Abstract
Competition over food and space is a primary driver of human–wildlife conflict. In the Cape Peninsula, South Africa, chacma baboons (Papio ursinus) have adapted to a human-modified environment, sleeping on the urban edge and raiding anthropogenic food sources on a daily basis. Human monitors, who herd baboons away from residential areas, are currently the preferred method of conflict mitigation. However, this method is costly and suffers from short-term interruptions, wherein the unexpected absence of monitors may lead to unprepared residents using lethal force to deter raiding baboons. Elsewhere in the chacma baboon distribution (in nonconflict areas), artificial food patches have been shown to alter troop movements drastically by eliciting consistent leadership behavior from alpha males. We investigated whether an artificial patch could be used to draw baboons away from the urban environment in the absence of monitors. First, we introduced an artificial food patch into natural land within a troop’s range and monitored movement and activity patterns. Although the troop utilized the patch, there was not a significant decline in use of the urban space as they continued to favor food in urban waste sites. Maintaining the patch, we then restricted access to these waste sites using wire-mesh fencing and observed a significant reduction in the time the troop spent within the urban space. In both experimental phases we observed consistent leadership, with dominant individuals arriving first at the patch and monopolizing food items thereon. Thus, we recommend the combined strategy of reducing raiding incentives in conjunction with provisioning as a short-term, cost-effective strategy to alter a baboon troop’s movement patterns and raiding frequency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human encroachment on natural habitat has resulted in worldwide resource competition between people and wildlife (Dickman 2010). Conflict arising from this competition translates into ecosystem changes (Cooper and Ginnett 2000; Ripple and Beschta 2007; Woodroffe et al. 2005), local population extinctions (Nyhus and Tilson 2004), and financial loss (Mishra 1997; Roper et al. 1995). Where a species has a large niche overlap with humans, this conflict can be frequent, as is often found with conflicts involving nonhuman primates (hereafter primates). Typically, these conflicts are over human food sources, which primates are able to thrive on, acquiring food through their intelligence, social cooperation, communication, agility, manual dexterity, and behavioral flexibility (Else 1991). Supplemented with human food, primates exhibit increased growth rates (Strum 2010) and are able to allocate more time to rest and social behavior (Altmann and Muruthi 1988; Forthman-Quick 1986). The genera Macaca (Riley and Priston 2010), Cercopithecus (Saj et al. 1999), and Papio (Hill 2000; Naughton-Treves 1997) are particularly adept raiders that are subsequently involved in conflict with humans. This conflict peaks where these species forage in crop fields and food stores, and directly deplete either the food supplies or livelihood of humans (Marchal and Hill 2009; Naughton-Treves et al. 1998).
In the Cape Peninsula, South Africa, an example of human–primate conflict exists between urban and rural inhabitants and the chacma baboon (Papio ursinus). The peninsula’s remaining natural landscape is characterized by Fynbos, the local vegetation type, and supports a baboon population of 475 (Beamish 2010) that is geographically isolated from conspecifics by human development surrounding the peninsula. Fynbos is a component of the Cape Floristic Region, one of the world’s 18 biodiversity hotspots and a UNESCO World Heritage Site and is characteristically nutrient poor (Cowling et al. 1996). This is reflected in a preference that peninsula baboons show for alien vegetation and urban food (Hoffman and O’Riain 2010). In fact, of the 16 baboon troops that occur on the peninsula, 10 have home ranges that overlap with permanent human settlements (Hoffman unpubl. data) and 14 regularly incorporate human-derived foods, e.g., fruit, vegetables, sugar, eggs, and bread, in their diets (Kaplan pers. obs.). The nutritional contrast between Fynbos and high-caloric urban food, which is higher in protein, fat, and carbohydrates, greatly increases raiding incentive. In the quest for these urban food items, baboons readily raid waste areas, cars, houses, and shops, and in some cases will take food directly from people. Although protected by legislation (Western Cape Nature Conservation 2000), the damage to property and stress for local residents ultimately results in injury and mortality to baboons as people counteract raiding behavior with aggression, e.g., poisoning and shooting (Beamish 2010).
To date, a variety of methods have been employed to reduce food loss to baboons across their distribution, including crop guarding (Naughton-Treves 1997), taste aversion (Forthman et al. 2005), translocation (Strum 2005), and culling (Katsvanga et al. 2006). In the peninsula, mitigation of raiding behavior is currently achieved through human herders or monitors. Teams of men assigned to raiding troops are equipped with 2-way radios and tasked with deterring troops from urban areas by whistling and shouting. Although largely successful (van Doorn 2009), the monitor program is costly and has an annual operating budget of approximately US$1 million. Difficulty in procuring funds has led to frequent interruptions to the monitor program for periods lasting 5–20 d (O’Riain pers. obs.). During these periods, baboons that have been herded previously are afforded unlimited access to urban environments, and conflict between baboons and residents—who are invariably caught unaware in the absence of monitors—escalates rapidly. Developing a management solution for these periods is thus critical to reducing property damage and injury/mortality to baboons. The solution must be cost effective and easy to implement at short notice because it may need to be administered in several troops simultaneously.
Conceptually, reducing the frequency of raiding behavior in these brief, but critical, periods can be achieved either by increasing the cost of foraging in the urban environment, i.e. the central tenet of the monitors’ approach, or by increasing the incentive to forage in natural environments by providing a benefit for this behavior. Food provisioning in natural environments could potentially provide this incentive, influencing a troop’s movement patterns and altering its space use. Although long-term provisioning has been proposed as an important tool in primate conservation (Asquith 1989), we advocate caution in experimenting with provisioning primates, owing largely to increases in body mass (olive baboons: Altmann and Alberts 2005; black bears: Partridge et al. 2001; red deer: Rodriguez-Hidalgo et al. 2010), fecundity (olive baboons: Warren et al. 2011; red deer: Rodriguez-Hidalgo et al. 2010), offspring survival (olive baboons: Warren et al. 2011; red deer: Schmidt and Hoi 2002; eagles: Margalida et al. 2007), and population size (eagles: McCollough et al. 1994) observed across a range of species. If realized in peninsula baboons, increased growth rates or fecundity could worsen human–baboon conflict by increasing the space required by extant troops. However, a specific provisioning methodology can (1) influence the movement patterns of baboon troops while (2) minimizing potential for increased growth rates and fecundity (King et al. 2008). King et al. examined movement decisions in chacma baboon troops in a desert environment (Tsaobis Nature Reserve, Namibia) that experiences no human–baboon conflict. When presented with a high-quality, dense food patch, dominant males led the whole troop to the patch and then monopolized the limited resources thereon. Thus incentive to the troop leader influenced the movement patterns of the whole troop, despite very few troop members receiving any nutritional incentive to follow the dominant individuals to the food patches and suffering consensus costs (Conradt and Roper 2003, 2005). Despite these consensus costs to subordinates, researchers observed troop fission rarely, occurring only on 6/80 occasions and always in the largest focal troop.
We test the hypothesis that implementation of the provisioning strategy of King et al. will reduce spatial overlap, and thus conflict, between humans and baboons in the Cape Peninsula. Based on the appeal of a reliable food resource and the findings of King et al., we predicted that after finding an introduced feeding patch, the troop would continue to visit that patch (prediction 1), led by dominant individuals (prediction 2) who would monopolize that patch (prediction 3). We also predicted that these visits would reduce the time the troop spends in the urban environment (by virtue of the patch’s placement in natural land: prediction 4). However, in contrast to Namibia, where baboons’ alternative to a provisioned food source was natural forage, Cape Peninsula baboons have access to urban food items, such as bread, fruit, vegetables, cereals, eggs, and even sugar (typically superior in nutritional value, and protein and fat contents than a uniform provisioned food, i.e., corn). These items are acquired from sources such as dustbins (where baboons often consume discarded food), human handouts, shops, and kitchens and could potentially moderate (lower) a troop leader’s incentive to visit an experimental feeding patch. We therefore, in addition to presenting the experimental patch, identified the urban food sources being raided by the baboons and secured them with barriers to prevent baboon access. After this modification, we predicted that the time baboons spent in the urban space would be further reduced (prediction 5). Finally, given that the baboons under investigation are already heavily reliant on localized, high-energy food sources in the urban space, we predicted that these changes in troop movement patterns and habitat use would not affect activity budgets (prediction 6).
Methods
Study Site and Subjects
Simon’s Town (34°11′39.62″S; 18°25′55.44″E) is located on the eastern coast of the Cape Peninsula, South Africa and is a composite of residential areas, a naval barracks, and a homeless shelter. Natural land on the periphery of the town is composed of a mixture of indigenous Fynbos (shrub-like community of plants, i.e., Proteacae, Ericaceae, and Restionaceae: Cowling et al. 1996) and alien vegetation that includes a small thicket of Eucalyptus and alien grasses. Ranging in these areas, the relatively small Waterfall troop is composed of 21 individuals (September 2009: 1 adult male, 1 subadult male, 9 adult females, 10 juveniles, and 1 infant). We compiled identikits for all adult individuals in this study and found the baboons already habituated to the close proximity of observers, presumably through their prolonged exposure to humans. That is, the troop sleeps in natural land on cliffs outside the urban edge but typically descends into the low-lying urban areas on a daily basis to forage for human derived food items (O’Riain, pers. obs.). This troop was the only routinely raiding peninsula troop that was not assigned a team of monitors at the time of the experiment, and residents in the area have expressed ongoing distress at the frequent troop incursions and the resultant damage to property and loss of food.
Experimental Procedure
The experiment (August 13, 2009–September 11, 2009) was divided into 3 consecutive phases (A = 10 d, B = 9 d, and C = 9 d). We collected baseline data of activity budget and movement patterns in phase A while in phase B we introduced a spatially discrete 10 × 10 m supplementary food patch within the troop’s home range sensu King et al. (2008). Patch location (34°11′36.45″S, 18°25′09.08″E) was a trade-off between distance from the urban edge and proximity to the core of the troop’s home range (Fig. 3). We provisioned the patch daily before sunrise with 880 g (80 g/adult member) of dry corn kernels (according to the high-contest patch of King et al. to make our experiments directly comparable; see also Devas 2005). We distributed kernels evenly (ca. 26 kernels/m2) throughout the patch by scattering a handful at 1-m intervals and collected any remaining kernels after sunset. Importantly, corn also provides a means to quantify foraging bite rate as baboons characteristically transfer individual kernels from the ground to their mouths. In phase C, we retained the experimental patch, but increased the incentive to use it by restricting troop access (using wire-mesh fencing) to the 3 major urban raiding sites identified in the baseline phase.
Data Collection
Observations commenced at sunrise (07:00 h) and ended at sunset (18:00 h). Numerous physical barriers, e.g., fences and walls, in the urban area confounded continuous troop follows on foot, necessitating a novel approach to record detailed troop movements. One observer (O1) was positioned at an elevated observation post on a ridge overlooking the urban space (Fig. 3). From this location O1 could observe and accurately plot the troop’s movement throughout the urban area. Using 2-way radios, O1 directed a second observer (O2) who followed the troop on foot and collected behavioral data. O1 thus recorded all spatial data and instances of raiding while O2 recorded focal and scan data. We discarded data collected on days with sustained heavy rainfall (phase A: 3 d; phase B: 2 d; phase C: 1 d) because these conditions have been shown to reduce range use substantially in peninsula baboons (Hoffman and O’Riain 2010) and because troop visibility to the elevated observer was greatly limited.
Focals
On average, O2 conducted N = 42.36 ± SD 2.28 (range = 16–49) 10-min continuous focal watches (Altmann 1974) on randomly selected adult troop members throughout the day. Each focal recorded habitat type and time spent foraging (actively searching for, manipulating, and ingesting food), grooming (affiliative manipulation of recipient’s pelage by groomer), locomoting (movement resulting in a shift in space while not engaged in foraging), and resting (sedentary position without foraging or grooming).
Scans
We conducted instantaneous scan samples (Altmann 1974) at 15-min intervals throughout the day during all phases. Each scan recorded the number of individuals foraging, locomoting, resting, and grooming as defined in the focal protocol and plotted the location of the estimated troop center on an aerial photograph. The clear delineation of land types (Fig. 3) within the troop’s home range allowed O1 to plot locations consistently with a high degree of accuracy, further improved by continuous communication with O2 at ground level.
Habitat Use
To determine the troop’s active and core areas of use in each phase, we developed fixed kernel estimates (ArcView 3.3, Home Range Extension: Rodgers and Carr 1998) based on troop locations collected every 15 min during active hours. The area of active use was delineated within the 95% probability contour, while the core use area was delineated within the 50% probability contour.
Raided Food Items
During phase A, observers obtained data (where and when) on the raiding behavior of the troop. Raided food item (RFI) intake was then systematically quantified for the troop during phases B and C, wherein 1 RFI was defined as a single movement of urban food from hand to mouth. Individual identification of raiding baboons was not always possible owing to their rapid movements and the tendency of subordinate baboons to retreat from view of dominant individuals when they obtained a RFI. We therefore found measurement of the troop’s collective raiding success to be more meaningful. Where clear identification of a food item was possible, its quantity was recorded but where it was not (N = 253/816 cases), the RFI was scored as 1 unit. Although this method provided only a rough estimate of the troop’s total RFI intake, a consistent approach allowed for meaningful comparisons across phases.
Dominance
We recorded the number and direction of agonistic events ad libitum throughout the study (sensu King et al. 2008) and compiled actor–recipient matrices from active supplants and displacements. We used Matman (De Vries et al. 1993) to determine a dominance hierarchy, which we found to be linear (h = 0.76, N = 189, p < 0.001).
Experimental Patch Protocol
When the troop entered the patch, we suspended normal observations. O1 relocated to the patch’s periphery immediately before the first baboon arrived, while O2 followed the troop to the patch. Continuous video recordings taken by a video camcorder (Canon ZR700) mounted on a tripod 10 m from the patch supplemented all patch observations. We recorded the arrival order of adults to the patch and the identity of all individuals within the patch at 1-min scans (mean = 41.67 ± SD 1.14 scans per visit; N = 250). We recoded the foraging bite rates of all adults on the patch (1 bite is measured as a baboon moving 1 corn kernel from the ground to its mouth), sampled at random (15.5 ± SD 1.45 bite rates per patch visit; N = 93). This allowed us to estimate mean individual foraging benefits to visiting the patch (mean bite rate * mean time spent on the provisioned patch), which was equivalent to the average corn intake of that individual per day. We could not measure accurately individual consensus costs (Conradt and Roper 2005; King et al. 2008) incurred as a result of visiting the experimental patch, i.e., lost foraging opportunities on urban food sources. We therefore examined relative differences in RFIs between phases B and C to assess whether the troop’s incentive to visit the patch may have been increased by restricting access to urban food sources.
Statistical Procedures
We used Wilcoxon-matched pairs tests (Statistica ver. 8.0) to assess differences in behavior and habitat use between the 3 phases of the study and a Mann-Whitney U test (Statistica ver. 8.0) to test for differences in the number of RFIs obtained between phases B and C. We used standard binomial statistics to test whether any individual led the troop to the patch significantly more than others and whether arrival order was random. We used a 1-way, single factor ANOVA (and Tukey HSD post hoc test) to examine differences in corn consumption across individuals. We analyzed arrival orders using a linear mixed model (LMM) conducted in MLwiN version 2.18 (Rasbash et al. 2003), where we included individual dominance rank, grooming relationship to the individual that arrived first, experimental phase (B, C), and sex (male, female) as fixed effects. We incorporated “experimental day” and “individual ID” as random effects to control for non-independence of repeated observations of individuals over experimental days. We used backward elimination in selecting the minimal adequate model and included only the factors that contributed significantly (p < 0.05) to the explanatory power (Akaike 1974). The significance of fixed terms is presented as Wald statistics evaluated against the χ2 distribution.
Results
Patch Use and Dominance
Four days elapsed between placing the supplementary food patch and its discovery by the troop. After it had discovered the patch, the troop visited its location significantly more in phases B (5/9 d) and C (7/9 d) than in phase A (0/10 d) (Fisher exact test: p AvsB = 0.0108; p AvsC < 0.0001), supporting our first prediction.
We found that individual arrival order to the patch was predicted by dominance rank (LMM: χ2 = 31.73, df = 1, p < 0.001; Table I, Fig. 1), with either the alpha male or subadult male (ranked second) leading the troop to the patch on all but 2 occasions (supporting prediction 2). We found that individual grooming relationships with the alpha male determined follower behavior, and was a nonlinear effect. That is, the stronger the grooming relationship with the dominant male the sooner the individual arrived on the patch (LMM: χ2 = 19.08, df = 2, p < 0.001; Table I). Once the troop was at the patch, we found that the foraging benefit differed significantly across individuals (ANOVA: F (10, 55) = 50.093, p < 0.001) with the alpha male and female consuming the most (Tukey HSD post hoc test: M1: p < 0.001; F2: p < 0.01) (Fig. 1: supporting prediction 3). Two mid-ranked (3rd and 4th) adult females attended the patch but did not remain for long periods, as reflected in their low corn yield (Fig. 1). Although we recorded detailed data only for adults, we consistently observed several juveniles (5.79 ± SD 0.57) present on the patch while food was present, i.e., from first adult arrival until the corn was completely depleted (mean = 33.6 min ± 9.8 min).
a Arrival orders to the supplementary patch during phases B and C, compared with dominance rank. The trend (prediction from Table 1) shows the tendency of more dominant individuals to arrive early. b Average daily corn yield plotted against dominance rank shows a clear monopoly by the 2 dominant troop members over phases B and C.
Habitat Use
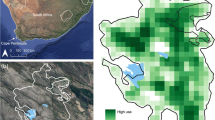
After introducing the patch in phase B, the troop’s average daily use of the urban environment decreased from 40% (±5.39) to 29.68% (±7.64) (N = 9; Z = 1.13; p = 0.26), although this change was not significant, i.e., we found no support for prediction 4). After restricting access to urban food items in phase C, the troop’s average daily use of the urban environment decreased to only 12% (±2.78), significantly less than in phase A (N = 9; Z = 2.310161; p < 0.05), supporting prediction 5 (Fig. 2).
Fixed kernel estimates showed a clear difference between troop range use in each phase (Fig. 3). During phase A the troop showed core area use of the navy barracks and Happy Valley home. In phase B, core area use extended to incorporate the patch and the Signal School, reflected in the increase seen in mean daily use of the Signal School from phase A (6 ± SD 11%) to phase B (11 ± SD 15%) and an increase in the troop’s core area size from phase A (0.04 km2) to phase B (0.13 km2). In phase C, restricted baboon access to the Signal School, Happy Valley home, and navy barracks resulted in a reduction in the core use area (0.06 km2) with focus shifting to natural habitat, reflected in the mean daily use of natural habitat increasing from phase B (70.32% ± 17.04) to phase C (88.06 ± 8.34).
a Habitat composition of study site: 1a, Fynbos; 1b, mixture of alien vegetation and Fynbos; 2a, sleep site 1; 2b, sleep site 2; 3, navy barracks; 4, Happy Valley home; 5, wetland; 6, residential zone; 7, procurement centre; 8, eucalyptus thicket; 9, Signal School. Sites 3, 4, and 9 are raiding hotspots as indicated by the frequency raided food items (all have large, accessible kitchens and waste areas). The star indicates the position of O1. The hollow square denotes the position (not size) of the supplementary patch. b–d Fixed kernel estimates of the troop’s range use (narrow white: 95% probability contour; bold white: 50% probability contour). b Phase A: baseline. c Phase B: location of introduced supplementary patch marked with a solid square. d Phase C: supplementary patch plus restriction of urban food availability.
RFIs and Activity Patterns
After identification of raiding locations in phase A, we subsequently recorded a significant decrease in mean RFIs obtained per day from phase B to phase C (Z = 2.2; N = 6; p < 0.001; Fig. 4).
Despite reductions in urban environment use and RFIs obtained, scan data showed no significant differences (Wilcoxon matched-pairs) in the mean daily time spent in any behavioral category during phase A (baseline) and phase B (foraging: Z = 0.77, N = 9, p = 0.44; locomotion: Z = 1.36, N = 11, p = 0.17; resting: Z = 0.42; N = 11, p = 0.68; grooming: Z = 0.42, N = 11, p = 0.68), supporting prediction 6. These values remained approximately constant in phase C, except for reduced locomotion from 20.55% (± SD 4.17%) in phase B to 15.66% (± SD 4.58%) in phase C (Z = 2.55, N = 9, p = 0.01). This trend was qualitatively confirmed by our focal data.
Discussion
Our work confirms the findings of King et al. (2008). That is, after its discovery the troop visited the supplementary food patch regularly (prediction 1), with the dominant male consistently arriving first at the patch (prediction 2) and grooming relationships with the dominant male predicted the subsequent arrival order of other troop members. Moreover, foraging benefits were highly skewed in favor of the most dominant troop members, i.e., the dominant male and female: prediction 3. Because of their high social rank, dominant troop members have priority of access to urban food sources, a high yield of raided food items and relatively high reproductive potential (Strum 2005, 2010). Thus, the skewed foraging benefits we observed on the patch were likely to have had a negligible impact on these dominant individuals’ fecundity. Crucially, as a test of a management tool, deliberate placement of the supplementary food patch, within a raiding baboon troop’s home range, but as far from the urban edge as possible, attracted that troop and subsequently induced multiple visits over a period of 20 d. While this reduced the time the troop spent in urban areas, the reduction was not significant, contrary to prediction 4.
After the placement of the supplementary patch, the troop—more specifically, its leader(s)—was given an incentive to visit an area in their home range that consistently provided a nutritional benefit. This caused a change in ranging pattern and habitat use with their core range use shifting to include the natural areas around the patch. However, there was no significant change in the troop’s use of the urban area. Further, the proximity of the patch to one of the main raiding sites beyond the urban edge (viz. Signal School) resulted in an undesirable increase in use of that area from phase A to phase B and its inclusion into the troop’s core range use area. Conceptually, the urban space represents a collection of variable, but potentially lucrative, e.g., whole loaves of bread or packets of fruit, food patches. Our corn-supplemented patch was therefore only an incremental improvement to an already profitable foraging environment, and though appealing, it was insufficient to persuade the dominant male to forego foraging in the urban space. Thus, we restricted access to the 3 urban waste areas frequented by the troop during the first 2 phases of the experiment. In essence this increased the profitability of our patch, relative to the baboons’ collective foraging environment, thus raising its appeal to the dominant male. Only once this step was taken did we see a significant decline in raided food items in the troop’s diet, coupled with a significant decline in use of the urban space (prediction 5) and a shift in the troop’s core range use to include only the natural environment. It is evident that successfully reducing the baboons’ incentive for the urban space was achieved by simultaneously reducing the appeal of the urban area while increasing the appeal for natural habitat.
Placing the patch (phase B) also did not alter the study troop’s activity budget (supporting prediction 6). This is unsurprising because the study troop was already unintentionally provisioned by concentrated human food sources. Our corn patch effected only a marginal improvement on the troop’s foraging environment, offering only 1 additional food type and thus had a negligible impact on the baboons’ activity budget, already typical of a food-supplemented troop, i.e., reduced locomotion and foraging and increased socializing and resting (Altmann and Alberts 2003; Bronikowski and Altmann 1996; van Doorn et al. 2010). Interestingly, restricting access to key urban foraging sites (phase C) also had no impact on the troop’s activity budget. Although the dominant male may have received sufficient nutritional benefit from the patch to maintain his activity budget through to phase C, this was not true of the majority of the troop. Arguably, the length of phase C was insufficient to force a change in the troop’s collective activity budget. Extension of phase C may result in increased locomotion and foraging (except for a minority of dominant individuals), and this may drive temporary fission events. Indeed, restricting the amount of provisioned food to baboons has been shown to increase daily range size, the number and diversity of sleep sites, and the proportion of natural items in their diet (Boug et al. 1994).
The influence of artificial food patches on species’ ranging behavior (Fersterer et al. 2001; Sahlsten et al. 2010) makes them appealing as management tools. Although tempting to explore provisioning as a long-term solution to reduce baboon raiding frequency, 2 of its potential consequences lead us to argue against it. First, local ecosystem changes can occur as species (target and nontarget alike) aggregate around provisioned food, resulting, e.g., in overuse of natural vegetation (Cooper et al. 2006) and alterations in community composition (Casey and Hein 1983; Robb et al. 2008), both of which are highly undesirable in an ecosystem contained within a biodiversity hotspot. Second, the risk of troop fission remains tangible where low-ranking individuals suffer high consensus costs when they follow their leader to a food patch where they do not benefit (while foregoing plentiful, if scattered, foraging opportunities in the urban environment). To counter the chance of fission, by allowing low-ranking individuals access to supplemented food, the amount of provisioned food would need to be increased, or its distribution widened. Increasing the food quantity may increase the growth rates or fecundity of troop members, the majority of which enjoy neither priority of access at urban food sources nor high yields of RFIs, while increasing the patch size would undermine the dominant individuals’ ability to monopolize the food, which is arguably the reason the troop visits the patch so regularly. Both troop fissions and increased growth rates/fecundity would undermine baboon management efforts in the peninsula.
Restricting the duration of provisioning periods can influence troop movements while foregoing risks of both fission and increased growth rates/fecundity. Thus it is an ideal strategy over short time periods (the exact length of which depends on the troop’s fission–fusion dynamics and the contrast in incentive between the patch and urban food sources) when the principle conflict mitigation measure (viz., monitors) is suspended. Although we expect that these results will not differ drastically across seasons, cold and wet conditions (winter) may increase the baboons’ incentive to use the sheltered urban space. In the long term, as an adjunct to provisioning, we stress the importance of restricting access to concentrated sources of human food, e.g., municipal waste areas, in reducing baboons’ incentive for urban environments.
Long-term management should also consider an anomaly we observed. That is, although competitive exclusion is expected when a hierarchical species feeds on a concentrated resource (López-Bao et al. 2009), exceptions are known to occur (Grenier et al. 1999) and did so in our experiment. The exception took the form of juveniles being tolerated on the patch and collectively obtaining higher overall yield than any subadults and all but 1 adult female. Although we do not have genetic data for this troop, we do know that only a single adult male has been present in this troop over the previous 3 yr. Thus, there is a strong probability that all infants and juveniles in the Waterfall troop were sired by the current, lone, dominant male. Therefore, tolerating juveniles may be expected to occur by direct fitness benefits alone (Hamilton 1964). The short duration of the provisioning experiment (20 d) minimizes any potential effect on the growth and ultimately fecundity of juvenile troop members, especially considering that these juveniles would have otherwise been foraging on high calorific urban food sources. Nevertheless, this observation has implications for raiding behaviour, i.e., tolerance of juveniles at concentrated urban food sources and subsequent increased growth rates/decreased mortality and habituation and affiliation for human food, and should be explored further.
As the field of human–wildlife conflict mitigation advances, so the need for innovative approaches becomes apparent. This work demonstrates how specific knowledge of a species’ behavioral ecology can be applied to aid conservation efforts in the face of increasing urbanization. That is, we show how troop movements can be manipulated through the consistent leadership presented in chacma baboons. By providing an incentive to dominant individuals to enter natural habitat, we reduced the time the troop spent in the urban environment. Further, because this provisioning is a short-term strategy intended only to replace the monitor system during the frequent funding crises, or after an extensive fire in the home ranges of multiple troops, many of the inherent problems associated with provisioning, e.g., ecosystem impacts and fission events, are largely negated. However, the most convincing result to emerge was that a significant change in the troop’s movement pattern was achieved by reducing the incentive to forage in urban environments, solely through proper waste management. Thus, whereas short-term provisioning emerges as a low-cost, low maintenance strategy to reduce spatial overlap between humans and baboons, long-term approaches should focus on reducing baboons’ incentive for human environments, principally by reducing food availability.
References
Akaike, H. (1974). A new look at the statistical model identification. IEEE Transactions on Automatic Control, 19(6), 7716–7723.
Altmann, J. (1974). Observational study of behaviour: sampling methods. Behaviour, 49(3/4), 227–267.
Altmann, J., & Alberts, S. C. (2003). Variability in reproductive success viewed from a life-history perspective in baboons. American Journal of Human Biology, 15, 401–409.
Altmann, J., & Alberts, S. C. (2005). Growth rates in a wild primate population: ecological influences and maternal effects. Behavioral Ecology and Sociobiology, 57(5), 490–501.
Altmann, J., & Muruthi, P. (1988). Differences in daily life between semi provisioned and wild-feeding baboons. American Journal of Primatology, 15, 213–221.
Asquith, P. (1989). Provisioning and the study of free-ranging primates: history, effects and prospects. American Journal of Physical Anthropology, 32(S10), 129–158.
Beamish, E. K. (2010). Causes and consequences of mortality and mutilation in the Cape Peninsula baboon population, South Africa. M.Sc. thesis, University of Cape Town.
Boug, A., Biquand, S., Biquand-Guyot, V., & Kamal, K. (1994). The response of commensal hamadryas baboons to seasonal reduction in food provisioning. Revue d’Écologie (La Terre et La Vie), 49, 307–319.
Bronikowski, A. M., & Altmann, J. (1996). Foraging in a variable environment: weather patterns and the behavioral ecology of baboons. Behavioral Ecology and Sociobiology, 39, 11–25.
Casey, D., & Hein, D. (1983). Effects of heavy browsing on a bird community in deciduous forest. Journal of Wildlife Management, 47, 829–836.
Conradt, L., & Roper, T. J. (2003). Group decision-making in animals. Nature, 421, 155–158.
Conradt, L., & Roper, T. J. (2005). Consensus decision making in animals. Trends in Ecology and Evolution, 20, 449–456.
Cooper, S. M., & Ginnett, T. F. (2000). Potential effects of supplemental feeding of deer on nest predation. Wildlife Society Bulletin, 28(3), 660–666.
Cooper, S. M., Owens, M. K., Cooper, R. M., & Ginnett, T. F. (2006). Effect of supplemental feeding on spatial distribution and browse utilization by white-tailed deer in semi-arid rangeland. Journal of Arid Environments, 66, 716–726.
Cowling, R. M., MacDonald, I. A. W., & Simmons, M. T. (1996). The Cape Peninsula, South Africa, physiographical, biological and historical background to an extraordinary hotspot of biodiversity. Biodiversity and Conservation, 5, 527–550.
Devas, F. (2005). The influence of social relationships on foraging success in chacma baboons (Papio ursinus). Ph.D. thesis, University of Cambridge.
De Vries, H., Netto, W. J., & Hanegraaf, P. L. H. (1993). Matman—a program for the analysis of sociometric matrices and behavioural transition matrices. Behaviour, 125, 157–175.
Dickman, A. J. (2010). Complexities of conflict: the importance of considering social factors for effectively resolving human–wildlife conflict. Animal Conservation, 13(5), 458–466.
Else, J. G. (1991). Nonhuman primates as pests. In H. O. Box (Ed.), Primate responses to environmental change (pp. 115–165). London: Chapman & Hall.
Fersterer, P., Nolte, D. L., Ziegltrum, G. J., & Gossow, H. (2001). Effect of feeding stations on the home ranges of American black bears in western Washington. Ursus, 12, 51–53.
Forthman, D. L., Strum, S. C., & Muchemi, G. (2005). Applied conditioned taste aversion and the management and conservation of crop-raiding primates. In J. D. Paterson & J. Wallis (Eds.), Commensalism and conflict: The human–primate interface (pp. 420–443). Norman, OK: American Society of Primatologists.
Forthman-Quick, D. L. (1986). Activity budgets and the consumption of human food in two troops of baboons, Papio anubis, at Gilgil, Kenya. In J. C. Else & P. C. Lee (Eds.), Primate ecology and conservation (pp. 221–228). Cambridge, UK: Cambridge University Press.
Grenier, D., Barrette, C., & Crête, M. (1999). Food access by white-tailed deer (Odocoileus virginianus) at winter feeding sites in eastern Québec. Applied Animal Behaviour Science, 63, 323–337.
Hamilton, W. D. (1964). The genetical theory of social behaviour. Journal of Theoretical Biology, 7, 1–52.
Hill, C. M. (2000). A conflict of interests between people and baboons: crop raiding in Uganda. International Journal of Primatology, 21, 299–315.
Hoffman, T. S., & O’Riain, M. J. (2010). The spatial ecology of chacma baboons (Papio ursinus) in a human-modified environment. doi:10.1007/s10764-010-9467-6.
Katsvanga, C. A. T., Mudyiwa, S. M., & Gwenzi, D. (2006). Bark stripping and population dynamics of baboon troops after chemical control in pine plantations of Zimbabwe. African Journal of Ecology, 44, 413–416.
King, A. J., Douglas, C. M. S., Huchard, E., Isaac, N. J. B., & Cowlishaw, G. (2008). Dominance and affiliation mediate despotism in a social primate. Current Biology, 18, 1833–1838.
López-Bao, J. V., Rodríguez, A., & Palomares, F. (2009). Competitive asymmetries in the use of supplementary food by the endangered Iberian lynx (Lynx pardinus). PLoS ONE, 4(10), 1–10.
Marchal, V., & Hill, C. (2009). Primate crop-raiding: a study of local perceptions in four villages in North Sumatra, Indonesia. Primate Conservation, 24, 107–116.
Margalida, A., González, L. M., Sánchez, R., Oria, J., Prada, L., Caldera, J., et al. (2007). A long-term large-scale study of the breeding biology of the Spanish imperial eagle (Aquila adalberti). Journal of Ornithology, 148, 309–322.
McCollough, M. A., Todd, C. S., & Owen, R. B., Jr. (1994). Supplemental feeding program for wintering bald eagles in Maine. Wildlife Society Bulletin, 22(2), 147–154.
Mishra, C. (1997). Livestock depredation by large carnivores in the Indian trans-Himalaya: conflict perceptions and conservation prospects. Environmental Conservation, 24, 338–343.
Naughton-Treves, L. (1997). Farming the forest edge: vulnerable places and people around Kibale National Park, Uganda. Geographical Review, 87, 27–46.
Naughton-Treves, L., Treves, A., Chapman, C., & Wrangham, R. (1998). Temporal patterns of crop-raiding by primates: linking food availability in croplands and adjacent forest. Journal of Applied Ecology, 35, 596–606.
Nyhus, P. J., & Tilson, R. (2004). Characterizing human-tiger conflict in Sumatra, Indonesia: implications for conservation. Oryx, 38, 68–74.
Partridge, S. T., Nolte, D. L., Ziegltrum, G. J., & Robbins, C. T. (2001). Impacts of supplemental feeding on the nutritional ecology of black bears. Journal of Wildlife Management, 65, 191–199.
Rasbash, J., Steele, F., Browne, W., & Prosser, B. (2003). A user’s guide to MLwiN version 2.0. London: Institute of Education.
Riley, E. P., & Priston, N. E. C. (2010). Macaques in farms and folklore: exploring the human-nonhuman primate interface in Sulawesi, Indonesia. American Journal of Primatology, 71, 1–7.
Ripple, W. J., & Beschta, R. L. (2007). Restoring Yellowstone’s aspen with wolves. Biological Conservation, 138, 514–519.
Robb, G. N., McDonald, R. A., Chamberlain, D. E., & Bearhop, S. (2008). Food for thought: supplementary feeding as a driver of ecological change in avian populations. Frontiers in Ecology and Environment, 6, 476–484.
Rodgers, A. R., & Carr, A. P. (1998). HRE: The home range extension for ArcView! User’s manual. Centre for Northern Forest Ecosystem Research, Ontario Ministry of Natural Resources.
Rodriguez-Hidalgo, P., Gortazar, C., Tortosa, F. S., Rodriguez-Vigal, C., Fierro, Y., & Vicente, J. (2010). Effects of density, climate, and supplementary forage on body mass and pregnancy rates of female red deer in Spain. Oecologia, 164, 389–398.
Roper, T. J., Findlay, S. R., Lüps, P., & Sheperdson, D. J. (1995). Damage by badgers Meles meles to wheat Triticum vulgare and barley Hordeum sativum crops. Journal of Applied Ecology, 32, 720–726.
Sahlsten, J., Bunnefeld, N., Månsson, J., & Ericsson, G. (2010). Can supplementary feeding be used to redistribute moose? Wildlife Biology, 16, 85–92.
Saj, T., Sicotte, P., & Paterson, J. D. (1999). Influence of human food consumption on the time budget of vervets. International Journal of Primatology, 20, 977–994.
Schmidt, K. T., & Hoi, H. (2002). Supplemental feeding reduces natural selection in juvenile red deer. Ecography, 25(3), 265–272.
Strum, S. C. (2005). Measuring success in primate translocation: a baboon case study. American Journal of Primatology, 65, 117–140.
Strum, S. C. (2010). The development of primate raiding: implications for management and conservation. International Journal of Primatology, 31, 133–156.
van Doorn, A. C. (2009). The interface between socioecology and management of chacma baboons (Papio ursinus) in the Cape Peninsula, South Africa. PhD thesis. University of Cape Town.
van Doorn, A. C., O’Riain, M. J., & Swedell, L. (2010). The effects of extreme seasonality of climate and day length on the activity budget and diet of semi-commensal chacma baboons (Papio ursinus) in the Cape Peninsula of South Africa. American Journal of Primatology, 72, 104–112.
Warren, Y., Higham, J. P., Maclarnon, M. A., & Ross, C. (2011). Crop-raiding and commensalism in olive baboons: The costs and benefits of living with humans. In V. Sommer & C. Ross (Eds.), Primates of Gashaka (Developments in Primatology: Progress and Prospects 35 (pp. 359–384). New York: Springer.
Western Cape Nature Conservation (2000). Western Cape Nature Conservation Laws Amendment Act, Sections 25A-47, 43–54.
Woodroffe, R., Thirgood, S., & Rabinowitz, A. R. (2005). The impact of human–wildlife conflict on natural systems. In R. Woodroffe, S. Thirgood, & A. R. Rabinowitz (Eds.), People and wildlife: Conflict or coexistence? (pp. 1–12). Cambridge, UK: Cambridge University Press.
Acknowledgments
We thank The Simon’s Town Civic Association, Peter de Villiers, Mrs. Dollery, and the South African Navy for their assistance and cooperation; Stuart and Judy Whittaker for their hospitality; Sabine Muëller for her assistance in the field; Tali Hoffman for assistance in spatial analyses; and 2 anonymous reviewers for helpful comments and suggestions on the manuscript. This work was supported by a South African National Research Foundation (NRF) grant awarded to M. J. O’Riain. A. J. King was supported by an AXA Postdoctoral Fellowship and a Natural Environment Research Council (NERC) Fellowship (NE.H016600.2). The experiment was approved by the University of Cape Town’s ethics committee and adhered to the legal requirements of South Africa.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaplan, B.S., O’Riain, M.J., van Eeden, R. et al. A Low-Cost Manipulation of Food Resources Reduces Spatial Overlap Between Baboons (Papio ursinus) and Humans in Conflict. Int J Primatol 32, 1397–1412 (2011). https://doi.org/10.1007/s10764-011-9541-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-011-9541-8