Abstract
On the basis of distinguishing characteristics of various genetic markers, pelage color, tail tuft, and vocalizations, we describe a new species of the genus Tarsius Storr 1780. The new taxon Tarsius wallacei sp. nov. occupies a disjunct range in Central Sulawesi, Indonesia. The two isolated populations differ significantly in body size, but are alike in color, tail tuft dimensions, vocalizations, and genetic composition. Morphologically, the new species is similar to other Sulawesi lowland tarsiers. In the field, it can be distinguished from its congeners via a characteristic duet song and its yellow-brown pelage coloration and a copper-colored throat. Genetic analyses prove Y-chromosomal and mitochondrial DNA sequences and also microsatellite allele frequencies to be absolutely diagnostic.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Tarsius wallacei New Species
Holotype
MZB 31153, adult female, skin, skull, and skeleton, deposited in the Museum Zoologicum Bogoriense (MZB), Bogor, Indonesia. Collectors: S. Merker, H. Dahruddin, and W. Sinaga. Captured alive on April 12, 2008; died on May 10, 2008 in captivity at the MZB.
Paratype
MZB 31969, adult male, skin, skull, and skeleton, deposited in the Museum Zoologicum Bogoriense, Bogor, Indonesia. Collectors: S. Merker, H. Dahruddin, and W. Sinaga. Still juvenile when captured alive on April 13, 2008; died on February 12, 2009 in captivity at the MZB.
Type Locality
Uwemanje, Central Sulawesi, Indonesia. The types were captured in secondary forest ca. 500 m south of the village Uwemanje (0°58′21′′S, 119°49′38′′E, 450 m asl) and ca. 9 km south-southwest of the city center of Palu, the provincial capital of Central Sulawesi.
Hypodigm
Apart from the types, we mist-netted, measured, and released six additional males and seven females of the new species and recorded the duet songs of ca. 30 individuals in the wild. The hypodigm further comprises eight tarsiers from four social groups captured, measured, and released by one of us (M. Shekelle) in a previous study (Shekelle 2003). Our analysis included 10 duet fragments recorded from these four social groups living east of the small city of Tinombo (Shekelle 2003, 2008a).
Diagnosis
The new species of the genus Tarsius Storr, 1780 is a typical Sulawesi lowland tarsier—with a well-marked facial mask, a long and bushy tail tuft, postauricular white spots, and a characteristic duet song—and is thus clearly distinct from Philippine and Western tarsiers. It is distinguished from other Sulawesian taxa by unique features of its distribution, pelage, genotype, and vocalizations.
1) Tarsius wallacei occupies an exclusive range in Central Sulawesi not shared with any other tarsier taxa. 2) The new species is similar in size to other lowland tarsiers (cf. Morphometrics section later) and larger than the montane Tarsius pumilus Miller and Hollister, 1921. 3) As other tarsiers from mainland Sulawesi, it differs from the small-island species, e.g., Tarsius sangirensis Meyer, 1897, T. tumpara Shekelle et al., 2008 (2008a), and a population from Selayar Island that Groves (1998) argued was taxonomically distinctive at the species level, in its longer, denser, and darker tail fur. Multivariate analyses of tail tufts show the distinctiveness of Tarsius wallacei from all other recognized species from mainland Sulawesi. 4) The pelage of adults of Tarsius wallacei is usually yellow-brown (Fig. 1) and thus distinct from the grayer T. dentatus Miller and Hollister, 1921 and the usually brown T. lariang Merker and Groves 2006. However, as there is considerable intraspecific variation in pelage color of tarsiers, the diagnostic power of this feature is typically rather poor, especially in light of a growing number of recognized taxa. The only absolutely diagnostic coloration we observed is the conspicuous yellow to copper-colored throat of the new species. A well-marked eye-ring of the same color—more and invariably developed near the mesial rim of the eye (Fig. 1)—distinguishes adult Tarsius wallacei from adult T. dentatus; however, some adult T. lariang and almost all juvenile tarsiers of these three species show a similarly developed eye-ring. 5) The genotype of the new species is absolutely diagnostic. Its autosomal and gonosomal DNA is clearly distinct from that of other tarsiers for which analogous data are available. 6) Tarsius wallacei is characterized by a unique duet song that separates it from all other Sulawesian lowland tarsiers. As described by Shekelle (2008a), the structure of the duet of Tarsius wallacei is comparatively simple; 1 female phrase is followed by 2–4 male notes. In this way, Tarsius wallacei is most similar to the unnamed population of tarsiers in the Togian Islands and different from all other known forms. However, different from the Togian Island tarsiers, the female phrase of Tarsius wallacei is typically a 2-note phrase, whereas among Togian Island tarsiers it is always a 1-note phrase.
Adult male Tarsius wallacei captured at Uwemanje, Central Sulawesi, Indonesia (photo: S. Merker). This specimen is labeled as No. 6 in Table I
Etymology
We named the new species Tarsius wallacei in honor of Alfred Russel Wallace (1823–1913), British naturalist and co-discoverer of natural selection. During his studies and travels in the Malay Archipelago, Wallace was the first to become aware of a zoogeographic boundary (now known as the Wallace line) separating Australian from strictly Asian fauna. Wallace’s tarsier lives immediately east of this line.
Distribution
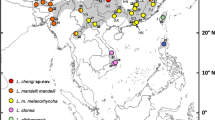
The new species occupies a discontinuous range within the province of Central Sulawesi, Indonesia (Fig. 2). Northern and southern populations are isolated from each other by the Palu Bay, the city of Palu, and the southern parts of the Isthmus of Palu, an area now inhabited by the parapatric species Tarsius dentatus.
According to our surveys, the northern population occurs within the following geographic limits: an unknown line just west of the village Tomini to the northeast (ca. 120°30′E), the coastlines of the Isthmus of Palu to the east and to the west, and an unknown line between the villages Ampibabo and Marantale to the south (ca. 0°30′S). Along its north-eastern boundary, this population borders the “Sejoli form” sensu Shekelle et al. (1997). At its southern limit, this population borders Tarsius dentatus. We captured tarsiers near the village Batusuya (0°24′14′′S, 119°46′30′′E) and near the small city of Tinombo (0°23′52′′N, 120°16′07′′E) and recorded duet songs throughout the range of this form. The northern population of the new species is equivalent to the Tinombo form of Shekelle et al. (1997). For that reason, we refer to it as Tarsius wallacei Tinombo form.
The southern population occurs southwest to west of Palu. It is definitely known from the type locality Uwemanje only and probably occurs within a very small area from Uwemanje to the west. Approximately 9 km to the south, we encountered Tarsius dentatus, and 24 km to the northwest, we found T. lariang. We call this population Tarsius wallacei Uwemanje form.
The hybrid biogeographic hypothesis (Shekelle and Leksono 2004) explains the current distribution of tarsiers on Sulawesi on the basis of microplate-tectonic shifts and subsequent sea-level fluctuations. Findings on the range limits of other tarsier species provide strong support for this theory (Merker et al. 2009). Therefore, the occurrence of the Uwemanje form of Tarsius wallacei on a microplate largely inhabited by T. lariang was quite unexpected. At this time, we can only speculate about the rationale for the range discontinuity of the new species. Merker et al. (2009) found evidence of Tarsius dentatus displacing T. lariang in parts of its range. A similar process, possibly effected by climate fluctuations, periodic droughts in the Palu area, and different colonization capacities of the two species, might underlie Tarsius dentatus having become a barrier to dispersal and gene flow between the northern and southern populations of T. wallacei.
Description and Comparison
External Characteristics
With head-and-body lengths of ca. 12 cm (Table I), Tarsius wallacei is of similar size to other known Sulawesi lowland tarsiers. However, the species comprises two geographical variants that are significantly different in size (Table I, Fig. 3). Skull lengths of the two types (Table II) are within the range of Tarsius dentatus and T. lariang (cf. Merker and Groves 2006). On average, ears of the new species are wider than in Tarsius dentatus and T. lariang (though not significantly); fingers and toes are shorter (Table I).
Head length (mean ± SD) of live adult Tarsius dentatus (n = 24), T. lariang (n = 36), and T. wallacei (n = 10). Different letters (a, b) denote highly significant differences between taxa (ANOVA, Tukey-HSD post hoc test, p < 0.005) when populations of Tarsius wallacei at Batusuya (Tinombo form, n = 4) and Uwemanje (Uwemanje form, n = 6) are treated as separate units
Except for an off-white ventrum, the pelage of the new species is mottled yellowish brown in color (Fig. 1). The mottled appearance is largely due to its gray undercoat and scattered patches of light gray to black hair tips. The transition from head to body is marked by a conspicuous copper-colored throat. Above and below eyes, there are distinct yellow to copper-colored patches that in most specimens form a nearly complete eye-ring, a characteristic that it shares with some, but not all specimens of Tarsius lariang. The paralabial pale zone is not uniformly expressed in all conspecifics; it varies in size and between white and off-white in color. As other Central Sulawesi species, Tarsius wallacei is typified by a dark tail with a thick and long tail pencil.
Morphometrics
We judged the age status of the subjects from external sex characteristics (descended testes in adult males, elongated nipples in adult females). Because sexual dimorphism in tarsier body length is insignificant (Merker 2003; cf. Merker 2003, 2006 and Shekelle 2003 for slight differences in body masses), we pooled morphometric data for males and females for further analyses. Table I provides live measurements of 10 adult Tarsius wallacei captured in March and April 2008. Mean body mass, head-and-body length, and head length of fully grown specimens from Batusuya are significantly smaller than in the conspecific Uwemanje form (t-test, see Table I). Several other morphometric data also show considerable population differences. This has two implications: 1) we computed species medians instead of means (Table I) and 2) one should interpret comparisons of morphological characters of these nocturnal primates with great care. In the following, we present two morphometric data analyses with different efficacies for taxonomic separation. The first concerns tarsier head lengths that differed significantly between the 2 forms, and the second analysis deals with characteristics of the tarsier tail, similar in both sampled populations of Tarsius wallacei.
-
A)
Head length The frequency distribution of head lengths within the new species is bimodal, with a significant difference between the 2 isolated forms (t-test, t = –3.646, p = 0.007, Table I, Fig. 3). To compare maximum head lengths among Tarsius wallacei, T. dentatus, and T. lariang, we thus performed a 1-way ANOVA in SPSS 17.0 for Windows and in addition applied the Brown-Forsythe robust test of equality of means with unequal variances. Both tests showed head lengths to vary significantly among the 3 species, thus supporting taxonomic separation (ANOVA, F(2,67) = 7.662, p = 0.001, Brown-Forsythe statistic = 5.236, p = 0.016). When we treated the 2 sampled populations of Tarsius wallacei separately, variances of the means were similar across all populations and species. The Uwemanje form does not differ in head length from Tarsius dentatus or T. lariang whereas tarsiers of the Tinombo form are characterized by significantly shorter heads than the other 2 species (ANOVA, F(3,66) = 11.681, p < 0.001, Fig. 3).
-
B)
Tail tuft fur length To evaluate an additional morphological feature potentially separating the new species from other Sulawesi tarsiers, we adopted an approach of Shekelle et al. (2008c). We measured overall tail lengths and tail tuft fur lengths at 100% (the distal end), 90%, 75%, and 50% of the length of the tail (Shekelle et al.2008c). Using the discriminant function (DF) analysis in SPSS 17.0 for Windows, we compared tail and tail tuft measurements from Tarsius wallacei with data obtained from all other recognized tarsier species of mainland Sulawesi. Differing from our study of head lengths, the 2 populations of Tarsius wallacei formed a continuous cluster of data points and were thus pooled for further tests. The DF analysis shows the new species to be well-separated from its congeners (Table III, Fig. 4). Based on tail and tail hair lengths, we correctly classified all 10 adult specimens of Tarsius wallacei. The same is true for the 2 specimens of Tarsius pumilus and 2 individuals of T. tarsier (Erxleben, 1777). Of Tarsius dentatus and T. lariang, 22.7% and 5.6%, respectively, were misclassified as the new species. Cross-validated analysis (using the leave-one-out option) generally confirmed original results, with 90% of Tarsius wallacei correctly classified and only 1 specimen assigned to T. pumilus (Table III). Tail length, tuft hair length at 100%, and tuft hair length at 50% proved to be the most important discriminators among species, with tail length being the main factor to single out the montane Tarsius pumilus, and the latter measurements being the most useful discriminators among lowland taxa (Fig. 4). Classification results remain virtually unaffected if hair lengths are adjusted for tail length. If tail length is left out of the analysis, 59.7% of original grouped cases (66.7% when hair lengths are adjusted for tail length) are correctly classified. These numbers are chiefly due to the relatively bushy tuft of the small-bodied and short-tailed Tarsius pumilus. In summary, although we did not attain complete resolution of taxonomic separation among Sulawesi tarsiers via this method, DF analysis of tail tufts supports the diagnosis of the new species. Our results underline its morphological uniqueness and are highly consistent with genetic and acoustic evidence as presented in this article.
Table III Predicted group membership after a discriminant function analysis of tail and tail hair length of 5 tarsier species from mainland Sulawesi Fig. 4 The first 2 discriminant functions of a discriminant function (DF) analysis of tail lengths and tail tuft fur lengths at 100%, 90%, 75%, and 50% of the length of the tail of 5 tarsier species from mainland Sulawesi. The discriminatory ability of the analysis is highly significant (p < 0.001, Wilks’ lambda = 0.22, χ 2 = 98.77, df = 20, using the first 4 canonical DF). Standardized DF coefficients of original variables in the first DF, which accounts for 64.7% of the total variation, are as follows: tail length = 0.035, fur length at 100% = –1.007, at 90% = 0.014, at 75% = 0.018, at 50% = 1.019. Standardized DF coefficients of the original variables in the second DF, which accounts for 32.5% of the total variation, are as follows: tail length = 0.981, fur length at 100% = –0.487, at 90% = 0.645, at 75% = –0.044, at 50% = –0.178
Genotype
Analyses of mitochondrial, Y-chromosomal, and autosomal genetic markers show a clear separation of Tarsius wallacei from T. dentatus and T. lariang.
We obtained small ear biopsies from 15 mist-netted specimens of both forms of the new species (n 1 = 7, n 2 = 8). Sample storage, DNA extraction, polymerase chain reaction (PCR) amplification, and data analyses followed the protocols and included the sequence and microsatellite data set given by Merker et al. (2009). We deposited sequence information for Tarsius wallacei in GenBank (Acc. No. HM115970-115991), and computed genetic distances among sequences using MEGA 4.0 (Tamura et al. 2007). In FINDMODEL (Los Alamos National Laboratory), we tested which models of sequence evolution best described our input data. Slightly different to the study by Merker et al. (2009), the inclusion of 6 unique cytochrome b (Cyt b) haplotypes of the new species led us to adopt the TN93 + G model of sequence evolution for this gene, with α(γ) = 0.19. We based analyses of the SRY gene (sex-determining region on the Y-chromosome) on the TN93 model with uniform substitution rates among sites. Genetic distances among Cyt b and SRY sequences of the 3 species are given in Table IV. The 6 Cyt b haplotypes of Tarsius wallacei fall into 2 major haplogroups (n 1 = 9, n 2 = 6 individuals) with an average genetic distance D A = 0.010 ± 0.003 between them. We found both haplogroups in each of the 2 sampling localities, Uwemanje and Batusuya. Thus, their distribution does not echo the geographic separation of the 2 forms but gives evidence of a polymorphism predating isolation. Each of the 3 species is characterized by a single unique SRY haplotype. The new taxon Tarsius wallacei differs from both T. dentatus and T. lariang at 10 nucleotide sites (Table IV). For comparison: Among all 6 Sulawesi macaque species whose SRY sequences are available from GenBank, orthologues comprise 4 variable sites (cf. Tosi et al. 2000).
We genotyped 95 individuals from 7 populations of Tarsius dentatus, T. lariang, and T. wallacei with 12 microsatellite markers (Merker et al. 2007). The sample locations include 5 localities described by Merker et al. (2009)—Laone, Kamarora, Make, Peana, and Koja—and 2 villages within the range of the new species: Batusuya and Uwemanje. With GENALEX 6 (Peakall and Smouse 2006), we computed a distance matrix and performed a principal coordinates analysis (PCoA) locating major axes of variation within this multivariate data set. The first 2 principal coordinates explain 73.1% of the variation. The new species is clearly separated from its congeners (Fig. 5).
These results confirm findings by Shekelle (2003) and Shekelle et al. (2008b), who examined 12S mtDNA of the Tinombo form and other Sulawesi tarsiers. They noted sequence variation among northern and central Sulawesi taxa to be largely congruent with geographic variation in duet songs and to be consistent with Shekelle and Leksono’s (2004) hybrid biogeographic hypothesis.
Vocalizations
As with nearly every known tarsier acoustic form, vocalizations are absolutely diagnostic of Tarsius wallacei, even with relatively simple analyses. The human ear can easily diagnose the vocalizations of the new species from all other tarsier acoustic forms with minimal training. Similarly, visual inspection of spectrograms is sufficient to differentiate the duet call of Tarsius wallacei (Figs. 6, 7, and 8) from all its congeners, particularly as regards female phrases. Shekelle (2008a) described the duet call of Tarsius wallacei. Each female note is a hook-shaped whistle, beginning at ca. 12–13 kHz and descending to about 5 kHz in about 0.4 s. Usually, 2 notes are made in rapid succession, the intervening gap being only about 0.1 s. Very frequently, the first of these 2 notes is a peculiarly modulated whistle that begins at ca. 13 kHz, descends to below 10 kHz, rises again to a point higher than the initial frequency, and finally descends to nearly 5 kHz, all in the span of only 0.3–0.4 s. There is no other note similar to this among all known tarsier acoustic forms, and in spectrographic analysis, it almost resembles a chevron-shaped male note superimposed upon the female’s hook-shaped whistle. Like all known tarsier acoustic forms north of the Isthmus of Palu, the male notes of Tarsius wallacei are wideband chevron-shaped chirps, and are not diagnostic without sophisticated spectrographic analyses or field playback experiments (Burton and Nietsch 2010; Shekelle 2008a). Shekelle et al. (1997, also in Shekelle 2003, 2008a) reported results of field playback tests of Tarsius wallacei that unambiguously differentiated this species from T. dentatus, T. sangirensis, and as yet unnamed acoustic forms classified as T. tarsier, i.e., Manado, Gorontalo, Sejoli, and Togian forms. Indeed, differentiation of Tarsius wallacei from any known tarsier taxa other than the Togian form is trivial: 1) T. pumilus does not duet (Grow and Gursky-Doyen 2010; Shekelle 2008b). 2) The duet of T. pelengensis Sody, 1949 is so similar to that of T. dentatus that visual inspection of spectrograms of duets, alone, is insufficient to differentiate the two (Burton and Nietsch 2010); thus, it is conclusively distinct from that of T. wallacei. Burton and Nietsch (2010) describe several acoustic forms from the southwest and southeast peninsulae. All of these populations are isolated from Tarsius wallacei by huge distances, so exhaustive comparisons are pointless. But their data, interestingly, include the Bantimurung form, which may be the best representative of Tarsius tarsier, the senior taxon of the species group. Burton and Nietsch note that the Bantimurung form is notable for using the lowest fundamental frequencies of any known acoustic form of Eastern Tarsiers, ca. 2 kHz, and a fast “note rate,” 51 notes per 10 s. Thus, Tarsius wallacei is unequivocally distinct from the senior taxon, Tarsius tarsier, in its duet form.
Spectrogram of a duet call of Tarsius wallacei (recorded in Batusuya). It depicts 7, 2-note, female phrases and 22 male notes. The male notes begin with a distinct chevron-shape, but gradually lose this shape by the latter part of the duet. Note a band of insect noise at ca. 6 kHz. Everything above this band is tarsier, and everything below it is avian, an apparent example of auditory partitioning that is characteristic of the Sulawesi dawn chorus. Note also that maximum fundamental frequencies are ca. 13 kHz, but harmonics are much higher, in excess of 20 kHz
Spectrograms of duet calls of Tarsius wallacei from 2 locations. a From Batusuya. The figure depicts an introductory 1-note female phrase, followed by 8, 2-note, female phrases. Owing to the position of the microphone and the tarsier pair, the female notes are seen here in virtual isolation from the male notes. b From Uwemanje. The figure depicts an introductory 1-note female phrase, followed by 7, 2-note, female phrases. Owing to the position of the microphone and the tarsier pair, the female notes are seen clearly, whereas the male notes are only faintly visible. Note the self-evident structural similarities between these 2 duet calls from geographically disjunct populations
Spectrograms of duet calls of Tarsius wallacei from a Batusuya and b Uwemanje. The figure depicts detailed close-ups of Fig. 7 showing the peculiar frequency modulations in the first note of the 2-note phrase, which are characteristic of female Tarsius wallacei. Note the self-evident differences between these 2 duets, particularly in the first note of each phrase. It is not yet known whether this is normal intrapopulation variation, or whether it represents incipient development of new acoustic forms
Previous Field Research
Shekelle (Shekelle et al. 1997; Shekelle 2003, 2008a) surveyed tarsiers near the small city of Tinombo, north of the Isthmus of Palu (0°23′52′′N, 120°16′07′′E, Fig. 2). This site was sampled as part of a transect design to reveal microevolutionary changes between Spectral tarsiers in the north and Dian’s tarsiers in central Sulawesi. The habitat around Tinombo is heavily degraded; tarsiers were located in an area of recently cleared agricultural land, with mixed agroforestry and secondary habitat. Shekelle et al. captured, measured, and released 8 tarsiers from 4 social groups in a span of 5 d. The specimens were small: 5 adult females weighed 88–113 g, and two adult males weighed 114–115 g. Overall, these data are very similar to body masses of Batusuya tarsiers sampled in this study (Table I). Examining phylogeographic patterns of mtDNA haplotypes, Shekelle et al. (2008b) found the Tinombo population to be paraphyletic, with respect to a haplotype found in the Marantale population to the south (Tarsius dentatus). They interpret this finding as evidence that male Tarsius dentatus might occasionally hybridize with female T. wallacei. Interestingly, Merker et al. (2009) found the same pattern of hybridization at a nearby parapatric boundary: evidence that Tarsius dentatus males occasionally hybridize with T. lariang females. We speculate that this mating pattern could have the effect of gradually expanding the range of Tarsius dentatus. Thus, the boundary of Tarsius lariang has been pushed south and west of the Palu-Koro fault (Merker et al. 2009), while the boundary with T. wallacei has been pushed north and west, resulting in the disjunct distribution we report on here.
Comments on the Necessity of Holistic Studies
The discontinuous range of Tarsius wallacei and its morphological heterogeneity make yet another argument for the efficacy of holistic experimental designs in speciation studies. Speciation is a by-product of evolution, and evolution has many mechanisms. Focusing on any 1 or 2 systems is bound to produce an unacceptably simplistic understanding of evolution and speciation. The comparison of results from genetics, bioacoustics, morphology, and biogeographic history provide a far fuller, far more satisfying understanding of evolutionary history than would any 1 or 2 of these data sets in isolation. Interpreting results of our morphometric analyses and geopositional data in view of the—thus far strongly supported—hybrid biogeographic hypothesis (Shekelle and Leksono 2004) might have led us to assume taxonomic distinctiveness between the 2 forms of Tarsius wallacei. However, acoustic and genetic analyses provided strong evidence that both forms belong to the same species. We found variation on different levels of organization—partly congruent, partly conflicting among different types of analysis. This mixture of patterns and possible interpretations gives us reason for strongly advocating holistic studies with complementary data sets to allow us to comprehend and describe tarsier diversity fully.
Conservation
Gursky et al. (2008) made provisional conservation assessments for Eastern Tarsier populations listed by Brandon-Jones et al. (2004) as being likely to be taxonomically distinct, including the Tinombo form (here described as Wallace’s tarsier, Tarsius wallacei). They followed the methods of the Indonesian Primate Conservation and Assessment Program (Supriatna et al. 2001). Their result was that the extent of occurrence (EOO), exclusive of the disjunct southern population, was ca. 3150 km2. Noting conservation concerns in the region, they suggested a conservation status of Endangered (EN B12bc). We recommend revisiting this assessment using the methods of Shekelle and Salim (2009), which improve on the previous methods by using more accurate, GIS-based EOO estimates. Nevertheless, we note that the proposed distribution of the southern population is very small and not likely to increase greatly the estimated EOO. Thus, the suggested status for the entire species is likely to remain EN, or VU, at best. Quickly assessing the vulnerability of the small and isolated southern population of Tarsius wallacei is obviously critical in conserving Central Sulawesi’s high tarsier diversity.
References
Brandon-Jones, D., Eudey, A. A., Geissmann, T., Groves, C. P., Melnick, D. J., Morales, J. C., et al. (2004). Asian primate classification. International Journal of Primatology, 25, 97–164.
Burton, J. A. & Nietsch, A. (2010). Geographical variation in duet songs of Sulawesi tarsiers: Evidence for new cryptic species in south and southeast Sulawesi. International Journal of Primatology. doi:10.1007/s10764-010-9449-8.
Erxleben, J. C. P. (1777). Systema Regni Animalis per Classes, Ordines, Genera, Species, Varietates, cum Synonymia et Historia Animalium. Classis I. Mammalia. Weygand, Leipzig.
Groves, C. P. (1998). Systematics of tarsiers and lorises. Primates, 39, 13–27.
Grow, N. B., & Gursky-Doyen, S. (2010). Preliminary data on the behavior, ecology, and morphology of Tarsius pumilus. International Journal of Primatology. doi:10.1007/s10764-010-9456-9.
Gursky, S., Shekelle, M., & Nietsch, A. (2008). The conservation status of Indonesia’s tarsiers. In M. Shekelle, I. Maryanto, C. Groves, H. Schulze, & H. Fitch-Snyder (Eds.), Primates of the oriental night (pp. 105–114). Jakarta: Indonesian Institute of Sciences (LIPI), LIPI Press.
Merker, S. (2003). Vom Aussterben bedroht oder anpassungsfähig?—Der Koboldmaki Tarsius dianae in den Regenwäldern Sulawesis. PhD dissertation, University of Göttingen, Germany.
Merker, S. (2006). Habitat-specific ranging patterns of Dian’s tarsiers (Tarsius dianae) as revealed by radiotracking. American Journal of Primatology, 68, 111–125.
Merker, S., & Groves, C. P. (2006). Tarsius lariang: A new primate species from Western Central Sulawesi. International Journal of Primatology, 27, 465–485.
Merker, S., Driller, C., Perwitasari-Farajallah, D., Zahner, R., & Zischler, H. (2007). Isolation and characterization of 12 microsatellite loci for population studies of Sulawesi tarsiers (Tarsius spp.). Molecular Ecology Notes, 7, 1216–1218.
Merker, S., Driller, C., Perwitasari-Farajallah, D., Pamungkas, J., & Zischler, H. (2009). Elucidating geological and biological processes underlying the diversification of Sulawesi tarsiers. Proceedings of the National Academy of Sciences USA, 106, 8459–8464.
Meyer, A. B. (1897). Säugethiere vom Celebes- und Philippinen-Archipel I. Abhandlungen und Berichte des Königlichen Zoologischen und Anthropologisch-Ethnographischen Museums zu Dresden, 6, 1–36.
Miller, G. S., & Hollister, N. (1921). Twenty new mammals collected by H. C. Raven in Celebes. Proceedings of the Biological Society of Washington, 34, 93–104.
Peakall, R., & Smouse, P. E. (2006). GENALEX 6: Genetic analysis in Excel. Population genetic software for teaching and research. Molecular Ecology Notes, 6, 288–295.
Shekelle, M. (2003). Taxonomy and biogeography of Eastern tarsiers. Ph.D.dissertation, Washington University, Saint Louis, MO.
Shekelle, M. (2008a). Distribution of tarsier acoustic forms, North and Central Sulawesi: With notes on the primary taxonomy of Sulawesi’s tarsiers. In M. Shekelle, I. Maryanto, C. Groves, H. Schulze, & H. Fitch-Snyder (Eds.), Primates of the oriental night (pp. 35–50). Jakarta: Indonesian Institute of Sciences (LIPI), LIPI Press.
Shekelle, M. (2008b). The history and mystery of the mountain tarsier, Tarsius pumilus. Primate Conservation, 23, 121–124.
Shekelle, M., & Leksono, S. M. (2004). Strategi konservasi di Pulau Sulawesi dengan menggunakan Tarsius sebagai flagship species [Conservation strategy in Sulawesi Island using Tarsius as flagship species]. Biota, 9(1), 1–10.
Shekelle, M., & Salim, A. (2009). An acute conservation threat to two tarsier species in the Sangihe Island chain [North Sulawesi, Indonesia]. Oryx, 43, 419–426.
Shekelle, M., Leksono, S. M., Ichwan, L. L. S., & Masala, Y. (1997). The natural history of the tarsiers of North and Central Sulawesi. Sulawesi Primate Newsletter, 4(2), 4–11.
Shekelle, M., Groves, C., Merker, S., & Supriatna, J. (2008a). Tarsius tumpara: A new tarsier species from Siau Island, North Sulawesi. Primate Conservation, 23, 55–64.
Shekelle, M., Morales, J. C., Niemitz, C., Ichwan, L. L., & Melnick, D. (2008b). Distribution of tarsier haplotypes for some parts of northern and central Sulawesi. In M. Shekelle, I. Maryanto, C. Groves, H. Schulze, & H. Fitch-Snyder (Eds.), Primates of the oriental night (pp. 51–69). Jakarta: Indonesian Institute of Sciences (LIPI), LIPI Press.
Shekelle, M., Groves, C., Gursky, S., Neri-Arboleda, I., & Nietsch, A. (2008c). A method for multivariate analysis and classification of tarsier tail tufts. In M. Shekelle, I. Maryanto, C. Groves, H. Schulze, & H. Fitch-Snyder (Eds.), Primates of the oriental night (pp. 71–84). Jakarta: Indonesian Institute of Sciences (LIPI), LIPI Press.
Sody, H. J. V. (1949). Notes on some primates, Carnivora and the babirusa from the Indo-Malayan and Indo-Australian regions. Treubia, 20, 121–190.
Storr, G. L. C. (1780). Prodromus Methodi Mammalium. Tübingen: Wolffer.
Supriatna J., Manansang, J., Tumbelaka, L., Andayani, N., Indrawan, M., Darmawan, L., Leksono, S. M., Djuwantoko, Seal, U., & Byers, O. (2001). Conservation Assessment and Management Plan for the Primates of Indonesia: Final Report. IUCN/SSC Conservation Breeding Specialist Group (CBSG), Apple Valley, MN.
Tamura, K., Dudley, J., Nei, M., & Kumar, S. (2007). MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular Biology and Evolution, 24, 1596–1599. (Publication PDF at http://www.kumarlab.net/publications).
Tosi, A. J., Morales, J. C., & Melnick, D. J. (2000). Comparison of Y chromosome and mtDNA phylogenies leads to unique inferences of macaque evolutionary history. Molecular Phylogenetics and Evolution, 17, 133–144.
Acknowledgments
We thank the Indonesian authorities LIPI, RISTEK, PHKA, and BKSDA as well as local administrations of several villages for granting research, capture, and export permits. We thank Dr. Joko Pamungkas, Director of the Primate Research Center at Bogor Agricultural University, and Wahyu Sudrajat for their administrative support. We also thank our long-term field assistants Amar, Baso, Leo, Raimon, Sapri, and Thony for their indispensable help in the field, and also Novik Nurhidayat, Yogy Simanjuntak, and Novita Anggraeni for assisting us in the laboratory. All work complied with international and Indonesian law and regulations. This study was part of a collaborative project among the University of Mainz, the Primate Research Center at IPB Bogor, and the Indonesian Institute of Sciences, LIPI. The work was supported through research grants from the Deutsche Forschungsgemeinschaft (DFG, Me2730/1-2, Me2730/1-3, to S. Merker).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Merker, S., Driller, C., Dahruddin, H. et al. Tarsius wallacei: A New Tarsier Species from Central Sulawesi Occupies a Discontinuous Range. Int J Primatol 31, 1107–1122 (2010). https://doi.org/10.1007/s10764-010-9452-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-010-9452-0