Abstract
Translocation programs releasing animals into the wild need to assess the potential risks associated with the exchange of parasites and other pathogens between native and translocated species. We assessed the composition of the parasite communities in sympatric native and introduced primates. Over a 3-yr period we monitored the gastrointestinal parasites of 3 primate species living in the isolated ecosystem of Rubondo Island National Park, Tanzania: translocated chimpanzees (Pan troglodytes) and guerezas (Colobus guereza) and the indigenous vervets (Chlorocebus aethiops pygerythrus). We detected Troglodytella abrassarti and Enterobius cf. anthropopitheci only in chimpanzees and Chilomastix mesnili in chimpanzees and guerezas. In vervets, we recorded Anatrichosoma sp. and Subulura sp., previously reported in Rubondo chimpanzees. We found Blastocystis sp., Giardia sp., Iodamoeba buetschlii, Entamoeba coli, Entamoeba spp., Trichuris sp., Strongyloides spp., spirurids (cf. Protospirura muricola), and undetermined strongylids in all 3 primate species. Considering the absence of Protospirura muricola in other wild populations of chimpanzees and guerezas, it has probably been acquired from the native vervets, as have Anatrichosoma sp. and Subulura sp. Lower parasite load in Rubondo chimpanzees, in comparison with wild populations at other study sites of this species, might be due to their stay in captivity in Europe before being released on the island. Despite a lack of any apparent health problems from infections in introduced Rubondo primates, parasite monitoring during reintroduction/introduction projects is necessary to decrease potential risks resulting from the exchange of parasites between translocated and native species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An overall decrease in biodiversity bringing hundreds of species to the brink of extinction (or beyond) is undoubtedly one of the most serious effects of global change. To enhance the survival of endangered species, translocation programs, including introductions, reintroductions, and restocking, have been launched worldwide (IUCN 1987, 1998). However beneficial these attempts are, they inherently bring several potentially adverse effects. One of the most serious risks is the introduction of new pathogens into the environment by the translocation of animals, which can result in undesirable consequences for indigenous species (Deem et al. 2001; McCallum and Dobson 1995; Viggers et al. 1993; Woodford and Rossiter 1993). This risk increases if the released animals have been kept in captivity before their release (Cunningham 1996). Conversely, released animals may be affected in their new environment by a wide range of local pathogens (Davidson and Nettles 1992; Viggers et al. 1993; Woodford and Rossiter 1993). Introduction of new hosts may also influence existing host-parasite relationships in the area (Cunningham 1996). For example, the introduction of brushtail possum (Trichosurus vulpecula) has provided a new and widespread host for bovine tuberculosis with resultant transmission of the disease to farm cattle and deer in New Zealand (Cowan and Rhodes 1992; Viggers et al. 1993).
Translocation programs focused on primates are conducted in the tropics worldwide (Beck et al. 2007; IUCN 2002). Released primates widely interact not only with the indigenous animals, especially with primates, but also with a plethora of their parasites and other pathogens. Studies of parasite fauna of free-ranging African primates are widespread (Ashford et al. 2000; Gillespie et al. 2004, 2005a; Huffman et al. 1997, 2009; Legesse and Erko 2004; Mbora and Munene 2006; Muehlenbein 2005; Okanga et al. 2006). Some studies have provided an assessment of the parasite community of sympatric primate species (Pan troglodytes troglodytes and Gorilla gorilla gorilla: Landsoud-Soukate et al. 1995; Pan troglodytes and Papio spp.: McGrew et al. 1989a; Pan troglodytes and Papio cynocephalus anubis: Murray et al. 2000; Pan troglodytes troglodytes, Gorilla gorilla gorilla and Cercocebus agilis: Lilly et al. 2002; Cercopithecus (aethiops) sabaeus and Erythrocebus patas patas: McGrew et al. 1989b; Procolobus rufomitratus and Cercocebus galeritus: Mbora and Munene 2006). However, studies of the gastrointestinal parasites of primates released into the wild are scarce, although some researchers have focused on orangutans (Collet et al. 1986; Kilbourn et al. 2003; Mul et al. 2007). To the best of our knowledge, there are no studies comparing the parasite fauna of released primates and sympatric indigenous primate species.
We monitored and compared the gastrointestinal parasite fauna of 2 released primate species (chimpanzees: Pan troglodytes; guerezas: Colobus guereza) and 1 indigenous species (vervets: Chlorocebus aethiops pygerythrus) living in the isolated ecosystem of Rubondo Island National Park, Tanzania. We identified parasites that were specific to a particular host species, parasites that occurred in all primates, and parasites that the released species may have acquired from the indigenous fauna. We also report on parasite prevalence, parasite richness, and seasonality of parasite infections. Finally, we compare the composition of parasite communities of Rubondo primates with the populations of the same species at other wild sites to clarify possible changes associated with their introduction.
Methods
Study Site and Primate Populations
The 240-km2 Rubondo Island is situated at the southwest corner of Lake Victoria (2°18′S, 31°50′E). The island became a forest reserve in 1928. In 1965 the Tanzanian government declared Rubondo a game reserve and in 1977 it was formally declared a National Park, together with 11 small surrounding islets and a large portion of the surrounding lake. Approximately 70% of the habitat comprises mixed evergreen and semideciduous forest. Grassland areas occur sporadically over the island, but are more dominant in the far southern regions (Moscovice et al. 2007). The climate of Rubondo Island includes an annual rainy season from October to May with 2 peaks, 1 in December and 1 in April/May. The dry months from June to September have little or no rainfall. Average annual rainfall is ca. 1200 mm, and the temperature varies between 17 and 29°C.
The original policy for Rubondo was that the island should be a sanctuary for Tanzanian game species threatened with extinction. It was considered suitable for this because there were no large predators, a variety of habitats were represented, it was relatively easy to protect because the lake forms a natural boundary around the island, and there were a large number of unoccupied ecological niches. In the 1960s and 1970s the Frankfurt Zoological Society (FZS) introduced several mammal species onto the island including 17 chimpanzees (Pan troglodytes) and 20 guerezas (Colobus guereza) (Borner 1985; Grzimek 1970; Kiwango 2002). The records about these introductions are very patchy and incomplete. Released guerezas (5 males and 15 females) originated from Mt. Meru (Tanzania). No systematic research has been conducted on the guerezas since their release and there is no official estimate of their current numbers or groups. They are known to occur mainly in the far southern region. The 17 chimpanzees (9 females and 8 males) were introduced in 4 events from 1967 to 1969. They were all wild born, originating from several West African countries. Before their release they spent 3.5 mo–9 yr in European zoos or circuses. Most likely these chimpanzees were treated for gastrointestinal parasite infections with anthelminthic and antiprotozoal drugs in captivity and shortly before their release (Huffman et al. 2008). Despite unfavorable factors during release (Grzimek 1970), the chimpanzees survived and adapted well to Rubondo (Borner 1985; Huffman et al. 2008; Moscovice 2006). Chimpanzees are totally reliant upon the island’s natural vegetation for their subsistence, as are the guerezas, and their number has at least doubled (Moscovice et al. 2007). The population and individual home range estimates for Rubondo chimpanzees are larger than the estimates at any other forested chimpanzee study site (Moscovice 2006). They are thought to live in 1 or 2 groups and avoid the southern parts of the island with grassland (Huffman et al. 2008; Petrželková et al. unpubl. data).
The only indigenous Rubondo primate species is the vervet (Chlorocebus aethiops pygerythrus), which occurs in several troops across the entire island. No population estimates of this species on the island are available, and the number of groups is not known. They are commonly seen in the forest and nearby human dwellings.
Sample Collection and Parasitological Analyses
We collected fecal samples of chimpanzees, vervets, and guerezas from mid-July 2006 to mid-July 2008. We identified samples to the primate species level only, because no primate groups on Rubondo have been completely habituated and individually identified. However, unidentified samples can provide basic presence or absence information for long-term monitoring of unhabituated populations (Gillespie 2006). We obtained chimpanzee samples while systematically looking for them daily or when found under night nests throughout their known range on the island. We collected guereza samples irregularly from a single group occurring in the southern region and also from 2 males from the central east coast. We collected samples of vervets when encountered while following chimpanzees and guerezas or when we encountered a vervet troop independently. Occasionally, we collected samples also from the troops living nearby human dwellings. We collected feces ≤12 h old. We stored the feces in plastic bags until we brought them back to camp, where we processed them immediately.
We fixed 5 g (chimpanzees) or 2 g (vervets and guerezas) of feces in 10% formalin solution in 20-ml vials and periodically transported the samples to the Department of Parasitology, Veterinary and Pharmaceutical University, Brno, Czech Republic. Before microscopic examination, we homogenized each sample and strained it through a sieve into a Falcon conical tube (P-lab, Czech Republic), diluted it with 0.025 M phosphate buffer solution, and centrifuged it for 10 min at 2000 rpm (MPV-340, swing up head). We resuspended the remaining sediment with 5 ml of 10% formalin. We examined the resuspended sediment microscopically via flotation with modified Sheather’s solution (Sheather 1923) and by merthiolate-iodine-formalin concentration (MIFC) methods (Blagg et al. 1955). We added Lugol´s solution to the examined drop onto a microscope slide to color cysts and dilute fecal debris. We identified parasites on the basis of egg or cyst color, shape, contents, and size (Ash and Orihel 2007; Jessee et al. 1970).
Climatological Data Collection
We obtained rainfall data from Tanzanian National Parks (TANAPA) collected on the island and used this to assess the effect of season on parasite infections in chimpanzees and vervets. We classified months as “wet” if cumulative rainfall was >50 mm and “dry” if it was ≤50 mm (Huffman et al. 1997).
Data Analysis and Statistics
We report sample parasite prevalence (percent of samples with a given parasite taxa) and sample parasite richness (the number of unique parasite taxa recovered from a sample) for each primate population. Because samples could not be attributed to individuals and there was the possibility that the same individual was sampled more than once on a particular day, we also used day samples by combining the results from all samples collected within the same day (Petrželková et al. 2010). We calculated day sample parasite prevalence (percent of day samples with a given parasite taxa) and day sample parasite richness (the number of unique parasite taxa recovered from the day sample) for chimpanzees and vervets. We did not report day sample parasite prevalence and parasite richness for guerezas, because of an insufficient quantity of samples.
We conducted comparisons of both sample and day sample prevalence of each parasite taxa between dry and wet seasons via Fisher exact tests for chimpanzees and vervets separately. We made sequential Bonferroni adjustments of p-values for these tests (Rice 1989). We performed Mann-Whitney U tests to compare sample and day sample parasite richness between seasons in chimpanzees and vervets. We did not include guerezas in these analyses because sample size was too small. We analyzed the data via STATISTICA, version 8.0 (StatSoft, Inc. 2008).
Results
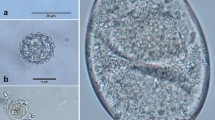
The following descriptions are based on 366 fecal samples collected from chimpanzees (n = 206; 53 day samples), guerezas (n = 49; 8 day samples), and vervets (n = 111; 47 day samples). From all 3 primate species we detected cysts and vacuolar forms of Blastocystis sp., cysts of Giardia sp., amoebae Entamoeba coli, Iodamoeba buetschlii, and Entamoeba spp. (Fig. 1b, c, e, f; Table I). We found flagellate trophozoites or the pear-shaped cysts of Chilomastix mesnili only in chimpanzee and guereza samples (Fig. 1d; Table I). We detected trophozoites of the entodiniomorph ciliate Troglodytella abrassarti, typical for chimpanzees, only in chimpanzees (Fig. 1a; Table I). We recorded the following developmental stages of nematodes in all 3 species of primates: eggs of Trichuris sp., spirurid eggs (cf. Protospirura muricola), undetermined strongylid eggs, eggs and larvae of Strongyloides spp., and various developmental stages of free-living nematodes (Fig. 1h, i, j, k; Table I). We found a low prevalence of Subulura sp. eggs in chimpanzees and vervets (Fig. 1m; Table I). We detected eggs of pinworm Enterobius cf. anthropopitheci (Hasegawa et al. 2005) only in chimpanzees, while eggs of Anatrichosoma sp. occurred only in vervets (Fig. 1g, l; Table I). The oocysts of Eimeria sp. and polysporocystid undetermined adeleid coccidia (Fig. 1n) were present in chimpanzees and vervets, and we considered them to be spurious parasites, i.e., those that are not typically found in a given host and only pass through the digestive tract (Zajac and Conboy 2006).
Parasites recovered from the feces of primates on Rubondo Island. a Troglodytella abrassarti (trophozoite). b Blastocystis sp. (cyst). c Giardia sp. (cyst). d Chilomastix mesnili (cyst). e Entamoeba coli (cyst). f Iodamoeba buetschlii (iodine stained cyst). g Enterobius cf. anthropopitheci (egg). h Strongylida fam. gen. (egg). i Strongyloides sp. (embryonated egg). j Spirurida fam. gen. (cf. Protospirura muricola) (egg). k Trichuris sp. (egg). l Anatrichosoma sp. (egg). m Subulura sp. (egg). n Oocyst of adeleid coccidia with 7 visible sporocysts (spurious parasite). Scale bars: a = 50 μm, l = 40 μm, b–f = 10 μm, all others = 20 μm.
We found significant differences in sample prevalence between the dry and wet season only for Chilomastix mesnili in chimpanzees and for strongylids in vervets (Table II). We did not find any differences in day sample prevalence between the dry and wet season for any parasite taxa for any primate species (Table II).
The median sample parasite richness was 2 for chimpanzees (range 0–7), 4 for vervets (range 0–8), and 2 for guerezas (range 0–7). There were no significant differences in sample parasite richness between the wet and dry season in chimpanzees or vervets (Mann-Whitney tests: Z = −1.11, p = 0.56; Z = 1.82, p = 0.07, respectively). The median day sample parasite richness for chimpanzees was 3 (range 0–8) and 5 for vervets (range 0–9). Similarly to sample parasite richness, we did not find any significant differences in day sample parasite richness between the wet and dry season in chimpanzees or vervets (Mann-Whitney tests: Z = 0.06, p = 0.95; Z = 0.82, p = 0.42, respectively).
Discussion
The isolated ecosystem of Rubondo Island offers a unique opportunity to investigate the possible impact of species introductions on the parasite ecology of both introduced and indigenous primates. The origin and history of primate populations on Rubondo likely influenced the composition of their parasite community. The value for parasite richness that we calculated for chimpanzees from Rubondo was higher than for chimpanzees from Kibale, Uganda (Muehlenbein 2005). We suggest that the lower parasite load of Rubondo chimpanzees might be related to their unusually large home range, which is even larger than the large home range reported for savanna chimpanzees at Mt. Assirik (Baldwin et al. 1982), by their stay in captivity before release, particularly because of the unavailability of intermediate hosts, e.g., Bertiella sp., Probstmayria sp. (Ashford et al. 2000; Krief et al. 2005), or by parasite treatment given to animals before introduction, e.g., strongylid nematodes (Petrželková et al. 2010). On the contrary, parasite richness for samples reported for colobines and guenons at other sites was the same or lower than in Rubondo guerezas and vervets (Piliocolobus tephrosceles, Colobus guereza, Cercopithecus ascanius: Gillespie et al. 2005b; Procolobus rufomitratus: Chapman et al. 2007; Colobus vellerosus: Teichroeb et al. 2009). Torchin et al. (2003) described a similar situation in which the number of parasites species in native populations of 26 host species was higher than in exotic/introduced populations.
Host-specific Troglodytella abrassarti and Enterobius anthropopitheci occurred only in chimpanzees at Rubondo. They are monoxenous parasites with fecal-oral transmission commonly recorded in free-ranging chimpanzees (Ashford et al. 2000; Hugot 1993; Krief et al. 2005; McGrew et al. 1989a; Muehlenbein 2005). The maintenance of infections is also known in captivity, as documented by the common presence of Troglodytella abrassarti in captive chimpanzees (Pomajbiková et al. 2010). The pinworm infections in captive chimpanzees are mostly caused by human-derived Enterobius vermicularis (Murata et al. 2002; Nakano et al. 2006), but Hasegawa and Udono (2007) reported a long-term maintenance of Enterobius anthropopitheci in captive chimpanzees in Japan. Therefore, we conclude that Troglodytella abrassarti and Enterobius anthropopitheci have been maintained by Rubondo chimpanzees since their original capture from the wild in West Africa.
Amoebas Entamoeba coli, Iodamoeba buetschlii, and several other species of the Entamoeba occur in all Rubondo primates without any apparent health problems. Findings reported here as Entamoeba spp. might represent Entamoeba hartmanni, E. histolytica, E. dispar, or E. chattoni. Microscopic differentiation of these species based on cyst morphology is not reliable, and molecular analyses are needed to determine species (Levecke et al. 2007; Verweij et al. 2003). Several authors found amoebas in wild chimpanzees (Ashford et al. 2000; Kuntz and Myers 1969; Muehlenbein 2005), and Appleton et al. (1994) noted a very high prevalence in Cercopithecus mitis. Only Entamoeba coli and E. histolytica occur in guerezas in Uganda and Ghana (Gillespie et al. 2005a; Teichroeb et al. 2009). These protozoa have a direct life cycle, immediate infectivity, and low host specificity, and primates are known as their reservoir (Pedersen et al. 2005; Verweij et al. 2003).
Chilomastix mesnili, a flagellate with questionable pathogenicity, was present only in chimpanzees and guerezas. Despite numerous parasitological studies on primates, this species seems to occur only rarely in wild primates (Pan troglodytes: Kuntz and Myers 1969, Landsoud-Soukate et al.1995; Reynolds 2005; Cercopithecus ascanius: Gillespie et al.2004). Several other species have been described from vertebrates and invertebrates (Kulda and Nohynkova 1978), but Chilomastix from primates, pigs, and humans are thought to belong to the same species with possible transmission between different host species (Hegner 1924; Levine 1985; Solaymani-Mohammadi et al.2004). The epidemiology of these flagellates remains largely unknown, making it difficult for us to propose the possible reasons for difference in sample prevalence between seasons in our study.
We recorded a relatively high prevalence of Blastocystis sp. in all Rubondo primates. Despite intensive research, there are only a few reports with lower prevalence of Blastocystis sp. in wild chimpanzees and vervets (Ashford et al. 2000; Legesse and Erko 2004; Muehlenbein 2005; Reynolds 2005), and there is only one report in captive guerezas (Teichroeb et al. 2009). Recent surveys showed that Blastocystis sp. is common in captive primates (Stensvold et al. 2009). Cysts of Blastocystis sp. can be easily overlooked in fecal debris because of their small size, and thus the scarcity of reports in wild populations could be a result of methods used for detection (Stensvold et al. 2007; Teichroeb et al. 2009). From 13 morphologically identical subtypes of Blastocystis sp., 7 subtypes have been isolated from nonhuman primates; 5 of these subtypes occur in humans (Parkar et al. 2010; Stensvold et al. 2007, 2009; Teichroeb et al. 2009; Yoshikawa et al. 2004, 2009). Some subtypes of Blastocystis sp. cause chronic infections in humans (Stensvold et al. 2007), but little is known about the potential host specificity and pathogenesis in primates (Stensvold et al. 2009; Yoshikawa et al. 2009). Ongoing identification of the subtypes of Blastocystis sp. in primates of Rubondo Island should help to clarify subtypes and the possibility of transmission and distribution in each primate population (Petrášová and Petrželková, unpubl. data). To the best of our knowledge, no researchers have conducted molecular studies on Blastocystis sp. from wild African primates.
The relatively high prevalence of Giardia sp. in vervets and guerezas warrants attention. The presence of the cysts of Giardia sp. in wild primates has typically been related to higher levels of contact with humans and livestock (Graczyk et al. 2002; Nizeyi et al. 1999; Salzer et al. 2007; Wolfe et al. 1998). Higher prevalence has been reported also in sanctuaries and zoological gardens, where it is probably associated with a higher density of hosts and human contact (Levecke et al. 2007; Teichroeb et al. 2009). Domestic animals are not permitted on the island; however, some troops of vervets approach human dwellings, although the chimpanzees have little to no contact with areas inhabited by humans. The host spectrum of zoonotic genotypes (assemblages) A and B of Giardia duodenalis includes both human and nonhuman primates (Gorilla gorilla beringei: Graczyk et al. 2002; Alouatta pigra: Vitazkova and Wade 2006; Alouatta clamitans: Volotao et al. 2008; Colobus vellerosus: Teichroeb et al. 2009). Although Rubondo chimpanzees lived in close contact with humans before their release, only molecular analyses can clarify if the infections include zoonotic genotypes (assemblages) of Giardia sp. found in the Rubondo primates.
Trichuris infections seem to be common in free-ranging primates (Ashford et al.2000; Huffman et al.2009; McGrew et al.1989a; Murray et al.2000), and generally all primate species have been considered to be infected with Trichuris trichiura, which occurs also in humans (Mbora and Munene 2006; Mudakikwa et al.1998; Munene et al.1998; Sleeman et al.2000). However, the morphology and size of the eggs found indicated possibly >1 species occurring in Rubondo primates. In an extensive morphological and morphometric assessment of whipworm eggs, Reptová (2008) found in various primate species living in captivity and the wild that Trichuris eggs from Rubondo chimpanzees and vervets were larger and separated from the major set of eggs obtained from other primates, including Rubondo guerezas. The whipworm eggs from Rubondo chimpanzees and vervets clustered together with the eggs of Trichuris suis obtained from pigs. Several authors have indicated that the Trichuris eggs found in primates are either morphologically dissimilar to T. trichiura (Nkurunungi 1999) or larger than those of T. trichiura (Dupain et al.2009; Hasegawa et al.1983). However, whipworm egg size is highly variable (Yoshikawa et al.1989), and therefore solid species determination based only on egg size is insufficient. Only morphological features of adult males or molecular analysis will provide reliable identification of trichurid species and the origin of infection (Beer 1976; Cutillas et al.2009; Spakulova 1994). The low prevalence of Trichuris sp. in Rubondo chimpanzees is very similar to reports for other wild chimpanzees (Ashford et al.2000; McGrew et al.1989a; Murray et al.2000). The higher prevalence of Trichuris sp. in Rubondo guerezas and vervets is comparable with results from other populations of these primates (Appleton et al.1994; Gillespie et al.2005a; Teichroeb et al.2009). High prevalence of Trichuris sp. was reported to be associated with increased host density and frequency of contact between uninfected and infected hosts in the group (Gillespie et al.2005b).
Petrželková et al. (2006, 2010) identified the spirurids occurring in Rubondo chimpanzees as Protospirura muricola based on adult morphology. We found morphologically identical spirurid eggs also in guerezas and vervets and we suggest that all primates at Rubondo probably harbor the same species of spirurid. Considering the absence of Protospirura muricola in other populations of chimpanzees and colobines, it seems likely that translocated chimpanzees and guerezas acquired this spirurid via ingestion of intermediate insect hosts (Anderson 2000) from the indigenous vervets or rodents after their arrival on the island. Protospirura muricola is a relatively nonpathogenic parasite in rodents, but it can cause severe, sometimes fatal, disease in captive primates (Foster and Johnson 1939; Ruch 1959). However, the prevalence of this spirurid remains low in Rubondo primates and does not seem to pose an obvious health risk for them.
Strongyloides sp. and strongylid nematodes have been reported to be the most prevalent parasites in wild chimpanzee populations (Ashford et al.2000; Huffman et al.2009; Lilly et al.2002; Muehlenbein 2005; Murray et al.2000), making the low prevalence of strongylids in Rubondo chimpanzees striking. It is possible that this phenomenon could be caused by their large home range and anticipated low host density (48 km2, 2–8 times greater than other groups) and probably longer travel distances per day (Moscovice 2006; Nunn et al.2003). Similar findings of overall low parasite infection and the absence of strongylids associated with a large home range have been reported for chimpanzees at Mt. Assirik, Senegal (McGrew et al.1989a). The prevalence of strongylids and Strongyloides spp. was much higher in Rubondo vervets and slightly more so in guerezas than in chimpanzees, but similar to that in other populations of these primate species reported elsewhere (Gillespie et al.2004, 2005a; Mbora and Munene 2006; Munene et al.1998; Okanga et al.2006). The morphology of Strongyloides and strongylid eggs does not allow species level determination, but the presence of eggs and larvae of Strongyloides in the feces suggests that Rubondo primates may be infected with both S. stercoralis and S. fuelleborni. Molecular analyses suggest that Strongyloides fuelleborni has diversified during geographical dispersal and the evolution of host primates (Hasegawa et al.2009). Similarly, the application of DNA fingerprinting revealed that strongylid Oesophagostomum bifurcum from different primate species represents distinct groups (Gasser et al.2009). Therefore it is possible that each species of Rubondo primate carries a host-specific species/genotype of strongylids and Strongyloides sp.
We found a higher sample prevalence (but not day sample prevalence) of strongylids during the rainy season only in vervets. Individual sampling would be necessary to confirm this pattern, because several authors observed higher prevalence of strongylid nematodes during rainy season also in chimpanzees and bonobos at other sites (Dupain et al. 2002; Huffman et al. 1997, 2009). The apparent lack of seasonality in strongylids of Rubondo chimpanzees and guerezas might be caused by their lower prevalence in comparison to vervets.
Petrželková et al. (2006, 2010) previously reported Anatrichosoma sp. and Subulura sp. in Rubondo chimpanzees. However, we found the eggs of Anatrichosoma sp. only in vervets and the eggs of Subulura sp. in both chimpanzees and vervets. Because there are no other reports of Anatrichosoma sp. and Subulura sp. in chimpanzees, we suggest that Rubondo chimpanzees might have obtained these parasites from vervets directly (Anatrichosoma sp.) or via intermediate insect hosts (Subulura sp.). The eggs of Anatrichosoma sp. are usually found by scraping the nasal mucosa of rhesus monkeys, baboons, and vervets (Conrad and Wong 1973; Long et al. 1976). Eggs of Anatrichosoma sp. are rarely found in the feces, and usual microscopic examination may not accurately reflect their actual prevalence (Orihel 1970). Nine species of Subulura have been reported in primates, and Subulura distans has been reported in Cercopithecus spp. (Cameron 1930; Yamashita 1963). However, we cannot exclude the possibility that both taxa represent spurious parasites passed through the digestive tract of Rubondo chimpanzees (Zajac and Conboy 2006), because chimpanzees occasionally hunt and eat vervets on Rubondo (Petrželková pers. obs.), and the prevalence of these parasites in chimpanzees was very low (Petrželková et al. 2006, 2010; this study).
Six Eimeria, 7 Isospora, and 1 Cyclospora species are known in primates, and all need sporogony time to be infectious for the host (Duszynski et al. 1999). We detected eimeriid and adeleid oocysts similar to oocysts reported previously in reptiles and birds in the feces of vervets (Daszak and Ball 1998; Kawazoe and Gouvea 1999). Because the oocysts found in feces were sporulated (spore-forming process), we considered them to be spurious parasites (Zajac and Conboy 2006). Oocysts of coccidia probably entered vervets with their insect prey or via food contaminated with bird fecal material.
Except for 2 clearly distinguishable chimpanzee specific parasites, the majority of the parasite taxa reported here was found in all 3 studied primate species. Rubondo chimpanzees probably lost some of their parasites before or during the introduction. However, both released primate species appear to have acquired ≥1 parasite from the native fauna. Molecular analyses are needed to clarify further the conspecificity of the parasites reported here. At present, there seem to be no obvious health problems associated with the parasites infecting the introduced primates transmitted from local fauna on Rubondo. However, continuous parasite monitoring at ongoing or future reintroduction/introduction sites is highly recommended to decrease the risk of negative consequences associated with possible exchanges of parasites between introduced and native fauna.
References
Anderson, R. C. (2000). Nematode parasites of vertebrates: Their development and transmission. Wallingford: CABI.
Appleton, C. C., Krecek, R. C., Verster, A., Bruorton, M. R., & Lawes, M. J. (1994). Gastro-intestinal parasites of Samango monkey, Cercopithecus mitis, in Natal, South Africa. Journal of Medical Primatology, 23, 52–55.
Ash, L. R., & Orihel, T. C. (2007). Atlas of human parasitology. Singapore: American Society for Clinical Pathology Press.
Ashford, R. W., Reid, G. D. F., & Wrangham, R. W. (2000). Intestinal parasites of the chimpanzee Pan troglodytes in Kibale Forest, Uganda. Annals of Tropical Medicine and Parasitology, 94, 173–179.
Baldwin, P. J., McGrew, W. C., & Tutin, C. E. G. (1982). Wide-ranging chimpanzees at Mt. Assirik, Senegal. International Journal of Primatology, 3, 367–385.
Beck, B., Walkup, K., Rodrigues, M., Uwin, S., Travis, D., & Stoinski, T. (2007). Best practice guidelines for the re-introduction of Great Apes. Gland: IUCN/SSC Primate Specialist Group of the World Conservation Union.
Beer, R. J. S. (1976). The relationship between Trichuris trichiura (Linnaeus, 1758) of man and Trichuris suis (Schrank, 1788) of the pig. Research in Veterinary Science, 20, 47–54.
Blagg, W., Schloegel, E. L., Mansour, N. S., & Khalal, G. I. (1955). A new concentration technic for the demonstration of protozoa and helminth eggs in feces. The American Journal of Tropical Medicine and Hygiene, 4, 23–28.
Borner, M. (1985). The rehabilitated chimpanzees of Rubondo Island. Oryx, 19, 151–154.
Cameron, T. W. M. (1930). The species of Subulura Molin in primates. Journal of Helminthology, 8, 49–58.
Chapman, C. A., Saj, T. L., & Snaith, T. V. (2007). Temporal dynamics of nutrition, parasitism, and stress in colobus monkeys: implications for population regulation and conservation. American Journal of Physical Anthropology, 134, 240–250.
Collet, J., Galdikas, B. M. F., Sugarjito, J., & Jojosudharmo, S. (1986). A coprological study of parasitism in orangutans (Pongo pygmaeus) in Indonesia. Journal of Medical Primatolology, 15, 121–129.
Conrad, H. D., & Wong, M. M. (1973). Studies of Anatrichosoma (Nematoda: Trichinellida) with descriptions of Anatrichosoma rhina sp. n. and Anatrichosoma nacepobi sp. n. from nasal mucosa of Macaca mulatta. Journal of Helminthology, 47, 289–302.
Cowan, P. E., & Rhodes, D. S. (1992). Restricting the movements of brushtail possum (Trichosurus vulpecula) on farmland with electric fencing. Wildlife Research, 19, 47–58.
Cunningham, A. A. (1996). Disease risks of wildlife translocations. Conservation Biology, 10, 349–353.
Cutillas, C., Callejon, R., de Rojas, M., Tewes, B., Ubeda, J. M., Ariza, C., et al. (2009). Trichuris suis and Trichuris trichiura are different nematode species. Acta Tropica, 111, 299–307.
Daszak, P., & Ball, S. J. (1998). Description of the oocysts of three new species of Eimeria (Apicomplexa: Eimeriidae) from Iguanid lizards (Sauria: Iguanidae) of Central and South America. Memórias do Instituto Oswaldo Cruz, 93, 471–475.
Davidson, W. R., & Nettles, V. F. (1992). Relocation of wildlife: identifying and evaluating disease risks. Transactions of the North American Wildlife & Natural Resources Conference, 57, 466–473.
Deem, S. L., Karesh, W. B., & Weisman, W. (2001). Putting theory into practice: wildlife health in conservation. Conservation Biology, 15, 1224–1233.
Dupain, J., van Elsacker, L., Nell, C., Garcia, P., Ponce, F., & Huffman, M. A. (2002). New evidence for leaf swallowing and Oesophagostomum infection in bonobos (Pan paniscus). International Journal of Primatology, 23, 1053–1062.
Dupain, J., Nell, C., Petrželková, K. J., Garcia, P., Modrý, D., & Gordo, F. P. (2009). Gastrointestinal parasites of bonobos in the Lomako Forest, Democratic Republic of Congo. In M. A. Huffman & C. Chapman (Eds.), Primate parasite ecology: The dynamics of host-parasite relationships (pp. 297–310). Cambridge: Cambridge University Press.
Duszynski, D. W., Wilson, W. D., Upton, S. J., & Levine, N. D. (1999). Coccidia (Apicomplexa: Eimeriidae) in the primates and the scandentia. International Journal of Primatology, 20, 761–797.
Foster, A. O., & Johnson, C. M. (1939). A preliminary note on identity, life cycle, and pathogenicity of an important parasite of captive monkeys. The American Journal of Tropical Medicine and Hygiene, 19, 265–277.
Gasser, R. B., de Gruijter, J. M., & Polderman, A. M. (2009). The utility of molecular methods for elucidating primate-pathogen relationships—the Oesophagostomum bifurcum example. In M. A. Huffman & C. Chapman (Eds.), Primate parasite ecology: The dynamics of host-parasite relationships (pp. 47–62). Cambridge: Cambridge University Press.
Gillespie, T. R. (2006). Noninvasive assessment of gastrointestinal parasite infections in free-ranging primates. International Journal of Primatology, 27, 1129–1143.
Gillespie, T. R., Greiner, E. C., & Chapman, C. A. (2004). Gastrointestinal parasites of the guenons of western Uganda. The Journal of Parasitology, 90, 1356–1360.
Gillespie, T. R., Greiner, E. C., & Chapman, C. A. (2005a). Gastrointestinal parasites of the colobus monkeys of Uganda. The Journal of Parasitology, 91, 569–573.
Gillespie, T. R., Chapman, C. A., & Greiner, E. C. (2005b). Effects of logging on gastrointestinal parasite infections and infection risk in African primates. Journal of Applied Ecology, 42, 699–707.
Graczyk, T. K., Bosco-Nizeyi, J., Ssebide, B., Thompson, R. C. A., Read, C., & Cranfield, M. R. (2002). Anthropozoonotic Giardia duodenalis genotype (Assemblage) A infections in habitats of free-ranging human-habituated gorillas, Uganda. The Journal of Parasitology, 88, 905–909.
Grzimek, B. (1970). Among animals of Africa. New York: Stein & Day.
Hasegawa, H., & Udono, T. (2007). Chimpanzee pinworm, Enterobius anthropopitheci (Nematoda: Oxyuridae), maintained for more than twenty years in captive chimpanzees in Japan. The Journal of Parasitology, 93, 850–853.
Hasegawa, H., Kano, T., & Mulavwa, M. (1983). A parasitological survey on the feces of pygmy chimpanzees, Pan paniscus, at Wamba, Zaire. Primates, 24, 419–423.
Hasegawa, H., Ikeda, Y., Fujisaki, A., Moscovice, L. R., Petrželková, K. J., Kaur, T., et al. (2005). Morphology of chimpanzee pinworms, Enterobius (Enterobius) anthropopitheci (Geldoelst, 1916) (Nematoda: Oxyuridae), collected from chimpanzees, Pan troglodytes, on Rubondo Island, Tanzania. The Journal of Parasitology, 91, 1314–1317.
Hasegawa, H., Hayashida, S., Ikeda, Y., & Sato, H. (2009). Hyper-variable regions in 18S rDNA of Strongyloides spp. as markers for species-specific diagnosis. Parasitology Research, 104, 869–874.
Hegner, R. W. (1924). Giardia and Chilomastix from monkeys, Giardia from the wild cat and Blantidium from sheep. The Journal of Parasitology, 11, 75–78.
Huffman, M. A., Gotoh, S., Turner, L. A., Hamai, M., & Yoshida, K. (1997). Seasonal trends in intestinal nematode infection and medicinal plant use among chimpanzees in the Mahale Mountains, Tanzania. Primates, 38, 111–125.
Huffman, M. A., Petrželková, K. J., Moscovice, L. R., Mapua, M. I., Bobáková, L., Mazoch, V., et al. (2008). Introduction of chimpanzees onto Rubondo Island National Park, Tanzania. In P. S. Soorae (Ed.), Global re-introduction perspectives: re-introduction case-studies from around the globe (pp. 213–215). Abu Dhabi: IUCN/SSC Re-introduction Specialist Group.
Huffman, M. A., Pebsworth, P., Bakuneeta, C., Gotoh, S. G., & Bardi, M. (2009). Chimpanzee-parasite ecology at Budongo Forest (Uganda) and the Mahale Mountains (Tanzania): Influence of climatic differences on self-medicative behavior. In M. A. Huffman & C. Chapman (Eds.), Primate parasite ecology: The dynamics of host-parasite relationships (pp. 331–350). Cambridge: Cambridge University Press.
Hugot, J. P. (1993). Redescription of Enterobius anthropopitheci (Gedoelst, 1916) (Nematoda, Oxyurida), a parasite of chimpanzees. Systematic Parasitology, 26, 201–207.
IUCN (International Union for Conservation of Nature). (1987). The IUCN position statement on translocation of living organisms. Gland: IUCN/SSC Re-introduction Specialist Group.
IUCN. (1998). Guidelines for re-introductions. Gland: IUCN/SSC Re-introduction Specialist Group.
IUCN. (2002). Guidelines for nonhuman primate re-introductions. Gland: IUCN/SSC Re-introduction Specialist Group.
Jessee, M. T., Schilling, P. W., & Stunkard, J. A. (1970). Identification of intestinal helminth eggs in old world primates. Laboratory Animal Care, 20, 83–87.
Kawazoe, U., & Gouvea, H. (1999). Description of Pythonella scleruri n. sp. (Apicomplexa: Eimeriidae) from a Brazilian bird rufous-breasted-leaftosser Scerurus scansor, 1835 (Passeriformes: Furnariidae). Memórias do Instituto Oswaldo Cruz, 94, 157–159.
Kilbourn, A. M., Karesh, W. B., Wolfe, N. D., Bosi, E. J., Cook, R. A., & Andau, M. (2003). Health evaluation of free-ranging and semi-captive orangutans (Pongo pygmaeus pygmaeus) in Sabah, Malaysia. Journal of Wildlife Diseases, 39, 73–83.
Kiwango, J. (2002). Ecological survey RINP. Unpublished report of Tanzanian National Parks.
Krief, S., Huffman, M. A., Sevenet, T., Guillot, J., Bories, B., Hladik, C. M., et al. (2005). Non-invasive monitoring of the health of Pan troglodytes schweinfurthii in the Kibale National Park, Uganda. International Journal of Primatology, 26, 467–490.
Kulda, J., & Nohynkova, E. (1978). Flagellates of the human intestine and of the intestines of other species. In J. P. Kreiner (Ed.), Parasitic protozoa (pp. 1–138). New York: Academic.
Kuntz, R. E., & Myers, B. J. (1969). Parasitic protozoa, commensals and helminths of chimpanzees imported from the Republic of the Congo. Proceedings of the 2nd International Congress of Primatology, 3, 184–190.
Landsoud-Soukate, J., Tutin, C. E., & Fernandez, M. (1995). Intestinal parasites of sympatric gorillas and chimpanzees in the Lope Reserve, Gabon. Annals of Tropical Medicine and Parasitology, 89, 73–79.
Legesse, M., & Erko, B. (2004). Zoonotic intestinal parasites in Papio anubis (baboon) and Cercopithecus aethiops (vervet) from four localities in Ethiopia. Acta Tropica, 90, 231–236.
Levecke, B., Dorny, P., Geurden, T., Vercammen, F., & Vercruysse, J. (2007). Gastrointestinal protozoa in non-human primates of four zoological gardens in Belgium. Veterinary Parasitology, 148, 236–246.
Levine, N. D. (1985). Veterinary protozoology. Iowa: Iowa State University Press.
Lilly, A. A., Mehlman, P. T., & Doran, D. (2002). Intestinal parasites in gorillas, chimpanzees, and humans at Mondika Research Site, Dzanga-Ndoki National Park, Central African Republic. International Journal of Primatology, 23, 555–573.
Long, G. G., Lichtenfels, J. R., & Stookey, J. L. (1976). Anatrichosoma cynamolgi (Nematoda: Trichinellida) in rhesus monkeys, Macaca mulatta. The Journal of Parasitology, 62, 111–115.
Mbora, D. N. M., & Munene, E. (2006). Gastrointestinal parasites of critically endangered primates endemic to Tana River, Kenya: Tana River red colobus (Procolobus rufomitratus) and crested mangabey (Cercocebus galeritus). The Journal of Parasitology, 92, 928–932.
McCallum, H., & Dobson, A. (1995). Detecting disease and parasite threats to endangered species and ecosystem. Tree, 10, 190–194.
McGrew, W. C., Tutin, C. E. G., Collins, D. A., & File, S. K. (1989a). Intestinal parasites of sympatric Pan troglodytes and Papio spp., at two sites—Gombe (Tanzania) and Mt. Assirik (Senegal). American Journal of Primatology, 17, 147–155.
McGrew, W. C., Tutin, C. E. G., & File, S. K. (1989b). Intestinal parasites of two species of free-living monkeys in far western Africa, Cercopithecus (aethiops) sabaeus and Erythrocebus patas patas. African Journal of Ecology, 27, 261–262.
Moscovice, L. R. (2006). Behavioral ecology of chimpanzees (Pan troglodytes) on Rubondo Island, Tanzania: habitat, diet, grouping and ranging at a release site. Dissertation Abstracts International, B 67(6), AADAA.
Moscovice, L. R., Issa, M. H., Petrželková, K. J., Keuler, N. S., Snowdon, C. T., & Huffman, M. A. (2007). Fruit availability, chimpanzee diet, and grouping patterns on Rubondo Island, Tanzania. American Journal of Primatology, 69, 487–502.
Mudakikwa, A. B., Sleeman, J. M., Foster, J. W., Madder, L. L., & Patton, S. (1998). An indicator of human impact: gastrointestinal parasites of mountain gorillas (Gorilla gorilla beringei) from the Virunga Volcanoes Region, Central Africa. In C. K. Baer (Ed.), Proceedings of the American Association of Zoo Veterinarians/American Association of Wild Veterinarians Joint Conference (pp. 436–437). Philadelphia: American Association of Zoo Veterinarians.
Muehlenbein, M. P. (2005). Parasitological analyses of the male chimpanzees (Pan troglodytes schweinfurthii) at Ngogo, Kibale National Park, Uganda. American Journal of Primatology, 65, 167–179.
Mul, I. F., Paembonan, W., Singleton, I., Wich, S. A., & van Bolhuis, H. G. (2007). Intestinal parasites of free-ranging, semicaptive and captive Pongo abelii in Sumatra, Indonesia. International Journal of Primatology, 28, 407–420.
Munene, E., Otsyula, M., Mbaabu, D. A. N., Mutahi, W. T., Muriuki, S. M. K., & Muchemi, G. M. (1998). Helminth and protozoan gastrointestinal tract parasites in captive and wild-trapped African non-human primates. Veterinary Parasitology, 78, 195–201.
Murata, K., Hasegawa, H., Nakano, T., Noda, A., & Yanai, T. (2002). Fatal infection with human pinworm, Enterobius vermicularis, in a captive chimpanzee. Journal of Medical Primatology, 31, 104–108.
Murray, S., Stem, C., Boudreau, B., & Goodall, J. (2000). Intestinal parasites of baboons (Papio cynocephalus anubis) and chimpanzees (Pan troglodytes) in Gombe National Park. Journal of Zoo and Wildlife Medicine, 31, 176–178.
Nakano, T., Okamoto, M., Ikeda, Y., & Hasegawa, H. (2006). Mitochondrial cytochrome c oxidase subunit 1 gene and nuclear rDNA regions of Enterobius vermicularis parasitic in captive chimpanzees with special reference to its relationship with pinworms in humans. Parasitology Research, 100, 51–57.
Nizeyi, J. B., Mwebe, R., Nanteza, A., Cranfield, M. R., Kalema, G. R. N. N., & Graczyk, T. K. (1999). Cryptosporidium sp. and Giardia sp. infections in mountain gorillas (Gorilla gorilla beringei) of the Bwindi Impenetrable National Park, Uganda. The Journal of Parasitology, 85, 1084–1088.
Nkurunungi, J. B. (1999). A survey of the gastro-intestinal helminths of the wild mountain gorilla (Gorilla gorilla beringei Matschie) and man in Bwindi Impenetrable National Park of southwestern Uganda. Proceedings of the Ecological Monitoring Programme Workshop: Research as an important tool of ecological monitoring in Bwindi Impenetrable National Park, Uganda, 9–11.
Nunn, C. L., Altizer, S., Jones, K. E., & Sechrest, W. (2003). Comparative tests of parasite species richness in primates. The American Naturalist, 162, 597–614.
Okanga, S., Muchemi, G., Maingi, N., Mogoa, E., & Munene, E. (2006). Gastrointestinal parasites of free-ranging colobus monkeys (Colobus angolensis palliatus) in Kwale District, Kenya coast. African Journal of Ecology, 44, 410–412.
Orihel, T. C. (1970). Anatrichosomiasis in African monkeys. The Journal of Parasitology, 56, 982–985.
Parkar, U., Traub, R. J., Vitali, S., Elliot, A., Levecke, B., Robertson, I., et al. (2010). Molecular characterization of Blastocystis isolates from zoo animals and their animal-keepres. Veterinary Parasitology, 169, 8–17.
Pedersen, A. B., Altizer, S., Poss, M., Cunningham, A. A., & Nunn, C. L. (2005). Patterns of host specificity and transmission among parasites of wild primates. International Journal for Parasitology, 35, 647–657.
Petrželková, K. J., Hasegawa, H., Moscovice, L. R., Kaur, T., Mapua, M. I., & Huffman, M. A. (2006). Parasitic nematodes in the chimpanzee population on Rubondo Island, Tanzania. International Journal of Primatology, 27, 767–777.
Petrželková, K. J., Hasegawa, H., Appleton, C. C., Huffman, M. A., Archer, C. E., Moscovice, L. R., et al. (2010). Gastrointestinal parasites of the chimpanzee population introduced onto Rubondo Island National Park, Tanzania. American Journal of Primatology, 72, 307–316.
Pomajbiková, K., Petrželková, K. J., Profousová, I., Petrášová, J., Kisidayová, S., Varadyová, Z., et al. (2010). A survey of entodiniomorphid ciliates in chimpanzees and bonobos. American Journal of Physical Anthropology, 142, 42–48.
Reptová, Z. (2008). Morfologicka variabilita vajicok hlistic rodu Trichuris u primatov. [Morphologic variability of eggs of genus Trichuris in primates]. VMD thesis, The University of Veterinary and Pharmaceutical Sciences, Brno, Czech Republic.
Reynolds, V. (2005). The chimpanzees of the Budongo forest. Ecology, behaviour, and conservation. Oxford: Oxford University Press.
Rice, W. R. (1989). Analyzing tables of statistical tests. Evolution, 43, 223–225.
Ruch, T. C. (1959). Diseases of laboratory primates. Philadelphia: W.B. Saunders.
Salzer, J. S., Rwego, I. B., Goldberg, T. L., Kuhlenschmidt, M. S., & Gillespie, T. R. (2007). Giardia sp. and Cryptosporidium sp. infections in primates in fragmented and undisturbed forest in Western Uganda. The Journal of Parasitology, 93, 439–440.
Sheather, A. L. (1923). The detection of intestinal protozoa and mange parasites by a flotation technique. Journal of Comparative Pathology, 36, 266–275.
Sleeman, J. M., Meader, L. L., Mudakikwa, A. B., Foster, J. W., & Patton, S. (2000). Gastrointestinal parasites of mountain gorillas (Gorilla gorilla beringei) in the Parc National des Volcans, Rwanda. Journal of Zoo and Wildlife Medicine, 31, 322–328.
Solaymani-Mohammadi, S., Rezaian, M., Hooshyar, H., Mowlavi, G. R., Babaei, Z., & Anwar, M. A. (2004). Intestinal protozoa in wild boars (Sus scrofa) in Western Iran. Journal of Wildlife Diseases, 40, 801–803.
Spakulova, M. (1994). Discriminant analysis as a method for the numerical evaluation of taxonomic characters in male trichurid nematodes. Systematic Parasitology, 29, 113–119.
Stensvold, C. R., Arendrup, M. C., Jespersgaard, C., Molbak, K., & Nielsen, H. V. (2007). Detecting Blastocystis using parasitologic and DNA-based methods: a comparative study. Diagnostic Microbiology and Infectious Disease, 59, 303–307.
Stensvold, C. R., Alfellani, M. A., Norskov-Lauritsen, S., Prip, K., Victory, E. L., Maddox, C., et al. (2009). Subtype distribution of Blastocystis isolates from synanthropic and zoo animals and identification of a new subtype. International Journal for Parasitology, 39, 473–479.
Teichroeb, J. A., Kutz, S. J., Parkar, U., Thompson, R. C. A., & Sicotte, P. (2009). Ecology of the gastrointestinal parasites of Colobus vellerosus at Boabeng-Fiema, Ghana: possible anthropozoonotic transmission. American Journal of Physical Anthropology, 140, 498–507.
Torchin, M. E., Lafferty, K. D., Dobson, A. P., McKenzie, V. J., & Kuris, A. M. (2003). Introduced species and their missing parasites. Nature, 421, 628–630.
Verweij, J. J., Vermeer, J., Brienen, E. A. T., Blotkamp, C., Laeijendecker, D., van Lieshout, L., et al. (2003). Entamoeba histolytica infections in captive primates. Parasitology Research, 90, 100–103.
Viggers, K. L., Lindenmayer, D. B., & Spratt, D. M. (1993). The importance of disease in reintroduction programmes. Wildlife Research, 20, 678–698.
Vitazkova, S. K., & Wade, S. E. (2006). Parasites of free-ranging black howler monkeys (Alouatta pigra) from Belize and Mexico. American Journal of Primatology, 68, 1089–1097.
Volotao, A. C. C., Souza Junior, J. C., Grassini, C., Peralta, J. M., & Fernandes, O. (2008). Genotyping of Giardia duodenalis from southern brown howler monkeys (Alouatta clamitans) from Brazil. Veterinary Parasitology, 158, 133–137.
Wolfe, N. D., Escalante, A. A., Karesh, W. B., Kilbourn, A., Spielman, A., & Lal, A. A. (1998). Wild primate populations in emerging infectious disease research: the missing link? Emerging Infectious Diseases, 4, 149–158.
Woodford, M. H., & Rossiter, P. B. (1993). Disease risks associated with wildlife translocations projects. Revue Scientifique et Technique—Office International des Epizooties, 12, 115–135.
Yamashita, J. (1963). Ecological relationships between parasites and primates. I. Helminth parasites and primates. Primates, 4, 1–96.
Yoshikawa, H., Yamada, M., Matsumoto, Y., & Yoshida, Y. (1989). Variations in egg size of Trichuris trichiura. Parasitology Research, 75, 649–654.
Yoshikawa, H., Morimoto, K., Wu, Z., Yap, E. H., Singh, M., & Hashimoto, T. (2004). Problems in speciation in the genus Blastocystis. Trends in Parasitology, 20, 251–255.
Yoshikawa, H., Wu, Z., Pandey, K., Pandey, B. D., Sherchand, J. B., Yanagi, T., et al. (2009). Molecular characterization of Blastocystis isolates from children and rhesus monkey in Kathmandu, Nepal. Veterinary Parasitology, 160, 295–300.
Zajac, A., & Conboy, G. A. (2006). Veterinary clinical parasitology. Ames: Blackwell.
Acknowledgments
The present research was supported by the Grant Agency of the Academy of Sciences of the Czech Republic (KJB600930615) and Grant Agency of the Czech Republic (524/06/0264, 206/09/0927). J. Petrášová was partially supported by the Internal Grant Agency of the University of Veterinary and Pharmaceutical Sciences (IGA 77/2007/FVL). We express our sincere appreciation to the Government of Tanzania, the Tanzania Commission for Science and Technology, Tanzania National Parks, and the Tanzania Wildlife Research Institute for their support and for granting permission to conduct this research in Tanzania. We thank our Tanzanian trackers for their participation in the collection of fecal samples and their help and wonderful companionship in the field. Our best wishes go to Rubondo Island National Park Wardens and staff for their kind hospitality and important logistical support. We thank our 2 anonymous reviewers and the editor for their helpful comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Petrášová, J., Modrý, D., Huffman, M.A. et al. Gastrointestinal Parasites of Indigenous and Introduced Primate Species of Rubondo Island National Park, Tanzania. Int J Primatol 31, 920–936 (2010). https://doi.org/10.1007/s10764-010-9439-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-010-9439-x