determined with a colorimetric assay with Greiss reagent (0.1% N-(1-naphthyl) ethylenediamine dihydrochloride, 1% sulphanilamide and 2.5% phosphoric acid) as described by Green et al. 1982. Equal volumes of supernatant and Greiss reagent were mixed, and this mixture was incubated for 10 min at room temperature in the dark. Absorbance was noted at 540 nm with Perkin–Elmer Lambda 20 spectrophotometer. The concentration of nitrite in the supernatant was determined from a sodium nitrite standard curve and expressed as percentage of vehicle-treated group.
Estimation of reduced glutathione
Reduced glutathione in brain was estimated according to the method described by Ellman (Ellman et al. 1961). Supernatant (1 ml) was precipitated with 1 ml of 4% sulfosalicylic acid and cold digested at 4°C for 1 h. The sample was centrifuged at 1,200g for 15 min at 4°C. To 1 ml of this supernatant, 2.7 ml of phosphate buffer (0.1 M, pH 8) and 0.2 ml of 5,5-dithiobis (2-nitrobenzoic acid) (DTNB) were added. The yellow color developed was read immediately at 412 nm using Shimadzu spectrophotometer. The results were calculated using molar extinction coefficient of chromophore (1.36 × 104 M−1 cm−1) and expressed as percentage of control.
Catalase estimation
Catalase activity was assayed by the method of Luck (1971), where in breakdown of hydrogen peroxides (H2O2) is measured at 240 nm. Briefly, assay mixture consisted of 3 ml of H2O2 phosphate buffer and 0.05 ml of supernatant of tissue homogenate (10%), and change in absorbance was recorded at 240 nm. The results were expressed as mm of H2O2 decomposed per milligram of protein/min.
Protein estimation
The protein content was measured by Biuret method using bovine serum albumin as standard (Gornall et al. 1949).
Mitochondrial complex enzymes estimation
Isolation of mice brain mitochondria
Second group of animals were used for mitochondrial isolation as described in the method of Berman and Hastings 1999. The brain regions were homogenised in isolated buffer. Homogenate was centrifuged at 13,000g for 5 min at 4°C. Pellet was re-suspended in isolation buffer with ethylene glycol tetraacetic acid (EGTA) and spun again at 13,000g at 4°C for 5 min. The resulting supernatant was transferred to new tubes and topped off with isolation buffer with EGTA and again spun at 13,000g at 4°C for 10 min. Pellet containing pure mitochondria was re-suspended in isolation buffer without EGTA.
NADH dehydrogenase activity
Complex-I was measured spectrophotometrically by the method of King and Howard 1967. The method involves catalytic oxidation of NADH to NAD+ with subsequent reduction in cytochrome c. The reaction mixture contained 0.2 M glycyl glycine buffer pH 8.5, 6 mM NADH in 2 mM glycyl glycine buffer and 10.5 mM cytochrome c. The reaction was initiated by addition of requisite amount of solubilised mitochondrial sample and followed absorbance change at 550 nm for 2 min.
Succinate dehydrogenase activity
Succinate dehydrogenase was measured spectrophotometrically according to King 1967. The method involves oxidation of succinate by an artificial electron acceptor, potassium ferricyanide. The reaction mixture contained 0.2 M phosphate buffer pH 7.8, 1% BSA, 0.6 M succinic acid, and 0.03 M potassium ferricyanide. The reaction was initiated by the addition of mitochondrial sample and absorbance change was followed at 420 nm for 2 min.
Cytochrome oxidase assay
Cytochrome oxidase activity was assayed in brain mitochondria according to the method of Sottocasa et al. (1967). The assay mixture contained 0.3 mM reduced cytochrome c in 75 mM phosphate buffer. The reaction was started by the addition of solubilised mitochondrial sample and absorbance change was recorded at 550 nm for 2 min.
Mitochondrial redox activity
The MTT assay is based on the reduction of (3-(4,5- dimethylthiazol-2-yl)-2,5-diphenyl-H-tetrazolium bromide (MTT) by hydrogenase activity in functionally intact mitochondria. The MTT reduction rate was used to assess the activity of the mitochondrial respiratory chain in isolated mitochondria by the method of Liu et al. (1997). Briefly, 100 μl mitochondrial samples was incubated with 10 μl MTT for 3 h at 37°C. The blue formazan crystals were solubilised with dimethylsulphoxide and measured by an ELISA reader at 580 nm filter.
Statistical analysis
The data were analysed using analysis of variance followed by Tukey’s test. All the values are expressed as mean ± SEM. In all tests, the criterion for statistical significance was P < 0.05.
Results
Effect of pioglitazone, caffeic acid and their combination on the wheel activity
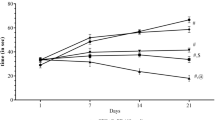
Animals exposed to a 6 min test session daily in the running wheel activity apparatus for 21 days showed a persistent decline in the number of counts per minute (wheel rotation per 6 min) (Fig. 1). Pioglitazone (5 and 10 mg/kg) and caffeic acid (5 and 10 mg/kg) drug treatment for 3 weeks significantly improved running wheel activity when compared with control group (Fig. 1). Further, combination of lower doses of pioglitazone (5 mg/kg) and caffeic acid (5 mg/kg) significantly synergies their protective effect (increased running wheel activity) as compared to their effect alone (Fig. 1).
Effect of pioglitazone, caffeic acid and their combination on running wheel activity. ªP < 0.05 as compared to naive, b P < 0.05 as compared to control (chronic fatigue mice), c P < 0.05 as compared to pioglitazone (5 mg/kg), d P < 0.05 as compared to caffeic acid (5 mg/kg) (one-way ANOVA followed by Tukey’s test). P5 pioglitazone (5 mg/kg), P10 pioglitazone (10 mg/kg), CA5 caffeic acid (5 mg/kg), CA10 caffeic acid (10 mg/kg), P5 + CA5 pioglitazone (5 mg/kg) + caffeic acid (5 mg/kg)
Effect of pioglitazone, caffeic acid and their combination on the locomotor activity
The initial locomotor activity recorded on day zero was not statistically different from each other in all the treatment groups. Animals exposed to running wheel activity apparatus for 6 min test session daily, decreased locomotor activity on day 8th, 15th and 22nd when compared with their respective naive group (Table 1). Pioglitazone (5 and 10 mg/kg) and caffeic acid (5 and 10 mg/kg) drug treatment improved locomotor activity when compared with the control group (Table 1). The combination of lower doses of pioglitazone (5 mg/kg) and caffeic acid (5 mg/kg) significantly synergist their protective effect (improved locomotor activity) which was significant when compared with their individual effects (Table 1).
Effect of pioglitazone, caffeic acid and their combination on the anxiety levels in the elevated plus maze test
Each 6 min test session on running wheel apparatus for 21 days significantly caused anxiety-like behaviour (decreased number of entries and time spent in open arm) as compared to naive animals (Table 2). Pioglitazone (5 and 10 mg/kg) and caffeic acid (5 and 10 mg/kg) drug treatment significantly attenuated anxiety-like behaviour (increased entries and time spent in open arm) as compared to control group (Table 2). Further, combination of lower dose of pioglitazone (5 mg/kg) and caffeic acid (5 mg/kg) significantly caused antianxiety-like effect which was significant when compared with their effect alone (Table 2).
Effect of pioglitazone, caffeic acid and their combination on the brain lipid peroxidation, nitrite level, and catalase enzyme and GSH levels
Six minute test session on running wheel apparatus for 21 days significantly increased lipid peroxidation and nitrite concentration, as well as depleted glutathione and catalase enzyme activity when compared with naive group on 22nd day (Figs. 2, 3). Pioglitazone (5 and 10 mg/kg) and caffeic acid (10 mg/kg) drug treatment significantly attenuated lipid peroxidation, nitrite concentration, restored catalase and glutathione levels when compared with control group (Figs. 2, 3). Further, combination of lower dose of pioglitazone (5 mg/kg) and caffeic acid (5 mg/kg) produced antioxidant effect which was significant when compared with their effect alone (Figs. 2, 3).
Effect of pioglitazone, caffeic acid and their combination on brain lipid peroxidation and nitrite concentration. ªP < 0.05 as compared to naive, b P < 0.05 as compared to control (Chornic fatigue mice), c P < 0.05 as compared to pioglitazone (5 mg/kg), d P < 0.05 as compared to caffeic acid (5 mg/kg) (one-way ANOVA followed by Tukey’s test). P5 pioglitazone (5 mg/kg), P10 pioglitazone (10 mg/kg), CA5 caffeic acid (5 mg/kg), CA10 caffeic acid (10 mg/kg), P5 + CA5 pioglitazone (5 mg/kg) + caffeic acid (5 mg/kg)
Effect of pioglitazone, caffeic acid and their combination on reduced glutathione and catalase levels. ªP < 0.05 as compared to naive, b P < 0.05 as compared to control (chronic fatigue mice), c P < 0.05 as compared to pioglitazone (5 mg/kg), d P < 0.05 as compared to caffeic acid (5 mg/kg) (one-way ANOVA followed by Tukey’s test). P5 pioglitazone (5 mg/kg), P10 pioglitazone (10 mg/kg), CA5 caffeic acid (5 mg/kg), CA10 caffeic acid (10 mg/kg), P5 + CA5 pioglitazone (5 mg/kg) + caffeic acid (5 mg/kg)
Effect of pioglitazone, caffeic acid and their combination on the on mitochondrial enzyme complexes
Six minute test session on running wheel apparatus for 21 days significantly impaired the mitochondrial enzyme complexes (I, II and IV) and mitochondrial redox activity (MTT assay) as compared to naive group (Fig. 4). Pioglitazone (10 mg/kg) treatment significantly restored mitochondrial enzyme (I, II and IV) complex activity as compared to the control group (Fig. 4). Caffeic acid (10 mg/kg) treatment significantly restored mitochondrial enzyme (I and II) complex activity as compared to the control group (Fig. 4). A combination of pioglitazone (5 mg/kg) and caffeic acid (5 mg/kg) significantly synergist their protective effect on mitochondrial enzyme (I, II and IV) (Fig. 4). However, treatment with pioglitazone (5 mg/kg) and caffeic acid (5 mg/kg) individually did not show any significant effect.
Effect of pioglitazone, caffeic acid and their combination on mitochondrial enzymes complex levels. ªP < 0.05 as compared to naive, b P < 0.05 as compared to control (chronic fatigue mice), c P < 0.05 as compared to pioglitazone (5 mg/kg), d P < 0.05 as compared to caffeic acid (5 mg/kg) (one-way ANOVA followed by Tukey’s test). P5 pioglitazone (5 mg/kg), P10 pioglitazone (10 mg/kg), CA5 caffeic acid (5 mg/kg), CA10 caffeic acid (10 mg/kg), P5 + CA5 pioglitazone (5 mg/kg) + caffeic acid (5 mg/kg)
Discussion
Persistent fatigue is characterised by impaired concentration, anxiety and headache which suggest the involvement of central nervous system in its pathophysiology (Vercoulen et al. 1998, Vercoulen et al. 1996a, b). A number of studies have provided the evidence for a model in which physical deconditioning helps in maintaining physical disability (White 2000; McCully and Natelson 1999; Lane et al. 1998). In the present study, daily 6 min test session for 21 days in the running wheel apparatus produced fatigue-like condition in animals, suggesting the reliability of the experimental model for research. The swim stress-induced fatigue model is useful, but lacks objectivity in its evaluation of immobility, because each experimenter evaluates it subjectively. Hence, in our study, we devised our own behavioural screening test which enabled us to quantify the escape behaviour. It is more specific in a way that activity can be directly measured in the form of number of wheel rotations. Thus, the subjective variation in measuring the immobility due to fatigue is completely ruled out. The wheel activity was significantly decreased on 21st day in control animals, indicating fatigue-like behaviour. The motor activity was also significantly impaired in chronic fatigue animals. Report suggests the deficiency of serum acylcarnitine in fatigue patients that induce a decrease in oxidative metabolism and higher levels of plasma lactate (Kuratsune et al. 1994; Wong et al. 1992). The metabolic defects may contribute to the reduced physical endurance of fatigue patients (Fulle et al. 2007). Supporting to reports (Short et al. 2002; Kroenke et al. 1988) reduced locomotor activity and anxiety-like behaviour was also observed in the present study. Increased cortisol level has been linked with anxiety-like behaviour and painful response in humans (Bristow and Holmes 2007). Central nervous system dopamine and serotonin has been found to be lowered in chronically stressed animals (Roth et al. 1982). Increased perceived stress and anxiety may cause the brain to increase adrenocorticotropic hormone release. Besides from above these factors neuroinflammation is also the major factor in disease pathology included chronic fatigue syndrome. There is an extensive literature on inflammatory response with microglial activation and the production of proinflammatory cytokines in neuroinflammatory/infectious and neurodegenerative diseases (Flachenecker et al. 2004; Kreutzberg 1996). Based on the extensive role of neuroinflammation, we used two antiinflammatory drugs, i.e. pioglitazone is a synthetic PPAR-γ agonist with additional antiinflammatory activity and caffeic acid, a well-known antiinflammatory with antioxidant property. In some of the recent studies, the neuroprotective effects of pioglitazone are also attributed to its antioxidant activity (Pathan et al. 2006). Keeping in mind, the beneficial effects of both the drugs, specifically as antioxidants, present study has been designed to explore the potential action of pioglitazone and caffeic acid combination against stress-induced behavioural, biochemical and cellular alterations. Since oxidative stress is reported to be the major pathogenic factor in fatigue (Fulle et al. 2003), we tried the combination of pioglitazone and caffeic acid in a hope that the combination will synergies the neuroprotective and antioxidant-like effects as compared to their effect per se. In the present study, pioglitazone and caffeic acid drug treatment significantly improved wheel running activity and locomotor activity when compared with control group. Pioglitazone or caffeic acid treatment during the 21 day exposure to chronic stress, recovered and attained the overall running wheel activity and locomotor ability patterns that were more similar to those of non-stressed (naive) mice. Further, pretreatment with pioglitazone and caffeic acid significantly prevented the anxiety levels as compared to control group. Whereas, the combination of both drugs (pioglitazone and caffeic acid) showed a significantly increased protective effect as compared to effect of the individual drugs indicating synergistic effect. These results provide the evidence that both drugs enhance the effect of each other on behavioural symptoms in chronic fatigue.
Oxidative stress is an emerging focus of research, in view of recent findings that it contributes to the pathology and clinical symptoms of chronic fatigue (Fulle et al. 2003; Jammes et al. 2005). Although it is uncertain whether oxidative stress is a primary cause or a result of this illness, recent studies have demonstrated that oxidative stress contributes to the pathology and clinical symptoms of chronic fatigue (Logan and Wong 2001). Theoretically, oxidative stress can be caused by an increase in the generation of reactive oxygen species, of which mitochondrial dysfunction is believed to be a main source, or it can be caused by a decline in the efficiency of antioxidant enzyme systems (Fulle et al. 2000). Recent studies have examined both these possibilities by looking for markers of oxidative stress and protective antioxidant systems. Mitochondrial dysfunction may be implicated in the genesis of chronic fatigue pathology. In the early 1990s, Kuratsune et al. showed that the low acylcarnitine content in the serum of CFS patients is related to reduce energy production by muscle mitochondria (Kuratsune et al. 1994). Pall et al. reported that the mitochondrial enzymes succinic dehydrogenase and cis-aconitase are inactivated by peroxynitrite (Pall 2000; Radi et al. 1994; Castro et al. 1994). In the present study, running wheel activity for 21 days significantly induced oxidative damage in brain shown by increased level of lipid peroxidation, nitrite concentration, decreased levels of glutathione and catalase. The results of the presents study are very similar with previous studies that chronic stress significantly induced oxidative damage in brain (Kennedy et al. 2005). To further extend our present experiment, we also estimated the mitochondrial complex enzymes levels in the whole brain and the observed significant decrease in the activity of the complex enzymes (I, II and IV) after chronic stress. Present study results are very similar with clinical reports that chronic stress significantly inhibited the mitochondrial enzymes activity (Madrigal et al. 2001).
Pretreatment with pioglitazone and caffeic acid significantly reversed the biochemical and mitochondrial changes induced due to chronic stress. This shows the antioxidant-like effect of both the drugs. Further, combination of both the drugs pioglitazone and caffeic acid significantly synergies their effect as compared to their effect alone. Some previous reports also documented the antioxidant-like effect of both the compounds (Gülçin 2006; Gumieniczek 2003).
Pioglitazone, an agonist of peroxisome proliferator-activated receptor-γ (PPAR-γ), used in treatment of type 2 diabetes. However, in last few years, it has become evident that the therapeutic effects of PPAR-γ ligands may reach far beyond their use as insulin sensitizers. The expression of PPAR-γ has been detected in various brain regions including cortex and hippocampus. Thus, role of PPAR-γ has been suggested in modulation of ageing, neurodegeneration, learning and memory (Moreno et al. 2004). Supporting the fact, neuroprotective effects of PPAR-γ agonists have been demonstrated in different experimental models of neurological disorders, including Alzheimer’s disease. Pioglitazone is also effective in traumatic spinal cord injury by anatomical and locomotor recovery (McTigue et al. 2007). Recently, PPAR-γ has been implicated as a regulator of cellular inflammatory responses. Studies have also reported the antioxidant effects of pioglitazone in intracerebroventricular streptozotocin-induced memory impairment in rats, therefore, suggesting its use in the treatment of chronic fatigue. However, a detailed characterisation of how PPAR-γ agonists the neuroinflammatory changes following chronic fatigue is still lacking. Several other studies have reported antioxidant effect of pioglitazone that accompanies its beneficial effect in diabetes (Dobrian et al. 2004; Ishida et al. 2004).
Caffeic acid (3,4-dihydroxycinnamic acid), is a natural phenolic compound, exerts neuroprotective effects against ischaemic brain injuries because of its potent antioxidant, antiinflammatory properties and free-radical scavenging action through LOX inhibition (Kart et al. 2009; Sul et al. 2009). Antioxidant activity is one of the well-known biological activities of caffeic acid (Prasad et al. 2009; Sul et al. 2009). The phenolic hydroxyl group of caffeic acid has been suggested to quench free radicals (Devipriya et al. 2008). Reports also suggest that caffeic acid is a potent inhibitor of NF-κB activation (Noelker et al. 2005), lipid peroxidation, lipoxygenase activities, protein tyrosine kinase and ornithine decarboxylase (Noelker et al. 2005; Zheng et al. 1995). Previous reports, also suggest the protective effect of caffeic acid against several types of neurotoxicity including low K+, Aβ-induced neurotoxicity glutamate-induced cell death as well as hypoxia-induced cerebral infarction (Noelker et al. 2005), significantly blocked H2O2-induced neurotoxicity, inhibition of caspase-1, 3 and 9 as well as inhibition of ROS (McEleny et al. 2004; Amodio et al. 2003). In addition, caffeic acid was able to modulate the 6-OHDA-induced release of cytochrome c in isolated liver mitochondria (Noelker et al. 2005; Chung et al. 2006).
In conclusion, the running wheel filled with water significantly induced chronic fatigue syndrome in mice. Pretreatment with pioglitazone and caffeic acid significantly showed protective effect against chronic fatigue-induced behavioural, biochemical and mitochondrial dysfunction. Further study confirms the antioxidant-like mechanism in the protective effect of both the drugs.
References
Afari N, Buchwald D (2003) Chronic fatigue syndrome: a review. Am J Psychiatry 160:221–236
Amodio R, De Ruvo C, Sacchetti A, Di Santo A, Martelli N, Di Matteo V, Lorenzet R, Poggi A, Rotilio D, Cacchio M, Esposito E (2003) Caffeic acid phenethyl ester blocks apoptosis induced by low potassium in cerebellar granule cells. Int J Dev Neurosci 21(7):379–389
Berman SB, Hastings TG (1999) Dopamine oxidation alters mitochondrial respiration and induces permeability transition in brain mitochondria: implications for Parkinson’s disease. J Neurochem 73:1127–1137
Bristow DJ, Holmes DS (2007) Cortisol levels and anxiety related behaviors in cattle. Physiol Behav 90:626–628
Castro L, Rodriguez M, Radi R (1994) Aconitase is readily inactivated by peroxynitrite, but not by its precursor, nitric oxide. J Biol Chem 269:29409–29415
Chung MJ, Walker PA, Hogstrand C (2006) Dietary phenolic antioxidants, caffeic acid and Trolox, protect rainbow trout gill cells from nitric oxide-induced apoptosis. Aquat Toxicol 80(4):321–328
Devipriya N, Sudheer AR, Menon VP (2008) Caffeic acid protects human peripheral blood lymphocytes against gamma radiation-induced cellular damage. J Biochem Mol Toxicol 22(3):175–186
Dobrian AD, Schriver SD, Khraibi AA, Prewitt RL (2004) Pioglitazone prevents hypertension and reduces oxidative stress in diet-induced obesity. Hypertension 43(1):48–56
Ellman GL, Courtney KD, Andres V, Featherston RMA (1961) New and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Flachenecker P, Bihler I, Weber F, Gottschalk M, Toyka KV, Rieckmann P (2004) Cytokine mRNA expression in patients with multiple sclerosis and fatigue. Mult Scler 10:165–169
Fulle S, Mecocci P, Fanò G, Vecchiet I, Vecchini A, Racciotti D, Cherubini A, Pizzigallo E, Vecchiet L, Senin U, Beal MF (2000) Specific oxidative alterations in vastus lateralis muscle of patients with the diagnosis of chronic fatigue syndrome. Free Rad Biol Med 29(12):1252–1259
Fulle S, Belia S, Vecchiet J, Morabito C, Vecchiet L, Fanò G (2003) Modification of the functional capacity of sarcoplasmic reticulum membranes in patients suffering from chronic fatigue syndrome. Neuromuscul Disord 13(6):479–484
Fulle S, Pietrangelo T, Mancinelli R, Saggini R, Fano G (2007) Specific correlations between muscle oxidative stress and chronic fatigue syndrome: a working hypothesis. J Muscle Res Cell Motil 28:355–362
García-Bueno B, Madrigal JL, Lizasoain I, Moro MA, Lorenzo P, Leza JC (2005) Peroxisome proliferator-activated receptor gamma activation decreases neuroinflammation in brain after stress in rats. J Biol Psychiatry 57(8):885–894
Gornall AG, Bardawill CJ, David MM (1949) Determination of serum proteins by means of the biuret reaction. J Biol Chem 177(2):751–766
Green LC, Wagner DA, Glgowski J, Skipper PL, Wishnok JS, Tannebaum SR (1982) Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Ann Biochem 126:131
Gülçin İ (2006) Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid). Toxicology 217(2–3):213–220
Gumieniczek A (2003) Effect of the new thiazolidinedione-pioglitazone on the development of oxidative stress in liver and kidney of diabetic rabbits. Life Sci 74(5):553–562
Gupta A, Vij G, Sharma S, Tirkey N, Rishi P, Chopra K (2009) Curcumin, a polyphenolic antioxidant, attenuates chronic fatigue syndrome in murine water immersion stress model. Immunobiology 214(1):33–39
Ishida H, Takizawa M, Ozawa S, Nakamichi Y, Yamaguchi S, Katsuta H, Tanaka T, Maruyama M, Katahira H, Yoshimoto K, Itagaki E, Nagamatsu S (2004) Pioglitazone improves insulin secretory capacity and prevents the loss of beta-cell mass in obese diabetic db/db mice: possible protection of beta cells from oxidative stress. Metabolism 53(4):488–494
Jammes Y, Steinberg JG, Mambrini O, Bregeon F, Delliaux S (2005) Chronic fatigue syndrome: assessment of increased oxidative stress and altered muscle excitability in response to incremental exercise. J Intern Med 257:299–310
Kalonia H, Kumar P, Kumar A, Nehru B (2009a) Effect of caffeic acid and rofecoxib and their combination against intrastriatal quinolinic acid induced oxidative damage, mitochondrial and histological alterations in rats. Inflammopharmacol 17:211–219
Kalonia H, Kumar P, Kumar A, Nehru B (2009b) Effects of caffeic acid, rofecoxib, and their combination against quinolinic acid-induced behavioral alterations and disruption in glutathione redox status. Neurosci Bull 25(6):343–352
Kart A, Cigremis Y, Ozen H, Dogan O (2009) Caffeic acid phenethyl ester prevents ovary ischemia/reperfusion injury in rabbits. Food Chem Toxicol 47(8):1980–1984
Kennedy G, Spence VA, McLaren M, Hill A, Underwood C, Belch JJF (2005) Oxidative stress levels are raised in chronic fatigue syndrome and are associated with clinical symptoms. Free Rad Biol Med 39(5):584–589
Kiaei M, Kipiani K, Chen J, Calingasan NY, Beal MF (2005) Peroxisome proliferator-activated receptor-gamma agonist extends survival in transgenic mouse model of amyotrophic lateral sclerosis. Exp Neurol 191:331–336
King TE (1967) Preparation of succinate dehydrogenase and reconstitution of succinate oxidase. Methods Enzymol 10:322
King TE, Howard RL (1967) Preparations and properties of soluble NADH dehydrogenases from cardiac muscle. Methods Enzymol 10:275
Kreutzberg GW (1996) Microglia: a sensor for pathological events in the CNS. Trends Neurosci 19:312–318
Kroenke K, Wood DR, Mangelsdroff D, Meier NJ, Powell JB (1988) Chronic fatigue in primary care: prevalence, patient characteristics, and outcome. JAMA 260:929–934
Kulkarni SK (1999) Handbook of experimental pharmacology, 3rd edn. Vallabh Parkashan, New Delhi
Kumar P, Padi SS, Naidu PS, Kumar A (2006) Effect of resveratrol on 3-nitropropionic acid-induced biochemical and behavioural changes: possible neuroprotective mechanisms. Behav Pharmacol 17(5–6):485–492
Kumar P, Kaundal RK, More S, Sharma SS (2009) Beneficial effects of pioglitazone on cognitive impairment in MPTP model of Parkinson’s disease. Behav Brain Res 197(2):398–403
Kuratsune H, Yamaguti K, Takahashi M, Misaki H, Tagawa S, Kitani T (1994) Acylcarnitine deficiency in chronic fatigue syndrome. Clin Infect Dis 18:S62–S67
Lane RJ, Barret CB, Woodrow D, Moss J, Fletcher R, Archard LC (1998) Muscle fibre characteristics and lactate responses to exercise in chronic fatigue syndrome. J Neurol Neurosurg Psychiatry 64:362–367
Liu H, Bowes RC, Van de Water B, Sillence C, Nagelkerke JF, Stevens JL (1997) Endoplasmic reticulum chaperones GRP78 and calreticulin prevent oxidative stress, Ca2+ disturbances, and cell death in renal epithelial cells. J Biol Chem 272(35):21751–21759
Logan AC, Wong C (2001) Chronic fatigue syndrome: oxidative stress and dietary modifications. Alt Med Rev 6(5):450–459
Lorusso L, Mikhaylova SV, Capelli E, Ferrari D, Ngonga GK, Ricevuti G (2009) Immunological aspects of chronic fatigue syndrome. Autoimmun Rev 8(4):287–291
Luck H (1971) Catalase. In: Bergmeyer HU (ed) Methods of enzyme analysis. Academic Press, New York, p 885
Madrigal JLM, Olivenza R, Moro MA, Lizasoain I, Lorenzo P, Rodrigo J, Leza JC (2001) Glutathione depletion, lipid peroxidation and mitochondrial dysfunction are induced by chronic stress in rat brain. Neuropsychopharmacology 24(4):420–429
Manuel KB, Moorkens G, Vertommen J, De Leeuw I (2001) Antioxidant status and lipoprotein peroxidation in chronic fatigue syndrome. Life Sci 462(68):2037–2049
McCully KK, Natelson BH (1999) Impaired oxygen delivery to muscle in chronic fatigue syndrome. Clin Sci 97:603–608
McEleny K, Coffey R, Morrissey C, Fitzpatrick JM, Watson RW (2004) Caffeic acid phenethyl ester-induced PC-3 cell apoptosis is caspase-dependent and mediated through the loss of inhibitors of apoptosis proteins. BJU Int 94(3):402–406
McTigue DM, Tripathi R, Wei P, Lash AT (2007) The PPAR gamma agonist pioglitazone improves anatomical and locomotor recovery after rodent spinal cord injury. Exp Neurol 205:396–406
Moreno S, Farioli-Vecchioli S, Ceru MP (2004) Immunolocalization of peroxisome proliferator-activated receptors in the adult rat CNS. Neuroscience 123:131–145
Nijs J, De Meirleir K (2004) Oxidative stress might reduce essential fatty acids in erythrocyte membranes of chronic fatigue syndrome patients. Nutr Neurosci 7:251–253
Noelker C, Bacher M, Gocke P, Wei X, Klockgether T, Du Y, Dodel R (2005) The flavanoide caffeic acid phenethyl ester blocks 6-hydroxydopamine-induced neurotoxicity. Neurosci Lett 383(1–2):39–43
Nomura S, Shimizu J, Kinjo M, Kametani H, Nakazawa T (1982) A new behavioral test for antidepressant drugs. Eur J Pharmacol 83:171–175
Pall ML (2000) Elevated, sustained peroxynitrite levels as the cause of chronic fatigue syndrome. Med Hypotheses 54:115–125
Pathan AR, Viswanad B, Sonkusare SK, Ramarao P (2006) Chronic administration of pioglitazone attenuates intracerebroventricular streptozotocin induced-memory impairment in rats. Life Sci 79(23):2209–2216
Prasad NR, Jeyanthimala K, Ramachandran S (2009) Caffeic acid modulates ultraviolet radiation-B induced oxidative damage in human blood lymphocytes. J Photochem Photobiol B 95(3):196–203
Radi R, Rodriguez M, Castro L, Telleri R (1994) Inhibition of mitochondrial electron transport by peroxynitrite. Arch Biochem Biophys 308:89–95
Roth KA, Mefford IM, Barchas JD (1982) Epinephrine, norepinephrine, dopamine and serotonin: differential effects of acute and chronic stress on regional brain amines. Brain Res 239(2):417–424
Sharma R, Kaundal RK, Sharma SS (2009) Amelioration of pulmonary dysfunction and neutrophilic inflammation by PPAR gamma agonist in LPS-exposed guinea pigs. Pulm Pharmacol Ther 22(3):183–189
Shimizu J, Nomura S, Hiroko K, Nakazawa T (1984) Effects of water temperature and weight of wheel load on water wheel turning behaviour of imipramine administered mice. Psychopharmacology 84:20–21
Short K, McCabe M, Tooley G (2002) Cognitive functioning in chronic fatigue syndrome and the role of depression, anxiety, and fatigue. J Psychosom Res 52(6):475–483
Singal A, Kaur S, Tirkey N, Chopra K (2005) Green tea extract and catechin ameliorate chronic fatigue-induced oxidative stress in mice. J Med Food 8(1):47–52
Singh A, Naidu S, Gupta S, Kulkarni S (2002) Effect of natural and synthetic antioxidants in a mouse model of chronic fatigue syndrome. J Med Food 5(4):211–220
Sottocasa GL, Kuylenstierna B, Ernster L, Bergstrand A (1967) An electron-transport system associated with the outer membrane of liver mitochondria: a biochemical and morphological study. J Cell Biol 32:415
Sul D, Kim HS, Lee D, Joo SS, Hwang KW, Park SY (2009) Protective effect of caffeic acid against beta-amyloid-induced neurotoxicity by the inhibition of calcium influx and tau phosphorylation. Life Sci 84(9–10):257–262
Tadokoro C, Kiuchi Y, Yamazaki Y, Nara K, Oguchi K, Kamijima K (1997) Behavioral stimulation without alteration of b and 5-HT receptors and adenylate cyclase activity in rat brain after chronic sertraline administration. Psychopharmacology 130:124–130
Takeda H, Tsuji M, Yamada T, Masuya J, Matsushita K, Tahara M, Iimori M, Matsumiya T (2006) Caffeic acid attenuates the decrease in cortical BDNF mRNA expression induced by exposure to forced swimming stress in mice. Eur J Pharmacol 534(1–3):115–121
Vercoulen JH, Hommes OR, Swanink CM, Jongen PJ, Fennis JF, Galama JM, van der Meer JW, Bleijenberg G (1996a) The measurement of fatigue in patients with multiple sclerosis: a multidimensional comparison with patients with chronic fatigue syndrome and healthy subjects. Arch Neurol 53:642–649
Vercoulen JH, Swanink CM, Fennis JF, Galama JM, van der Meer JW, Bleijenberg G (1996b) Prognosis in chronic fatigue syndrome: a prospective study of the natural course. J Neurol Neurosurg Psychiatry 60:489–494
Vercoulen JH, Swanink CM, Galama JM, Fennis JF, Jongen PJ, Hommes OR, van der Meer JW, Bleijenberg G (1998) The persistence of fatigue in chronic fatigue syndrome and multiple sclerosis: development of a model. J Psychosom Res 45:507–517
Watson GS, Cholerton BA, Reger MA, Baker LD, Plymate SR, Asthana S, Fishel MA, Kulstad JJ, Green PS, Cook DG, Kahn SE, Keeling ML, Craft S (2005) Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: a preliminary study. Am J Geriatr Psychiatry 13(11):950–958
White PD (2000) The role of physical inactivity in the chronic fatigue syndrome. J Psychosom Res 49:283–284
Wills ED (1966) Mechanism of lipid peroxide formation in animal tissue. Biochem J 99:667
Wong R, Lopaschuk G, Zhu G, Walker D, Catellier D, Burton D, Teo K, Collins-Nakai R, Montague T (1992) Skeletal muscle metabolism in the chronic fatigue syndrome. In vivo assessment by 31P nuclear magnetic resonance spectroscopy. Chest 102(6):1716–1722
Zheng ZS, Xue GZ, Grunberger D, Prystowsky JH (1995) Caffeic acid phenethyl ester inhibits proliferation of human keratinocytes and interferes with the EGF regulation of ornithine decarboxylase. Oncol Res 7(9):445–452
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, A., Vashist, A. & Kumar, P. Potential role of pioglitazone, caffeic acid and their combination against fatigue syndrome-induced behavioural, biochemical and mitochondrial alterations in mice. Inflammopharmacol 18, 241–251 (2010). https://doi.org/10.1007/s10787-010-0048-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-010-0048-2