Abstract
Differences among females in infant survival can contribute substantially to variance in fitness. Infant survival is a product of external risk factors and investment by kin, especially the mother, and is thus closely tied with the evolution of behavior and life history. Here we present a 9-yr study (2004–2012) of infant survival and sex ratio relative to age and dominance ranks of mothers and the presence of immigrant males in a free-ranging population of gray-cheeked mangabeys (Lophocebus albigena) in Kibale National Park, Uganda. We consider immigrant males because they are known to increase infant mortality in several other species. We found that infants of older mothers had higher survival than those of younger mothers but that high rank did not confer a significant benefit on infant survival. Female infants had higher survival than male infants. Young, low-ranking females had more male infants than young, high-ranking females, which had slightly more daughters, but this difference declined as females aged because low-ranking females had more daughters as they aged. With limited data, we found a significant relationship between the presence of male immigrants and infant mortality (falls and unexplained disappearances) to 18 mo. Our results suggest that infant survival in gray-cheeked mangabeys is most precarious when mothers must allocate energy to their own growth as well as to their infants, that sons of young mothers are at greatest risk, and that immigrant males can negatively affect infant survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In most populations, mortality of immatures greatly exceeds that of adults (Charnov 1993). Female reproductive success thus depends to a large extent on the ability of infants to survive to maturity. A major factor influencing the likelihood of surviving beyond infancy is differential maternal investment. The level of maternal investment available to offspring is dependent on access to food resources. First-time (primiparous) mothers are often still growing and must allocate their energy to both their offsprings’ and their own growth (Bercovitch et al. 1998; Setchell et al. 2002). As a result, younger mothers may not be able to invest sufficiently in their offspring to keep them alive. In a literature review, Pusey (2012) noted that in 15 of 21 studies of primates, younger or primiparous mothers reared their infants less successfully than older mothers. Similarly, in yellow baboons (Papio cynocephalus) and semi-free-ranging mandrills (Mandrillus sphinx), younger or primiparous mothers have smaller infants than older mothers (Setchell et al. 2001; Altmann and Alberts 2005). In nonprimate mammals such as red deer (Cervus elaphus) and red squirrels (Sciurus vulgaris), young females also have poorer offspring survival, and they also have smaller infants (Clutton-Brock 1984; Descamps et al. 2008). One mechanism for an age effect on infant growth and survival may be a change in milk quality or yield (Hinde and Capitanio 2010). As female rhesus macaques (Macaca mulatta) become older, stop growing, and have more pregnancies, the energy available in their milk increases (Hinde and Capitanio 2010).
Infant survival may also improve as females gain more experience with infant care. In a captive population of vervets (Chlorocebus pygerythrus), infants of primiparous mothers with allomothering experience had better survival than those of mothers without such experience (Fairbanks 1990). Similarly, in three species of captive lion tamarins (Leontopithecus spp.), allomothering experience improved infant survival for primiparous mothers in the first 30 d of life (French et al. 1996). Conversely, infant survival may be compromised by rough handling by others (Isbell et al. 2009; Kleindorfer and Wasser 2004), and less experienced mothers may be less able to prevent that.
Providing sufficient maternal investment may be particularly challenging for primiparous mothers bearing the sex that requires the greater maternal investment. In polygynous, sexually dimorphic mammals, male infants tend to require more maternal investment than females, as has been found in chimpanzees (Pan troglodytes: Nishida et al. 2003), mountain gorillas (Gorilla beringei: Robbins et al. 2007), Hanuman langurs (Semnopithecus entellus: Ostner et al. 2005), and rhesus macaques (Bercovitch et al. 2000). Greater maternal investment is indicated by longer interbirth intervals after the birth of sons (Bercovitch and Berard 1993; Bercovitch et al. 2000; Ostner et al. 2005; Robbins et al. 2007), longer lactational periods with sons (Ostner et al. 2005), greater body mass in sons (Bercovitch et al. 2000; Hinde 2009), and higher quality milk for sons (Hinde 2007). Although primiparous rhesus macaque mothers provide milk with more gross energy and protein to their sons, they are often unable to sustain that investment, and first-born sons die at a higher rate than first-born daughters (Hinde 2007).
In group-living animals, competitive ability can affect access to food, with higher-ranking females often having greater access (Barton 1993; Koenig 2000; Whitten 1983; cf. Heesen et al. 2013). Thus, higher-ranking females are expected to be able to provide greater maternal investment than lower-ranking females, with the consequence of achieving higher infant survival. For example, infants of high-ranking female olive baboons (Papio anubis) and chimpanzees at Gombe tend to have higher survival than infants of low-ranking females (Packer et al. 1995; Pusey et al. 1997).
From an ultimate perspective, Trivers and Willard (1973) proposed that mothers’ interactions with resource availability can select for adaptive determination of the sex of their infants. Thus, mothers in good condition, i.e., those with good access to food resources, were hypothesized to invest more in the sex that has more opportunity to be reproductively successful or to gain more from the mother’s condition. In most species, males can theoretically reproduce more often than females, so mothers in good condition should therefore produce predominantly sons, according to the Trivers-Willard hypothesis (Veeroja et al. 2010). Because high-ranking females tend to have better access to food resources, sons of high-ranking mothers are likely to be in better condition than sons of low-ranking mothers. Therefore, high-ranking mothers should also benefit more by producing sons (Clutton-Brock et al. 1984). However, the Trivers–Willard hypothesis does not take into full account the complexities of group living. In species where males disperse and females remain with the mother, and daughters acquire ranks near their mothers (as is typical of cercopithecines), it may be instead that high-ranking mothers benefit by producing daughters because daughters may assist them in competitive interactions (Silk 1983). Moreover, meta-analysis of birth sex ratios in primates reveal that sample size has a strong effect on reported sex ratios, with the difference between high- and low-ranking females in infant sex ratio close to zero when samples are large (Brown and Silk 2002; Silk and Brown 2004). Overall, there appears to be no consistent effect of maternal rank or condition on offspring sex in primates once sample size is considered (Silk and Brown 2004). There is, however, evidence for group-wide adjustment linked to dispersal patterns in that infant sex ratios are biased toward the helping sex in cooperatively breeding primate populations and toward the dispersing sex in noncooperatively breeding primate populations (Silk and Brown 2008).
Although infant survival may depend largely on mothers, male behavior may also affect infant survival. This is most obvious in species in which infanticide is a male sexual strategy (Palombit 2012) but males may also reduce infant survival indirectly, such as when an infant is inadvertently killed by being knocked off the branch during fights between males.
Here we present data on infant survival over a 9-yr period in four groups of wild gray-cheeked mangabeys (Lophocebus albigena). Gray-cheeked mangabeys are cercopithecine primates that live in multimale, multifemale groups in which females form the stable core of the group. Like many other cercopithecine primates, female gray-cheeked mangabeys remain in their natal groups throughout life and have stable, linear dominance hierarchies in which high-ranking females have greater access to foods (Chancellor and Isbell 2009a, b). In contrast, male gray-cheeked mangabeys disperse from their natal groups around sexual maturity and then attempt to immigrate into other groups where they are initially low-ranking and may rise in rank over time (Arlet et al. 2011; Olupot and Waser 2001).
Based on theory and evidence from other species, we predicted that infant survival would be 1) lower for younger mothers than for older mothers; 2) lower for low-ranking females than for high-ranking females; and 3) lower for male than female infants. Although infanticide has yet to be observed in these mangabeys, if it occurs, it is more likely to be committed by immigrant males. We do have direct observations of infants dying from falls during fights between males (M. E. Arlet pers. obs.). Thus, we also predicted that immigrant males in this study population would reduce infant survival via infanticide or falls from trees.
Methods
Study Area and Subjects
We collected data in Kibale National Park, Uganda (0°13′–0°41′N and 30°19′–30°32′E), near the Makerere University Biological Field Station. Kibale (795 km2) is a moist, evergreen, medium altitude forest with a mosaic of swamp, grassland, thicket, and colonizing forest (Chapman et al. 2010). The gray-cheeked mangabey population in Kibale has been studied intermittently since the 1970s. Despite a typically bimodal pattern of yearly precipitation, with more rain falling in March–April and September–November (Isbell 2012; Valtonen et al. 2013), there appears to be no birth seasonality in this population (Olupot and Waser 2013). We collected data on four habituated groups (BT1, CC, MK, and LC1), which ranged in size from 10 to 23 individuals (Table I).
Demographic Data
We collected demographic data from August 2004 until December 2012 (August 2004–July 2006: R. L. Chancellor; July 2006–December 2012: M. E. Arlet). During Chancellor’s tenure, each group was observed for an average of 3 d/mo (Chancellor and Isbell 2009a). During Arlet’s tenure, each group was followed for up to 6 consecutive days on a 5-wk rotation schedule (Arlet et al. 2009). During each sampling period for each group, we recorded demographic data on group composition and membership, female cycling stage, and, after August 2006, presence of immigrant males with the focal group.
Females were initially identified as individuals by R. L. Chancellor and her field assistants (March 2004–July 2004) using natural markings such as relative body size, nipple color and size, and tail characteristics (scars, shape, and thickness of hair). We used the same characteristics to recognize maturing females (and males) as the years progressed. The number of females (in four groups) ranged from 21 (2007–2008) to 26 (2009, 2011–2012), with a mean of 22.6 females per year. Analyses are drawn from a total of 38 adult females over the 9-yr period.
When a new infant was first seen in a group, we counted it as a birth. Because we did not follow the groups continuously, pregnancy losses, stillbirths, or very early mortality could have occurred without our knowledge. We defined infants as animals <18 mo old (Waser 1974). During this study, 76 infants were born in the four study groups, including 12 infants to primiparous mothers. Infants were considered to have died when they were no longer seen, as infants cannot survive by themselves. Seven infants died before we were able to identify their sex and thus we omitted them from analyses on infant sex ratio.
Immigrant males were adult males that joined the study groups during the years the study groups were under observation. If those males were still with a given group after 6 mo, we then considered them as resident males because by this time glucocorticoid profiles are similar to those of longer-term resident males (Arlet et al. 2009).
Age and Rank of Adult Females
We knew approximate birth dates (within 6 wk) for all 12 adult females that matured and gave birth for the first time during this study. These were all 6–8 yr old by the end of the study. We could also estimate birth months for two females that were still dependent on their mothers when our study began. We categorized the ages of the other older 24 females into 4-yr intervals (based on Strum and Western [1982] for baboons and modified here for mangabeys) that described the reproductive status of each female: young multiparous (8–12 yr), middle-aged multiparous (12–16 yr), old multiparous (16–20 yr), and very old multiparous (>20 yr). We estimated age classes based on relative size, skin condition of perineal sexual swelling, nipple length, and general marks of aging, such as wrinkled skin on the face and the neck, saggy skin around the face, and thinning hair.
We calculated female ranks based on 136–318 dyadic agonistic interactions per group involving females, including chases, i.e., aggressively pursuing another; approach–avoids, i.e., moving away from another who is approaching; supplants, i.e., taking the place of another; physical contacts, e.g., biting, tail-pulling, pushing; and nonphysical threats, e.g., facial threats (Chancellor and Isbell 2009a, b). We used a combination of focal and all-occurrences sampling to record agonistic interactions. We determined dominance relationships by the outcome of only those agonistic interactions in which we could determine a clear aggressor. We constructed dominance matrices for each group, with rank order determined by minimizing the number of reversals against the hierarchy, i.e., interactions below the diagonal. All groups had linear dominance hierarchies that rarely changed, the exceptions occurring when females rose in the dominance hierarchy as a consequence of deaths of high-ranking mothers or their daughters (Arlet et al. in preparation). We labeled those one or two females per group that won 54–92% of the encounters in their group during a given year as high-ranking and all other females as low-ranking because they lost those encounters. Between 2004 and 2012, six females changed from low- to high-ranking or vice versa, and they were classified in each year separately. For analysis, we also assigned ranks to females on an inverse scale where the highest ranking individual is 1, the second 0.5, the third 0.33, etc., and an even distribution where the highest rank is 1, the lowest is 0, and all other individuals are placed at regular intervals between them.
Data Analysis
We used a Cox proportional hazards model to model survival to the juvenile age (survival times for those that reached the juvenile age were considered to be censored). We treated survival times in the same group as correlated. We considered infant sex, age of the mother, parity, and rank of the mother as predictor variables and included the interaction of infant sex and mother’s rank. Although parity and age are associated in that primiparous females were young, the sample sizes for parity and age were different because we lacked data on parity for all females. We also considered the mean number of immigrant males in the group until the infant became a juvenile as a predictor. To test for the effect of mother’s age on infant sex and any rank–age interaction, we fitted a generalized linear mixed model (GLMM) to the infant sex data (logistic regression model with mother as a random factor with rank, age, and their interaction as predictors). In this model, we excluded data on infants whose sex was not known.
We examined the effect of male immigrants on infant mortality using the combined categories of deaths from unknown causes (disappearances) and falls during fights by calculating the time when immigrant males were present and when they were not. We excluded events when we were not able to determine whether immigrant males were present. In total this amounted to 12 events in 204.1 mo with immigrant males present and four events in 467.9 mo with immigrant males absent during all infants’ first 18 mo. We treated this as a rate with a Poisson distribution model (GLM).
We performed all analyses using the statistical software R (R Development Core Team 2011). We fitted GLMMs using the lme4 package (Bates et al. 2011) and the Cox proportional hazards model using the survival package (Therneau 2011).
Results
General Patterns of Infant Mortality
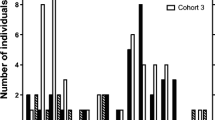
Thirty of 76 infants (39.5 %) died or disappeared during the study (Fig. 1). Seven of the 30 infants (23 %) died after becoming ill. Seven infants (23 %) died after falls from trees, all during fights between males. Thirteen infants (43 %) disappeared apparently healthy (when seen for the last time). Three infants (10 %) were confirmed victims of predation by chimpanzees. In these cases, we either witnessed the predator’s attack or were informed by other researchers or their staff. During our study period we did not witness any successful eagle attacks on females or infants.
Infant Survival Relative to Mother’s Age, Parity, and Rank
Infants of older mothers survived significantly longer than infants of younger mothers (Table II). Parity also had a significant effect on infant survival: multiparous mothers had higher infant survival than primiparous mothers (Table III). One female (IJ, a low-ranking female in Mikana group) gave birth after the estimated age of 20 yr, but her infant died soon after. Five of the seven infants that fell during male aggression were infants of mothers aged 6–8 yr, all of which were primiparous. There was no effect of maternal rank on infant survival (P = 0.955 for age model, P = 0.547 for parity model; Tables II and III).
Infant Survival Relative to Infant Sex and Mother’s Rank
Of the 76 infants born during the study in four groups, 69 were identified to sex. Thirty-nine were male (56.5 %) and 30 were female (43.5 %). A significantly higher percentage of female infants (18/30; 60 %) than male infants (17/39; 43 %) survived to 18 mo (five infants had not reached juvenile stage and so were not included in the test; Table II). However, there was no differential survival of male and female infants according to mother’s rank (P = 0.266 for age model, P = 0.250 for parity model; Tables II and III and Figs. 2 and 3). Male and female infant survival relative to mother’s rank also did not change substantially when we modeled rank using the alternatives of inverse (in the interaction, P = 0.338) and even rank distributions (in the interaction, P = 0.123).
Survival of male and female infants in high- vs. low-ranking female gray-cheeked mangabeys in four groups in Kibale National Park, Uganda for the years 2004-2012. HR f = daughters of high-ranking mothers; HR m = sons of high-ranking mothers; LR f = daughters of low-ranking mothers; LR m = sons of low-ranking mothers.
Infant Sex Relative to Mother’s Age, Parity, and Rank
Among primiparous females, 3 of 11 known infants (27.3 %) were daughters (1 infant died before its sex was determined), whereas among multiparous females, 26 of 58 infants (44.8 %) were daughters (the sex of six infants was not known). Among high-ranking mothers, 14 of 22 infants (64 %) were daughters whereas among low-ranking mothers, 16 of 47 infants (34 %) were daughters.
Modeling the sex of the infant using a GLMM revealed an interaction between mother’s age and rank. Low-ranking mothers had significantly more than 50 % male infants at 7.5 yr (the oldest age by which all females had had their first offspring; Table IV), but as they aged, the sex ratio changed significantly from farther away to closer to unity (Table IV). High-ranking mothers had significantly more female infants than low-ranking mothers at 7.5 yr (Table IV), and no significant change with aging could be detected in the sex ratio (Table IV).
Infant Survival Relative to Immigrant Males
From September 2006 until December 2012, the time period during which we recorded data on immigrant male presence in groups, immigrant males were present in groups on average 27 % of the time, encompassing the time period for 45 of the 76 infants born during the study. Immigrant males were present in six of seven cases when infants died after falls from trees during fights between males. After entering a new group, immigrant males often receive aggression from high-ranking males, including chasing, grabbing, and biting (Arlet et al. 2008). If the infant is not held by its mother or other caregiver while males chase each other, the infant can be accidentally pushed off the branch as males run past the infant, with deadly consequences. Out of 13 disappearances of apparently healthy infants, 6 (46 %) infants disappeared when an immigrant male was in the group, 3 (23 %) died when there were no immigrant males present, and 4 (31 %) died when immigrant male presence was unknown (Fig. 1). When we combined disappearances of infants when immigrant males were present with infant falls during male fights, we found that the infant death rate was significantly greater when immigrant males were present (Poisson rate data, P = 0.008).
Discussion
We studied four groups of gray-cheeked mangabeys for 9 yr, recording group composition at least once every 5 wk, and found interactions between mother’s rank, age, sex-specific infant survival, and sex ratio at birth. Infants of younger (and primiparous) mothers had poorer survival than infants of older (and multiparous) mothers, and male infants had poorer survival than female infants, but the rank of the mother did not affect infant survival. Young low-ranking females had more male infants than young high-ranking females, which had slightly more daughters, but this difference declined as females aged because low-ranking females had more daughters as they grew older. Finally, we found that infant mortality via disappearances and deaths by accidental falls during fights between males was significantly higher when immigrant males were present in groups.
Our results are consistent with studies that suggest that younger mammalian mothers are more limited in the energy they can allocate to reproduction because they may not have stopped growing (Bercovitch et al. 1998; Clutton-Brock 1984; Descamps et al. 2008; Hinde 2007; Hinde and Capitanio 2010; Setchell et al. 2002). Compared to older mothers, younger mothers may face a more severe trade-off between feeding (to grow and lactate) and still also needing to protect their young infants from harm, may be less able to defend their offspring as they themselves are more vulnerable because they are smaller, and may be less experienced in protecting their infants.
High status in female gray-cheeked mangabeys offers better access to preferred foods (Chancellor and Isbell 2009a). Although among low-ranking females almost 25 % fewer infants survived, we found that the benefit of high rank did not translate statistically into more surviving infants. Similarly, Pusey (2012) documented that in 15 of 20 other studies of primates, there was no significant rank effect on infant survival. The five studies that did show a rank effect on infant survival included olive baboons and chimpanzees, both at Gombe, and three captive populations of macaques. The fact that these are either localized or captive suggests that the effect of rank on infant survival may be enhanced under certain conditions. For example, in the wild, predation may be consistently low, or predators may be absent for periods of time (Isbell et al. 2009; Nishida et al. 2003; Wasser et al. 2004), or human presence may inhibit predators (Isbell and Young 1993), and captive environments eliminate predation altogether. Under conditions of low or no predation, rank effects on access to foods may be more pronounced. Conversely, when predation is high, rank effects may not be found. At Gombe, predation on baboons is apparently relatively low. Over 18 yr, an average of only about one infant per year was known to be killed by predators (J. Goodall pers comm. in Cheney and Wrangham 1987). At Mikumi National Park Tanzania, predation was apparently low, and rank effects on reproductive success in female yellow baboons existed but not when they experienced a period of intense leopard (Panthera pardus) predation (Wasser et al. 2004). Finally, among vervets in Amboseli National Park, Kenya, where predation was a consistently large source of mortality for all ages, rank effects on infant survival did not occur largely because infants of higher-ranking mothers were more likely to die of predation than infants of lower-ranking mothers (Cheney et al. 1988). Though predation of infant mangabeys has been reported (Olupot and Waser 2013; this study), better observations of predators are needed to know how frequently it occurs.
In our study, infant males were particularly vulnerable. All infants that died in a captive mangabey population were also males (Deputte 1991). Greater male mortality in infancy has also been reported in captive rhesus macaques and it may be related to needing more maternal investment than female infants but not receiving sufficient maternal investment to thrive (Hinde 2007). Greater male mortality in infancy has also been documented in humans (Drevenstedt et al. 2008). The observation that greater male mortality begins with infancy helps explain why gray-cheeked mangabey groups typically have fewer adult males than females (Olupot and Waser 2013).
Position in the female dominance hierarchy also appears to influence whether sons or daughters are born more often: high-ranking female mangabeys overproduced daughters whereas low-ranking mothers overproduced sons. The same pattern was found in yellow baboons in Amboseli (Altmann 1980). However, in a review of the literature, Bercovitch (2002) cautioned that rank-related sex biases at birth are unusual among primates and that when they do occur, they occur most often in captive populations where food is often abundantly available. It has been suggested that the stress caused by harassment or aggression toward low-ranking females and their infants contributes to rank-associated differential reproductive success in captive cercopithecines (Garcia et al. 2006; Schino and Troisi 2005; Silk et al. 1981). In that case, it may be adaptive for low-ranking mothers to produce fewer daughters, which have little chance to increase in rank and which, if they survive to become adults themselves, will also be harassed and may even have lower survival rates (Blomquist et al. 2011).
We witnessed no cases of infanticide during this 9-yr study, nor are there any published records of infanticide in this population. Nevertheless, on six occasions, infants disappeared shortly after males immigrated into study groups and in an earlier study two females were observed carrying dead infants shortly after new males entered the group (W. Olupot pers. comm.). We cannot exclude infanticide in these disappearances and deaths. We also documented seven infant deaths from falls while males were engaged in aggressive encounters. For six of these infant deaths, immigrant males were in the group at the time. We found that infant mortality was significantly higher when immigrant males were present but only when falls were combined with disappearances. Because fighting among males and the entry of immigrant males into groups both coincide with the presence of receptive females in groups, immigrant males are at least indirectly responsible for some infant deaths via their involvement in male–male competition.
References
Altmann, J. (1980). Baboon mothers and infants. Cambridge: Harvard University Press.
Altmann, J., & Alberts, S. C. (2005). Growth rates in a wild primate population: Ecological influences and maternal effects. Behavioral Ecology and Sociobiology, 57, 490–501.
Arlet, M. E., Molleman, F., & Chapman, C. A. (2008). Mating tactics in male grey-cheeked mangabeys (Lophocebus albigena). Ethology, 114, 841–852.
Arlet, M. E., Grote, M. N., Isbell, L. A., Molleman, F., & Carey, J. R. (2009). Reproductive tactics influence cortisol levels in individual male gray-cheeked mangabeys (Lophocebus albigena). Hormones and Behavior, 55, 210–216.
Arlet, M. E., Kaasik, A., Molleman, F., Isbell, L. A., Carey, J. R., & Mänd, R. (2011). Social factors increase fecal testosterone levels in wild male gray-cheeked mangabeys (Lophocebus albigena). Hormones and Behavior, 59, 605–611.
Barton, R. A. (1993). Sociospatial mechanisms of feeding competition in female olive baboons, Papio anubis. Animal Behaviour, 46, 791–802.
Bates, D., Maechler, M., & Bolker, B. (2011). lme4: Linear mixed-effects models using S4 classes. R package version 0.999375–41/r1341. Retrieved from http://R-Forge.R-project.org/projects/lme4/.
Bercovitch, F. B. (2002). Sex-biased parental investment in primates. International Journal of Primatology, 23, 905–921.
Bercovitch, F. B., & Berard, J. D. (1993). Life history costs and consequences of rapid reproductive maturation in female rhesus macaques. Behavioral Ecology and Sociobiology, 32, 103–109.
Bercovitch, F. B., Lebron, M. R., Martinez, H. S., & Kessler, M. J. (1998). Primigravidity, body weight, and costs of rearing first offspring in rhesus macaques. American Journal of Primatology, 46, 135–144.
Bercovitch, F. B., Widdig, A., & Nürnberg, P. (2000). Maternal investment in rhesus macaques (Macaca mulatta): Reproductive costs and consequences of raising sons. Behavioral Ecology and Sociobiology, 48, 1–11.
Blomquist, G. E., Sade, D. S., & Berard, J. S. (2011). Rank-related fitness differences and their demographic pathways in semi-free-ranging rhesus macaques (Macaca mulatta). International Journal of Primatology, 32, 193–208.
Brown, G. R., & Silk, J. B. (2002). Reconsidering the null hypothesis: Is maternal rank associated with birth sex ratios in primate groups? Proceedings of the National Academy of Sciences of the USA, 99, 11252–11255.
Chancellor, R. L., & Isbell, L. A. (2009a). Food site residence time and female competitive relationships in wild gray-cheeked mangabeys (Lophocebus albigena). Behavioral Ecology and Sociobiology, 63, 1447–1458.
Chancellor, R. L., & Isbell, L. A. (2009b). Female grooming markets in a population of gray-cheeked mangabeys (Lophocebus albigena). Behavioral Ecology, 20, 78–86.
Chapman, C. A., Chapman, L. J., Jacob, A. L., Rothman, J. M., Omeja, P., Reyna-Hurtado, R., Hartter, J., & Lawes, M. J. (2010). Tropical tree community shifts: Implications for wildlife conservation. Biological Conservation, 143, 366–374.
Charnov, E. L. (1993). Life history invariants: Some explorations of symmetry in evolutionary ecology. Oxford: Oxford University Press.
Cheney, D. L., Seyfarth, R. M., Andelman, S. J., & Lee, P. C. (1988). Reproductive success in vervet monkeys. In T. H. Clutton-Brock (Ed.), Reproductive success (pp. 384–402). Chicago: University of Chicago Press.
Cheney, D. L., & Wrangham, R. W. (1987). Predation. In B. B. Smuts, D. L. Cheney, R. M. Seyfarth, R. W. Wrangham, & T. T. Struhsaker (Eds.), Primate societies (pp. 227–239). Chicago: University of Chicago Press.
Clutton-Brock, T. H. (1984). Reproductive effort and terminal investment in iteroparous animals. American Naturalist, 123, 212–229.
Clutton-Brock, T. H., Albon, S. D., & Guiness, F. E. (1984). Maternal dominance, breeding success and birth sex ratios in red deer. Nature, 308, 358–360.
Deputte, B. L. (1991). Reproductive parameters of captive grey-cheeked mangabeys. Folia Primatologica, 57, 57–69.
Descamps, S., Boutin, S., Berteaux, D., & Gaillard, J. (2008). Age-specific variation in survival, reproductive success and offspring quality in red squirrels: Evidence of senescence. Oikos, 117, 1406–1416.
Drevenstedt, G. L., Crimmins, E. M., Vasunilashorn, S., & Finch, C. E. (2008). The rise and fall of excess male mortality. Proceedings of the National Academy of Sciences of the USA, 105, 5016–5021.
Fairbanks, L. A. (1990). Reciprocal benefits of allomothering for female vervet monkeys. Animal Behaviour, 40, 553–562.
French, J. A., Pissinatti, A., & Coimbra-Filho, A. F. (1996). Reproduction in captive lion tamarins (Leontopithecus): Seasonality, infant survival, and sex ratios. American Journal of Primatology, 39, 17–33.
Garcia, C., Lee, P. C., & Rosetta, L. (2006). Dominance and reproductive rates in captive female olive baboons, Papio anubis. American Journal of Physical Anthropology, 131, 64–72.
Heesen, M., Roghan, S., Ostner, J., & Schülke, O. (2013). Food abundance affects energy intake and reproduction in frugivorous female Assamese macaques. Behavioral Ecology and Sociobiology, 67, 1053–1066.
Hinde, K. (2007). First-time mothers bias milk composition in favor of sons. Current Biology, 17, R958–R959.
Hinde, K. (2009). Richer milk for sons but more milk for daughters: Sex-biased investment during lactation varies with maternal life history in rhesus macaques. American Journal of Human Biology, 21, 512–519.
Hinde, K., & Capitanio, J. P. (2010). Lactational programming? Mother’s milk energy predicts infant behavior and temperament and rhesus macaques (Macaca mulatta). American Journal of Primatology, 72, 522–529.
Isbell, L. A. (2012). Re-evaluating the ecological constraints model with red colobus monkeys (Procolobus rufomitratus tephrosceles). Behaviour, 149, 493–529.
Isbell, L. A., & Young, T. P. (1993). Human presence reduces predation in a free-ranging vervet monkey population in Kenya. Animal Behaviour, 45, 1233–1235.
Isbell, L. A., Young, T. P., Jaffe, K. E., Carlson, A. A., & Chancellor, R. L. (2009). Demography and life histories of sympatric patas monkeys (Erythrocebus patas) and vervets (Cercopithecus aethiops) in Laikipia, Kenya. International Journal of Primatology, 30, 103–124.
Kleindorfer, S., & Wasser, S. K. (2004). Infant handling and mortality in yellow baboons. Behavioral Ecology and Sociobiology, 56, 328–337.
Koenig, A. (2000). Competitive regimes in forest-dwelling Hanuman langur females (Semnopithecus entellus). Behavioral Ecology and Sociobiology, 48, 93–109.
Nishida, T., Corp, N., Hamai, M., Hasegawa, T., Hiraiwa-Hasegawa, M., Hosaka, K., Hunt, K. D., Itho, N., Kawanaka, K., Matsumoto-Oda, A., Mitani, J. C., Nakamura, M., Norikoshi, K., Sakamaki, T., Turner, L., Uehara, S., & Zamma, K. (2003). Demography, female life history, and reproductive profiles among the chimpanzees of Mahale. American Journal of Primatology, 59, 99–121.
Olupot, W., & Waser, P. M. (2001). Correlates of intergroup transfer in male grey-cheeked mangabeys. International Journal of Primatology, 19, 169–187.
Olupot, W., & Waser, P. M. (2013). Lophocebus albigena Grey-cheeked mangabey. In T. M. Butynski, J. S. Kingdon, & J. Kalina (Eds.), The mammals of Africa (Primates, Vol. II, pp. 206–209). London: Bloomsbury Publishing.
Ostner, J., Borries, C., Schülke, O., & Koenig, A. (2005). Sex allocation in a colobine monkey. Ethology, 111, 924–939.
Packer, C., Collins, D. A., Sindwimwo, A., & Goodall, J. (1995). Reproductive constraints on aggressive competition in female baboons. Nature, 373, 60–63.
Palombit, R. A. (2012). Infanticide: Male strategies and female counterstrategies. In J. C. Mitani, J. Call, P. M. Kappeler, R. A. Palombit, & J. B. Silk (Eds.), The evolution of primate societies (pp. 432–468). Chicago: University of Chicago Press.
Pusey, A. E. (2012). Magnitude and sources of variation in female reproductive performance. In J. C. Mitani, J. Call, P. M. Kappeler, R. A. Palombit, & J. B. Silk (Eds.), The evolution of primate societies (pp. 143–166). Chicago: University of Chicago Press.
Pusey, A., Williams, J., & Goodall, J. (1997). The influence of dominance rank on the reproductive success of female chimpanzees. Science, 227, 828–831.
R Development Core Team. (2011). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Retrieved from http://www.R-project.org/.
Robbins, A. W., Robbins, M. M., & Fawcett, K. (2007). Maternal investment of the Virunga mountain gorillas. Ethology, 113, 235–245.
Schino, G., & Troisi, A. (2005). Neonatal abandonment in Japanese macaques. American Journal of Physical Anthropology, 126, 447–452.
Setchell, J. M., Lee, P. C., Wickings, E. J., & Dixson, A. F. (2001). Growth and ontogeny of sexual size dimorphism in the mandrill (Mandrillus sphinx). American Journal of Physical Anthropology, 115, 349–360.
Setchell, J. M., Lee, P. C., Wickings, E. J., & Dixson, A. F. (2002). Reproductive parameters and maternal investment in mandrills (Mandrillus sphinx). International Journal of Primatology, 23, 51–68.
Silk, J. B. (1983). Local resource competition and facultative adjustment of sex ratios in relation to competitive abilities. American Naturalist, 121, 56–66.
Silk, J. B., & Brown, G. R. (2004). Sex ratios in primate groups. In P. M. Kappeler & C. P. van Schaik (Eds.), Sexual selection in primates: New and comparative perspectives (pp. 253–265). Cambridge University Press.
Silk, J. B., & Brown, G. R. (2008). Local resource competition and local resource enhancement shape primate birth sex ratios. Proceedings of the Royal Society of London B: Biological Sciences, 275, 1761–1765.
Silk, J. B., Clark-Wheatley, C. B., Rodman, P. S., & Samuels, A. (1981). Differential reproductive success and facultative adjustment of sex ratios among female bonnet macaques (Macaca radiata). Animal Behaviour, 29, 1106–1120.
Strum, S. C., & Western, J. D. (1982). Variation in fecundity with age and environment in olive baboons (Papio anubis). American Journal of Primatology, 3, 61–76.
Therneau, T. (2011). Survival: Survival analysis, including penalised likelihood. Original Splus->R port by Thomas Lumley. R package version 2.36–10.
Trivers, R. L., & Willard, D. E. (1973). Natural selection of parental ability to vary the sex ratio of offspring. Science, 179, 90–92.
Valtonen, A., Molleman, F., Chapman, C. A., Carey, J. R., Ayres, M. P., & Roininen, H. (2013). Tropical phenology: Bi-annual rhythms and interannual variation in an Afrotropical butterfly assemblage. Ecosphere, 4, 1–28.
Veeroja, R., Kirk, A., Tilgar, V., Säde, S., Kreitsberg, M., & Tõnisson, J. (2010). Conception date affects litter type and foetal sex ratio in female moose in Estonia. Journal of Animal Ecology, 79, 169–175.
Waser, P. M. (1974). Intergroup interaction in a forest monkey: The mangabey Cercocebus albigena. Dissertation. New York: The Rockefeller University.
Wasser, S. K., Norton, G. W., Kleindorfer, S., & Rhine, R. J. (2004). Population trend alters the effects of maternal dominance rank on lifetime reproductive success in yellow baboons (Papio cynocephalus). Behavioral Ecology and Sociobiology, 56, 338–345.
Whitten, P. L. (1983). Diet and dominance among female vervet monkeys (Cercopithecus aethiops). American Journal of Primatology, 5, 139–159.
Acknowledgments
We thank the Uganda Wildlife Authority, Uganda National Council for Science and Technology, and personnel at the Makerere University Biological Field Station in Kanyawara for permission to work in Kibale National Park. The study complied with all current laws of Uganda. We thank all the field assistants who worked with us during these years for their invaluable help: Kaseregenyu Richard, Katusabe Swaibu, Irumba Peter, Sabiti Richard, Akora Charles, and Koojo John. We thank Richard Wrangham and field assistants of Kibale Chimpanzee Project who informed us on chimpanzee predation on mangabeys. Thanks also to Linda-Liisa Veromann for help in entering behavioral data. This research was supported by the Leakey Foundation and the University of California, Davis, Department of Anthropology (to R. L. Chancellor), by NIH/NIA grants PO1 A6022500 and PO1 A608761 (to J. R. Carey), by the European Union through the European Social Fund (Mobilitas postdoctoral grant MJD56, to M. E. Arlet). R. Mänd and F. Molleman were supported by the Estonian Ministry of Education and Science (targeted financing projects number 0180004s09 and 0180122s08 and ESF 9215,7406, 7699, 7522, 8413, and GD6019), and the European Regional Development Fund (Center of Excellence FIBIR). Finally, we appreciate the diligent efforts of Joanna Setchell and two anonymous reviewers to improve this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arlet, M.E., Isbell, L.A., Molleman, F. et al. Maternal Investment and Infant Survival in Gray-Cheeked Mangabeys (Lophocebus albigena). Int J Primatol 35, 476–490 (2014). https://doi.org/10.1007/s10764-014-9754-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-014-9754-8