Abstract
Rivers border the geographic distributions of primate taxa, but the extent to which the rivers are effective barriers is debated. We here provide the first statistically substantiated analysis of the role of rivers as barriers to the distribution of primates in west and central Africa, as judged by the coincidence of the edge of distributions of forest-dwelling primates with rivers. We use data from the literature to analyze the distributions of 14 genera, 44 species, and 77 subspecies around 18 rivers, river groups, or stretches of rivers. Rivers bordered the distributions of more new taxa (subspecies) than of old taxa (genera), although within genera, age of origin of the taxon did not correlate with likelihood of rivers bordering distributions. Fewer taxa crossed wide rivers than crossed narrow ones. Some analyses indicated that more smaller-bodied genera were stopped by smaller rivers than were larger-bodied genera, but other analyses indicated no effect of body size. Genera with smaller geographic ranges crossed a smaller proportion of the rivers that they met than did genera with large ranges, implying a fundamental difference between small- and large-range taxa in their ability to disperse. Specialization might be the operating trait. Finally, although the aridity of the biogeographic gap in west Africa, the Dahomey Gap, is often argued to be the barrier to movement of species across the Gap, we found that for half the primate species that do not cross the Gap, the rivers that border the Gap could in fact be the effective barrier.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The issue of what determines the edge of a taxon’s distribution is a general one in biogeography, including the biogeography of humans. Barriers can be biological, as when pathogens or other species restrict extent of distribution. A well-known example of the former would be trypanosomiasis (sleeping sickness) preventing the use of horses in much of Africa, which arguably slowed the European conquest of Africa (Maudlin 2006). A checkerboard distribution of competing species on small islands, i.e., only 1 species per island, but the successful species differing between islands, indicates the latter (Diamond 1975). Barriers can also be geographical, as in the case of mountains and seas separating European peoples and languages (Barbujani and Sokal 1990).
Water commonly borders the distribution of terrestrial mammals, including primates. Thus rivers are obvious borders to the distributions of South American primates (Ayres and Clutton-Brock 1992; Hershkovitz 1977; Wallace 1876, 1880), southeast Asian primates (Meijaard and Groves 2006), and Malagasay lemurs (Goodman and Ganzhorn 2004). Similarly, across west and central Africa, rivers apparently bound the distribution of many primate species (Colyn 1988; Gautier-Hion et al. 1999; Grubb 1990). However, only for the Amazon have we found quantitative assessment of the extent to which rivers are barriers to primate distributions (Ayres and Clutton-Brock 1992), and the extent to which even the Amazon and its tributaries are barriers is debated (da Silva and Patton 1998; Gascon et al. 2000).
The longer a river has bordered a taxon’s distribution, or been near it, the more likely it is that geographic events, such as aridity or a change in the course of the river, will have allowed passage across the river (da Silva and Patton 1998). Age of rivers is difficult to know, but because taxa are usually older than are subtaxa, we can use taxonomic level as a surrogate for time. The older a taxon, the more likely it is too that the taxon will itself have crossed the river, e.g., on floating vegetation, the usually suggested mode for primates’ travel across seas (Abegg and Thierry 2002; Houle 1998; Tattersall 2006).
Not all rivers are equally effective barriers. Larger or faster flowing Amazonian rivers are stronger barriers than are smaller ones (Ayres and Clutton-Brock 1992), as are the lower sections of Malagasy (Madagascan) rivers compared to their headwaters (Goodman and Ganzhorn 2004). The same appears to be the case in Africa, where the R. Congo and its lower sections seem to be more effective barriers than are smaller rivers or headwaters (Grubb 1990). Again, though, only for the Amazon have we found quantitative assessment of the influence of size of a river on its apparent efficacy as a barrier to primate distributions (Ayres and Clutton-Brock 1992).
Not all taxa are equally likely to experience rivers as barriers. In Amazonia, small-bodied primate species experience rivers as barriers more than do large-bodied taxa, as indicated by the fact that size of the river correlates with the size of the largest species that had a river as a boundary (Ayres and Clutton-Brock 1992). Also in Ayres and Clutton-Brock’s analysis, larger-bodied species had fewer subspecies, indicating that the larger species were less likely to experience rivers as barriers. However, other analyses accounting for phylogeny have not found that body mass of primates correlates with taxonomic diversity (Conroy 2003). We have not found any other quantitative assessment of the relationship of body size of primates to likelihood of distributions being bordered by a river.
Ayres and Clutton-Brock suggested that the association with body size might explain why the smaller-bodied species had smaller geographic ranges. However, they did not directly test whether taxa with small ranges crossed fewer rivers. In addition, other studies have not found that body size matches geographic range size in primates in South America or elsewhere, including Africa (Harcourt 2000; Harcourt et al. 2005). Instead, primate taxa with small geographic ranges are by various measures generally more specialized than are those with large ranges (Harcourt 2000; Harcourt et al. 2002), a contrast in traits that presumably could influence propensity to cross rivers.
Rivers are assumed to be barriers when a species’ distribution stops at a river. Oates (1988) questions that assumption. He argues that often we do not know whether the distribution of species on one side of a river in fact reaches the river, and that even if it does, an adverse environment on the opposite side, not the river, could be the primary barrier. For instance, although the Cross River in eastern Nigeria has been highlighted as an unusually effective barrier for its size, Oates (1988) argued that aridity and a dense human population west of the river might be the obstacles preventing eastern species moving across the river. Oates made his arguments about the environment as a barrier with particular reference to west Africa, where the rivers are narrower than some of the central African rivers, and therefore the environment is indeed a likely limit to distribution. Nevertheless, Oates’ arguments are substantiated by Meijaard and Groves (2006), who showed that the environment differs on either side of most of the rivers, such as the Brahmaputra, that bound the distributions of mammalian species in mainland Southeast Asia. The Mekong is an exception: it bounds mammalian distributions even though the environment is similar east and west of it.
In sum, the extent to which rivers are barriers to the distribution of mammals, including primates, is debatable. We therefore here provide a statistically substantiated analysis of rivers as potential barriers to primate geographic ranges in west and central Africa. The region we analyze runs from Liberia in the west to eastern Democratic Republic of Congo. We ignored tropical east central Africa, because the extensive savannah in the region is such a clear barrier to the movement of forest primates that effects of rivers would be difficult to detect. In tropical west and central Africa, the far greater extent of forest leaves mostly rivers as the main potential barrier.
Within west and central Africa, we examine in extra detail one region, the Dahomey Gap of west Africa. This well-known biogeographic feature is a particular example of the difficulty of separating the nature of the environment either side of rivers from the rivers themselves as the barrier to movement. The Dahomey Gap is a relatively arid region of savannah and scrub forest in the region of Benin, Togo, and Ghana that separates the western Upper Guinea forests from the Congo Forest zone. It therefore separates many forest-dwelling taxa, so much so that the forests on either side of the Gap are recognized as different biogeographical provinces (Booth 1958; Happold 1987; Kingdon 1989; Lernould 1988; Oates 1988). It seems likely, indeed almost obvious, that the Gap’s aridity is the environmental barrier to the forest species. However, the Gap is bounded by rivers, the R. Volta in eastern Ghana on the Gap’s western border, and the R. Ouémé in central Benin on the eastern border. Is it therefore the aridity of the Gap that is the barrier to the forest species, or are the rivers that bound the Gap the effective barrier?
Further confounding the issue is the fact that the Gap is not a gap to all terrestrial taxa. Many forest-dwelling mammals, including primates, are common to both the Upper Guinea and the Congo forest blocks (Booth 1958; Happold 1987; Kingdon 1989; Lernould 1988). Indeed, some forest primate species currently inhabit the Gap (Campbell et al. 2008). The presence of the same forest-dwelling species on either side of the Gap, and even in it, is easily explained by the fact that the region of the Gap was forested at times in the past, including in the early to mid Holocene for a period of nearly 4000 yr (Dupont and Weinelt 1996; Salzmann and Hoelzmann 2005). Even now, rain forest extends into the Gap in western Togo, east of the R. Volta, and gallery forest exists along rivers in the Gap (Campbell et al. 2008; Oates 1988). If forest once extended all across the Gap, then it becomes possible that the Volta and the Ouémé were in fact the geographic barriers, not the present-day aridity of the Gap.
In investigating the extent to which river might be barriers to the distribution of primates in west and central Africa, we ask 6 questions:
-
1.
What is the extent to which rivers border the edge of primates’ geographic ranges?
-
2.
Are younger taxa (subspecies, species) more likely to be stopped in their distribution by rivers than are older taxa (species, genera)?
-
3.
Are larger rivers stronger barriers than are smaller ones?
-
4.
Are small-bodied primates more or less likely than large-bodied primates to experience rivers as barriers?
-
5.
Are primate genera that occupy small geographic ranges more or less likely than large-range genera to have rivers as boundaries to their distribution?
-
6.
Are the rivers on either side of the Dahomey Gap the effective biogeographic barrier to passage of primates, as opposed to the usually assumed aridity of the Gap?
Methods
Distribution of Primates in Relation to Rivers
In total, we collated data for 18 rivers, river complexes, or stretches of rivers; 14 genera; 44 species; and 77 subspecies.
We initially took information on the distribution of west and central African strepsirrhines, colobines, and apes from Grubb’s (1990) review. We modified his west African cercopithecine distributions using the far more detailed analysis of Oates (1988). Similarly, for central African catarrhine distributions, we also used the more detailed and updated review of Gautier-Hion et al. (1999).
We ignored primate taxa that inhabit savannah or savannah-woodland (Chlorocebus, Erythrocebus, Papio). In the west, these taxa can effectively bypass the rivers, by traveling across their minor headwaters, whereas in central Africa they do not reach several of the rivers in the data set because they barely enter the central African forests.
Extensive savannah lies just north of the lower sections of the R. Congo (named the R. Zaire for a period). In case the savannah, rather than the river, might be the barrier, we repeated analyses, omitting the data from these lower parts of the river. The result is a conservative test of the hypothesis that rivers are barriers, because a thin strip of forest stretches along the northern bank of the lower R. Congo (Gautier-Hion et al. 1999). Given that 2 species in the analysis, Cercopithecus cephus and C. pogonias, inhabit this northern riverine forest (Gautier-Hion et al. 1999), but do not cross the river, presumably other species in the analysis for which we do not have such precise information on distribution could do the same.
We excluded several taxa in Grubb (1990) and Gautier-Hion et al. (1999) because their distributions did not reach the river that was the potential barrier. Grubb termed these species “restricted distribution” or “isolate” taxa. We also excluded the several subspecies that Gautier-Hion et al. (1999) showed with oval distributions to indicate lack of good knowledge of their exact distribution.
Gautier-Hion et al. (1999) queried whether Allenopithecus occurred on both banks of the Congo, but we recorded it as crossing the Congo to favor the null hypothesis. In addition, Gautier-Hion et al. (1999) show a few subspecific hybrid zones. We recorded the respective species as occupying the zone, because they must have crossed any rivers bordering the zones. However, we ignored a queried subspecific hybrid zone on the Oubangui for Lophocebus albigena.
Finally, Oates (1988) argued that several taxa recorded with the R. Sanaga as a boundary in fact cross the Sanaga, or might do so. Gautier-Hion et al. (1999) confirm that several do so. Although Grubb (1990) suggested that Mandrillus leucophaeus occurred just south of the Sanaga, we did not record it as crossing, because many of the records of its southern presence may be false (Wolfheim 1983).
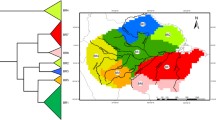
With regard to quantitative analysis of primates’ distributions in relation to rivers, we used the biogeographic zones in west and central Africa that Grubb (1990) identified based on their complement of mammals (Fig. 1; Table I). His zones enabled us to score rivers as potential boundaries, because rivers bounded his zones. To analyze quantitatively the distribution of primates in relation to rivers, we tested whether taxa were present in both or only one of adjacent zones, and recorded the numbers that were on only one side of the river or on both sides. Hence we obtained the proportion of taxa that abutted a river that experienced it as a barrier.
Schematic map of study site. Numbers are the Grubb (1990) biogeographic zones, and our variations on them (see Methods). All rivers mentioned in the text are shown (solid lines). Letters show countries, with borders indicated by faint dashed lines. From west to east: Liberia, Sierra Leone, Ghana, Togo, Benin, Niger, Cameroon, Gabon, Rep. Congo, Dem. Rep. Congo, Central African Rep.
We split 2 of Grubb’s zones, because major rivers, the R. Niger (zone 1c) and R. Oubangui (zone 3′), traversed the zones, yet must be counted as potential barriers. We used Oates (1988) to identify the distribution of the taxa with respect to the Niger, and Gautier-Hion et al. (1999) for the Oubangui. Otherwise we ignored rivers within zones. If taxa crossed these rivers, but we did not record the river and therefore the crossing, we have in one sense inflated the proportion of rivers that were barriers. However, no one expects all small rivers to be barriers. This analysis therefore needs to be understood as one of rivers large enough to be considered potential barriers.
We extended Grubb’s zone 4b, from east to west of the Cuango (Kwango in Gautier-Hion et al.), because the Cuango did not appear to be a barrier to any taxa in Gautier-Hion et al. (1999) whose full distribution was known. Grubb did not extend his zones 2b and 2c (effectively in Cameroon and Gabon, respectively) south to the R. Congo because mainly savannah lies between them and the Congo. However, forest exists along the northern border of the R. Congo, so we extended the 2 zones to the R. Congo. We ignored distributions between Grubb’s (1990) zones 2b and 4b (between the Sanaga and Ogooué, and southwest of the Kasai, respectively), considering that counts of distributions would repeat those between 2b and 4a (between the Congo and the Kasai). The zones for which savannah, rather than a river, could have been the geographic barrier to distribution are indicated in Table I.
The R. Congo borders several of Grubb’s (1990) biogeographical zones. Our use of >1 zone on either side of the R. Congo to estimate probability of the Congo being a barrier might seem to inflate the sample size, or to overestimate the significance of the Congo as a barrier. However, recording of >1 zone for a river takes into some account the length of the river available to be a barrier. If we allowed only 1 record per river, the 200-km Cross River, for example, would have been deemed as much of a potential barrier as the 3000-km Congo River. The number of pairs of zones per river is not equal to the lengths of the rivers, but sometimes the disparity is not great: the R. Congo, for instance, appears 6 times by comparison to the Cross River’s once.
Age of Taxa
We used 2 measures for the age of taxa. Simplistically, taxonomic level indicated age, with genera older than species, which were older than subspecies. For a more detailed analysis within genera, we took age of genera from Purvis (1995), again using genera as the most rigorous test.
Size of Rivers
To analyze the potential effect of size of rivers on their efficacy as barriers, we compared the proportion of taxa recorded to have a river as a boundary to their distribution (including and excluding the zones recorded as bordered by savannah):
-
1.
The largest river in the region, the R. Congo, vs. all other rivers in our data set
-
2.
R. Congo vs. its tributaries
-
3.
R. Congo and its tributaries ranked according to size in midpoint of zone edge, as judged from maps (Table I). We omitted 4 tributaries, because we could not reliably judge their width in relation to the others.
To control for phylogeny in this analysis of the effect of size of rivers, we also asked within genera (the taxonomic level least likely to have been stopped by any river) whether those genera whose ranges include or abut the R. Congo as well as other rivers were more or less likely to be stopped by the Congo than to be stopped by the other rivers.
Body Mass
To assess the influence of body mass on likelihood of taxa experiencing rivers as barriers, we plotted 1) body mass of genera (measured as median congeneric species’ mass) against proportion of rivers that were barriers and 2) body size of the largest genus with the river as a distributional boundary against size of river (Ayres and Clutton-Brock 1992). The generic median body masses that we used are listed in Harcourt (2000). We used genera as the data for reasons already stated. The phylogenetic influence is even more severe than for range size, because congeneric species are so similar in body mass.
Geographic Range Size
Geographic range size of taxa was obtained from distribution maps in the literature (Harcourt et al. 2002). Using only genera produces a small sample, but use of species has the potential to introduce some phylogenetic confounds, given how many multispecies genera are in the analysis. Also, because genera are less hindered by rivers than are shallower-level taxa, they provide a more severe test.
The Dahomey Gap
For details of distributions around the Dahomey Gap, we used Booth (1958), Grubb (1990), Happold (1987), and Oates (1988). Campbell et al. (2008) provide updated details of distribution of species within the Gap. They indicate an absence within the Gap of 2 species (Cercopithecus nictitans and Pan troglodytes), whereas from the earlier accounts we record them there. Even if not in the Gap now, the species must have been there previously, because they occur on either side of it. Indeed Campbell et al. (2008) suggest that the chimpanzee only recently became extinct in the Gap.
Taxonomy
We used the taxonomy of Gautier-Hion et al. (1999) for central African simians. We used Booth (1958), Happold (1987), Oates (1988), Grubb (1990), and Groves (2001) for west Africa, updating to each later author where possible. Updating was not always possible. For instance, Groves (2001) does not consider that enough information is available to distinguish Galago/Galagoides demidoff and G. thomasi properly.
For unnamed subspecies in Gautier-Hion et al. (1999), such as Procolobus pennanti sp., we ignored the subspecies, but included the information in the range of the species and genus. The one species with no subspecies was entered in the subspecies count as a single subspecies.
Analysis
We used JMP 8.0 (SAS Institute Inc 2009) for statistical analyses, presenting 2-tailed probabilities. To assess effect of age of taxon (question 2 at end of Introduction), we used matching model analysis and Wilcoxon matched-pairs, signed ranks tests. For size of river (question 3) and body size (question 4), we used Wilcoxon/Kruskal-Wallis tests and Spearman correlations. For geographic range size (question 5), we used Spearman correlations.
Results
Are Rivers Barriers?
If the presence of a primate taxon on one side of a river but not the other is accepted as evidence that the river was and is a barrier to distribution, then rivers are barriers to the distribution of primates in Africa. Many taxa at all 3 taxonomic levels of genus, species, and subspecies have distributional limits at or near rivers (Table I).
Do Younger Taxa Cross Fewer Rivers?
Rivers separate more subspecies than they do species or genera, and more species than genera (Table I; Fig. 2). In other words, with taxonomic level as the measure of age of a taxon, younger taxa have crossed fewer current-day rivers than have older taxa (matching model analysis: whole model, df = 15, F = 11.0, p < 0.0001; taxonomic levels, F = 56.2, p < 0.0001; rivers, F = 4.1, p = 0.001). Genera vs. species, subspecies; species vs. subspecies: Wilcoxon matched pairs, signed ranks test: N = 18, W = > 81, p < 0.0001 (per river, proportion of each taxonomic level that did not cross). Within genera, no effect of age was apparent (Spearman: N = 14, r s = 0.02, p > 0.9).
Proportions of 3 taxonomic levels for which rivers border their distributions. Data are the percent of members of each taxonomic level that does not cross the river (by river, or river section), between the biogeographic zones of Grubb (1990) (see Methods). Lines join data for each river, or section of river; column = median.
Are Larger Rivers More Effective Barriers?
Larger rivers separate more taxa than do smaller ones. Significantly fewer species and subspecies cross the R. Congo, the largest of tropical Africa’s rivers, than cross all other rivers in tropical west–central Africa (Tables I and IIA). Also, fewer species and subspecies cross the main river of the Congo than cross its tributaries (Tables I and IIB), and fewer genera and species cross broader stretches of the Congo and its tributaries than cross narrower stretches (Tables I and IIC).
The 3 lower stretches of the R. Congo are bordered on their northern edge by extensive savannah. If one omits these stretches (because grassland is a potential barrier to the forest taxa analyzed here), it is not so clear that rivers are a barrier, except perhaps to subspecies (Table IIA, B, C).
Controlling for phylogeny by asking whether, within genera, those whose ranges include or abut the R. Congo as well as other rivers are more or less likely to be stopped by the Congo than to be stopped by the other rivers, we found that of 13 such genera, 7 were more likely to have the Congo as a boundary to their range, and only 1 (Miopithecus) was more likely to have other rivers as boundaries. Of the remaining 5 genera, 4 crossed all rivers analyzed. When the savannah zones of the Congo were omitted, equally as many genera had the Congo as an apparent barrier as had another river as an apparent barrier.
Are Smaller-Bodied Taxa Less Able to Cross Rivers?
Whether body size correlates with the likelihood of rivers being a boundary to distributions depended on the comparison that we used. Although the proportion of rivers crossed by a genus does not correlate with generic body mass (Spearman: N = 14, r s = 0.02, p > 0.9), the degree to which genera experienced the R. Congo as a boundary compared to other rivers does correlate. The smallest-bodied genera were as often stopped by another river as by the R. Congo, whereas larger genera were more likely to be stopped by the Congo than by another river (percent sections of R. Congo not crossed minus percent other rivers not crossed. Spearman: N = 11 [not all genera reached the Congo], r s = 0.87, p < 0.001). If the lower sections of the Congo are excluded, this comparison still showed that smaller-bodied genera were more often stopped by smaller rivers (N = 10, r s = 0.86, p < 0.002).
Using the Ayres and Clutton-Brock method of analyzing the largest bodied taxon that was stopped by a river in relation to size of the river, no comparisons showed a significant correlation. The size of the largest genera stopped by the Congo was not larger than that of the largest genera stopped by other rivers (Wilcoxon/Kruskal-Wallis: N = 6, 12; z = 1.7, p > 0.8). The same lack of significance was true for the R. Congo compared to its tributaries (N = 6, 6; z = 1.1; p > 0.2), and for the Congo and its tributaries ranked by size (N = 8, r s = 0.4, p > 0.2). If the lower sections of the Congo bounded by savanna are removed, none of these 3 maximum body size comparisons was significant, but sample sizes are now small (N = 3 for R. Congo; N = 5 for ranked size of river and tributaries; all comparisons, p > 0.6).
These associations were not confounded by any association between range size and body mass, because there was none (Spearman: N = 14, r s = 0.2, p > 0.5).
Are Taxa with Small Geographic Ranges Less Able to Cross Rivers?
Genera with smaller geographic ranges were stopped by a greater proportion of the rivers that they met (Spearman: N = 14, r s = 0.82, p < 0.001). The relationship held when the lower stretches of the R. Congo, bordered on the north by savannah, were omitted from analysis (Spearman: N = 10, r s = 0.74, p < 0.02).
The Dahomey Gap: Boundary Rivers or Aridity as the Barrier?
Fourteen species reach, enter, or cross the Dahomey Gap (Fig. 3), and are thus potentially relevant to the question of whether the aridity of the Gap, or its boundary rivers are the barrier to taxa that do not cross the Gap. Eight of the species are not stopped by the Gap or its flanking rivers, the Volta and Ouémé. They therefore do not allow a distinction between aridity or rivers as the primary barrier. Of the other 6 species, 3 species cross the flanking rivers, but then their distribution stops within the Gap, implying that the aridity of the Gap is the barrier to their distribution. Two, Cercocebus torquatus and Cercopithecus erythrogaster, come from the east across not only the Ouémé, but also the Niger, and one, Cercopithecus petaurista, comes from the west across the Volta (Campbell et al. 2008; Nobime et al. 2009). Oates (1988) has Cercopithecus petaurista reaching the Ouémé, but subsequent work indicates that its eastern edge is west of the Ouémé, in western Togo (Campbell et al. 2008). The remaining 3 species, however, do not cross one of the rivers bordering the Gap. Consequently, the possibility exists that the river rather than the aridity is the effective barrier to movement across the Gap.
Forest primate distributions in relation to Dahomey Gap and adjacent rivers. Distributional data from Booth 1958; Campbell et al. 2008; Groves 2001; Happold 1987; Oates 1988. Updated taxonomy from Groves (Groves 2001). Numbers in boxes are the number of species per box. Savannah, savannah-woodland taxa not included, i.e., Chlorocebus, Erythrocebus, Papio. *Cercopithecus mona extends only ca. 100 km beyond the west bank of the R. Volta, and does not enter the Upper Guinea forest zone (Oates 1988).
Discussion
In west and central Africa, rivers border the distributions of several forest-dwelling primate taxa, as if the rivers are barriers to the distribution of these primates. Larger rivers border the distributions of more forest taxa than do smaller rivers. A logical assumption is that rivers, especially large rivers, are barriers to the distribution of these forest primates. However, the environment near rivers can differ from that farther away, and the environment can differ on either side of a river (Oates 1988). When we omitted from analysis the lower sections of the R. Congo, north of which is mostly savannah, not forest, the role of rivers as barriers was less obvious. Of course, omission of the lower portions of the Congo is omission of precisely the part of the river, the widest, that might be the most effective barrier.
A further consideration is that a thin strip of forest exists on the northern bank of the R. Congo in this savannah area, and neither of the 2 forest species that inhabit this strip extend to the forest south of the Congo (Gautier-Hion et al. 1999). Concomitantly, several forest species south of the river do not occur in the strip of riverine forest north of the R. Congo. With forest on both sides of the river, if thinly on the north side, and some of the forest species in this region not crossing the river in either direction, we suggest that the river is a main barrier in this region.
By contrast, in the case of the Dahomey Gap, an arid gap in the forest situated between 2 rivers, our analysis supports the previous ones listed in the Introduction in indicating that the aridity could be the effective barrier to perhaps 3 of the 6 species that reach the Gap but do not cross it. These 3 species entered the Gap, i.e., crossed ≥1 river, but nevertheless did not traverse the Gap. One of the species, though, Cercopithecus erythrogaster, lives so far within the Gap that it seems reasonable to ask whether in wetter times it did not get all the way across from the east, but then was stopped by the larger of the bordering rivers, the R. Volta. For 3 other species that reached 1 of the flanking rivers, the Volta, but did not cross the Gap, it is possible that the river was the effective barrier, rather than the aridity of the Gap.
We partially controlled for the possibility that a river could change its course so as to cut through a taxon’s distribution, by arguing that the more time available, the greater the likelihood of this sort of event. In addition, the more time available, the more likely it is that the taxon itself could cross a river. Indeed, genera were recorded as crossing rivers more than were species and especially subspecies.
Although Ayres and Clutton-Brock (1992) found that small-bodied taxa were less likely to cross Amazonian rivers than were large-bodied taxa, our findings were equivocal. This contrast correlates with the fact that in their data, smaller-bodied species had smaller geographic ranges. They did not correct for phylogeny, however, and other studies that did so have not found body size to be obviously related to range size of primates in South America or elsewhere, including Africa (Harcourt 2000; Harcourt et al. 2005), and nor was there a relation for the genera of west and central Africa in this study.
If it is not body size that restricts range size because of the inability of small-bodied animals to cross rivers, degree of specialization could be an acting biological trait. Taxa with small ranges are more specialized than those with large ones (Harcourt 2000; Harcourt et al. 2002), and this study indicated that the small-range taxa crossed fewer of the rivers that they encountered than did taxa with large ranges. We can only speculate as to why specialists might be less likely than generalists to cross a river. Given that rivers can be barriers to not just primates, but many other species as well (da Silva and Patton 1998; Grubb 1990; Meijaard and Groves 2006), with the result that the environment on either side of a river can be effectively different (Meijaard and Groves 2006), one possibility is that specialists can perceive the difference, and avoid it, or survive less well than generalists in the new environment if they do cross the river. Alternatively, perhaps specialists might be less likely than generalists to cross adverse habitat. An example would be the long-known reluctance of specialist, forest-dwelling bird species to move between fragments of forest (Stouffer et al. 2009; Turner 1996).
Two factors could bias the results of this study, one diminishing the apparent role of rivers as barriers and the other increasing the role. The first is the possibility that humans transported species across rivers. Wallace (1876) suggested that some Asian taxa east of what came to be termed the Wallace line, i.e., the line separating Asian from Australian faunas, were carried across the line by humans. Following that lead, Heaney (1986) and Meijaard and Nijman (2003) excluded several mammalian species from their analysis of the presence of mammals on islands in insular Southeast Asia because of the possibility that the species were transported to the islands by humans. Certainly, people have kept primates as pets, or food, or working beasts for centuries (Fuentes and Wolfe 2002). Single records or sightings of a species on the far side of a river that is the apparent distributional limit might be the result of transport by humans, especially perhaps if the sightings were near villages. If any taxa have been transported by humans across rivers, then of course we have underestimated the role of rivers as barriers to natural distributions.
The second potential bias is a possible circularity in the argument. Differences between subspecies can be minor. We think it possible that if those who determined the subspecific status of the populations had not known of the presence of the river, they might have considered the populations to be mere varieties. Subspecific status might have been conferred, we suggest, because the authors assumed that the individuals could not cross the river. The assumption would be strongest where the river was widest. In the current context, the result would be that the prevalence of rivers as barriers to subspecies is inflated. However, some genetic analysis is revealing detectable differences between intraspecific populations on either side of rivers. For instance, the mandrill (Mandrillus sphinx) populations north and south of the Ogoué R. are different enough that present estimates have them separated since 800,000 yr ago (Telfer et al. 2003).
In sum, the results of our quantitative analyses substantiate previous arguments that rivers can be barriers to the geographical distribution of forest-dwelling primates in west and central Africa. Certainly other geographic barriers could exist too, but even in the case of the most obvious one in the region, the aridity of the Dahomey Gap, at least some of the species could be stopped by the Gap’s flanking rivers, not by the aridity. As natural habitat is increasingly destroyed, our ability to discern natural barriers to distribution will be increasingly difficult.
References
Abegg, C., & Thierry, B. (2002). Macaque evolution and dispersal in insular south-east Asia. Biological Journal of the Linnean Society, 75, 555–576.
Ayres, J. M., & Clutton-Brock, T. H. (1992). River boundaries and species range size in Amazonian primates. The American Naturalist, 140, 531–537.
Barbujani, G., & Sokal, R. R. (1990). Zones of sharp genetic change in Europe are also linguistic boundaries. Proceedings of the National Academy of Sciences of the USA, 87, 1816–1819.
Booth, A. H. (1958). The Niger, the Volta, and the Dahomey Gap as geographic barriers. Evolution, 12, 48–62.
Campbell, G., Teichrob, J., & Paterson, J. D. (2008). Distribution of diurnal primate species in Togo and Bénin. Folia Primatologica, 79, 15–30.
Colyn, M. M. (1988). Distribution of guenons in the Zaïre-Lualaba-Lomami river system. In A. Gautier-Hion, F. Bourlière, & J.-P. Gautier (Eds.), A primate radiation: Evolutionary biology of the African guenons (pp. 104–124). Cambridge, UK: Cambridge University Press.
Conroy, G. C. (2003). The inverse relationship between species diversity and body mass: Do primates play by the "rules"? Journal of Human Evolution, 45, 43–55.
da Silva, M. N. F., & Patton, J. L. (1998). Molecular phylogeography and the evolution and conservation of Amazonian mammals. Molecular Ecology, 7, 475–486.
Diamond, J. M. (1975). Assembly of species communities. In M. L. Cody & J. M. Diamond (Eds.), Ecology and evolution of communities (pp. 343–444). Cambridge, MA: Harvard University Press.
Dupont, L. M., & Weinelt, M. (1996). Vegetation history of the savanna corridor between the Guinean and the Congolian rain forest during the last 150,000 years. Vegetation History and Archaeobotany, 5, 273–292.
Fuentes, A., & Wolfe, L. D. (Eds.). (2002). Primates face to face: The conservation implications of human–nonhuman primate interconnections. Cambridge, MA: Cambridge University Press.
Gascon, C., Malcolm, J. R., Patton, J. L., da Silva, M. N. F., Bogart, J. P., Lougheed, S. C., Peres, C. A., Neckel, S., & Boag, P. T. (2000). Riverine barriers and the geographic distribution of Amazonian species. Proceedings of the National Academy of Sciences of the USA, 97, 13672–13677.
Gautier-Hion, A., Colyn, M., & Gautier, J. P. (1999). Histoire naturelle des primates d'Afrique Centrale. Libreville, Gabon: ECOFAC.
Goodman, S. M., & Ganzhorn, J. U. (2004). Biogeography of lemurs in the humid forests of Madagascar: The role of elevational distribution and rivers. Journal of Biogeography, 31, 47–55.
Groves, C. P. (2001). Primate taxonomy. Washington, DC: Smithsonian Institution Press.
Grubb, P. (1990). Primate geography in the Afro-tropical rain forest biome. In G. Peters & R. Hutterer (Eds.), Vertebrates in the tropics (pp. 187–214). Bonn: Alexander Koenig Zoological Research Institute and Zoological Museum.
Happold, D. C. D. (1987). The mammals of Nigeria. Oxford: Clarendon Press.
Harcourt, A. H. (2000). Latitude and latitudinal extent: A global analysis of the Rapoport effect in a tropical mammalian taxon: primates. Journal of Biogeography, 27, 1169–1182.
Harcourt, A. H., Coppeto, S. A., & Parks, S. A. (2002). Rarity, specialization and extinction in primates. Journal of Biogeography, 29, 445–456.
Harcourt, A. H., Coppeto, S. A., & Parks, S. A. (2005). The distribution-abundance (i.e. density) relationship: Its form and causes in a tropical mammal order, Primates. Journal of Biogeography, 32, 565–579.
Heaney, L. R. (1986). Biogeography of mammals in southeast Asia: Estimates of rates of colonization, extinction and speciation. Biological Journal of the Linnean Society, 28, 127–166.
Hershkovitz, P. (1977). Living New World monkeys (Platyrrhini) (Vol. 1). Chicago: University of Chicago Press.
Houle, A. (1998). Floating islands: A mode of long-distance dispersal for small and medium-sized terrestrial vertebrates. Diversity and Distributions, 4, 201–216.
Kingdon, J. (1989). Island Africa: The evolution of Africa's rare animals and plants. Princeton, NJ: Princeton University Press.
Lernould, J.-M. (1988). Classification and geographical distribution of guenons: A review. In A. Gautier-Hion, F. Bourlière, & J.-P. Gautier (Eds.), A primate radiation: Evolutionary biology of the African guenons (pp. 54–78). Cambridge, UK: Cambridge University Press.
Maudlin, I. (2006). African trypanosomiasis. Annals of Tropical Medicine and Parasitology, 100, 679–701.
Meijaard, E., & Groves, C. P. (2006). The geography of mammals and rivers in mainland southeast Asia. In S. M. Lehman & J. G. Fleagle (Eds.), Primate biogeography (pp. 305–329). New York: Springer.
Meijaard, E., & Nijman, V. (2003). Primate hotspots on Borneo: Predictive value for general biodiversity and the effects of taxonomy. Conservation Biology, 17, 725–732.
Nobime, G., Sinsin, B., & Lernould, J.-M. (2009). Ecological factors determining the distribution of the red-bellied guenon Cercopithecus e. erythrogaster in Benin and Togo. International Journal of Biological and Chemical Sciences, 3, 606–611.
Oates, J. F. (1988). The distribution of Cercopithecus monkeys in West African forests. In A. Gautier-Hion, F. Bourlière, & J.-P. Gautier (Eds.), A primate radiation: Evolutionary biology of the African guenons (pp. 79–103). Cambridge, UK: Cambridge University Press.
Purvis, A. (1995). A composite estimate of primate phylogeny. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 348, 405–421.
Salzmann, U., & Hoelzmann, P. (2005). The Dahomey Gap: An abrupt climatically induced rain forest fragmentation in West Africa during the late Holocene. The Holocene, 15, 190–199.
SAS Institute Inc. (2009). JMP 8.0. Cary, North Carolina: SAS Institute Inc.
Stouffer, P. C., Strong, C., & Naka, L. N. (2009). Twenty years of understorey bird extinctions from Amazonian rain forest fragments: Consistent trends and landscape-mediated dynamics. Diversity and Distributions, 15, 88–97.
Tattersall, I. (2006). Historical biogeography of the strepsirhine primates of Madagascar. Folia Primatologica, 77, 477–487.
Telfer, P. T., Souquiere, S., Clifford, S. L., Abernethy, K. A., Bruford, M. W., Disotell, D. R., Sterner, K. N., Roques, P., Marx, P. A., & Wickings, E. J. (2003). Molecular evidence for deep phylogenetic divergence in Mandrillus sphinx. Molecular Ecology, 12, 2019–2024.
Turner, I. M. (1996). Species loss in fragments of tropical rain forest: A review of the evidence. Journal of Applied Ecology, 33, 200–209.
Wallace, A. R. (1876). The geographical distribution of animals (Vol. I). New York: Harper and Bros.
Wallace, A. R. (1880). Island life. London: Macmillan.
Wolfheim, J. H. (1983). Primates of the world: Distribution, abundance and conservation. Seattle: University of Washington Press.
Acknowledgments
We thank Lydia Beaudrot, John Oates, Joanna Setchell, Kelly Stewart, and an anonymous referee for critical commentary that markedly improved the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Harcourt, A.H., Wood, M.A. Rivers as Barriers to Primate Distributions in Africa. Int J Primatol 33, 168–183 (2012). https://doi.org/10.1007/s10764-011-9558-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-011-9558-z