Abstract
Studies of primate foraging efficiency during the exploration of new areas can provide important insights into the adaptive value of long-term spatial memory. After 6 yr of observation of a group of gray-cheeked mangabeys (Lophocebus albigena johnstonii) in Kibale National Park, Uganda, we observed exploration of a new area, followed 7 mo later by a group split. We recorded their ranging and foraging behavior for 22 mo after the first exploration. Controlling for weather variables, we found that mangabeys moved longer daily travel distances, explored more area per day, and had larger group spreads in the new area compared to the old area in both parent and daughter groups. The increase in search swath in the new area likely enabled the monkeys to counteract their lack of knowledge of food locations in the new area, as the efficiency in finding fruit in general did not differ between the old and new areas. We did, however, find a lower efficiency in finding fruit from preferred fig trees whose edibility could not be assessed by visual cues in the new area. Fig finding efficiency remained lower, even when we controlled for potential differences in fig density. In addition, mangabeys traveled and foraged less often on the ground in the new compared to the old area. However, when the monkeys became more familiar with the new area, terrestrial behavior increased. Our results are consistent with the hypothesis that when monkeys move into an area in which they have no experience, an absence of knowledge acquired via long-term spatial memory decreases their foraging efficiency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A large variety of animals use long-term spatial memory to find either artificial or natural food, including rats (Rattus norvegica): Tolman (1948), digger wasps (Ammophila campestris): Tinbergen (1972), chimpanzees (Pan troglodytes): Menzel (1973), sticklebacks (Gasterosteus aculeatus): Girvan and Braithwaite (1997), nutcrackers (Nucifraga Columbiana): Balda and Kamil (1998), capuchins (Cebus apella nigritus): Janson (1998), and orangutans (Pongo abelli): Scheumann and Call (2006). Several authors have discussed the advantages of such memory (Emery and Clayton 2004; Milton 1988; Stevens et al. 2005; Waser and Jones 1983). However, few researchers have investigated the advantages of long-term spatial memory in the natural habitat via empirical study (Isbell et al. 1990; Isbell and van Vuren 1996). One way to do so is to compare the foraging behavior of an animal in both familiar and unfamiliar areas. Natural events that allow for such comparisons include situations wherein juveniles explore neighboring areas before they disperse from their natal home range, e.g., gray-cheeked mangabeys (Lophocebus albigena): Janmaat et al. (2009), badgers (Meles meles): Roper et al. (2003), red squirrels (Tamiasciurus hudsonicus): Larsen and Boutin (1994), or when adult individuals explore new areas for breeding sites (extraterritorial prospecting: meerkats (Suricata suricatta): Doolan and MacDonald 1996; review on birds: Reed et al. 1999). However, in these particular cases it is difficult to interpret a change in foraging behavior, as it may be a result of learning and development in a young animal, or an effect of a switch from gregarious to solitary feeding. Further, adult foragers that are prospecting for new breeding sites are often more preoccupied with the inspection of breeding facilities—burrows, tree holes, nest locations—than with foraging (Doolan and MacDonald 1996). It is unclear how much these individuals already know about the area by the time they settle and resume normal foraging activities. Another factor that complicates matters is that most vertebrates cannot be followed easily on foot, limiting reliable measurements of foraging success, such as the number and size of food sources that are encountered per distance traveled. Many dispersal studies are therefore limited to measurements of mortality rates or breeding success (Isbell and van Vuren 1996; Larsen and Boutin 1994; Nilsson 1989) and cannot document underlying factors such as foraging efficiency.
However, primates are good candidates to investigate the importance of long-term spatial memory on foraging efficiency in the natural habitat because they are among the easiest vertebrates to follow on foot, provided that they are well habituated and live in accessible habitats. Primate groups have been observed to shift their home range to unfamiliar areas, e.g., vervets (Cercopithecus aethiops): Isbell et al. (1990) and baboons (Papio cynocephalus): Bronikowski and Altmann (1996). However, these events are rare and often correlate with reductions in habitat quality, which complicates the interpretation of potential differences in foraging efficiency in old and new areas. Shifts in ranging area may also occur after a group split, when one of the daughter groups moves into a new range: red-tail monkeys (Cercopithecus ascanius): Struhsaker and Leland (1988), blue monkeys (Cercopithecus mitis): Cords and Rowell (1986), Japanese macaques (Macaca fuscata): Sugiyama (1960), and rhesus macaques (Macaca mulatta): Chepko-Sade and Sade 1979). Habitat quality does not necessarily change after such shifts, but their interpretation is nevertheless complicated by differences in group size and intragroup competition before and after the split, or between daughter groups.
We had the unique opportunity to observe a group of rain forest primates (gray-cheeked mangabeys, Lophocebus albigena johnstonii) in Kibale National Park, Uganda, exploring a radically new area after an observation period of 6 yr. There were no obvious signs that the exploration was related to sudden changes in habitat quality such as habitat destruction by human interference. The exploration was followed 7 mo later by a group split. The 2 daughter groups were equal in size, and one quickly returned to the home range of the parent group, while the other used mostly the new range (Janmaat 2006; Janmaat et al. 2009). The exceptional timing of the home range shift and the even-sized group split enabled us to make two comparisons of foraging behavior: of the parent group in familiar vs. unfamiliar areas and of the 2 equal-sized daughter groups, one in a new and the other in an old area.
Because gray-cheeked mangabeys are known to use spatial memory to relocate fruit trees, we expected that our focal group would suffer a decrease in foraging efficiency when it entered an area in which the monkeys had little or no memory (Janmaat et al. 2006a). First, we investigated the effect of the move into the new area on daily travel distance, exploration rate, and group spread. Gray-cheeked mangabeys are noted for their exceptionally large group spread, which has been suggested to present a means of finding or exploiting food sources (Waser and Floody 1974). Waser (1985) suggested that factors such as an increased likelihood of exploiting food resources without competition from other group members would trigger mangabeys to become more peripheral. We therefore expected that the lack of knowledge of food sources in the new area would result in an increase in group spread, which would decrease overlap between individual search swaths by increasing interindividual distances. In addition, we expected that daily travel distances and area explored per day would increase as individuals attempted to increase daily area covered. In this way, new food sources would more likely be discovered by means of individual search using sensory cues or local enhancement (Crook 1965).
Second, we investigated the effect of the move into the new area on terrestrial behavior. Gray-cheeked mangabeys were initially reported as strictly arboreal monkeys that preferred to travel through the higher levels of forest canopy (Chalmers 1968). Yet, more recent studies investigating more habituated groups show that gray-cheeked mangabeys are observed to travel or forage on the ground at a variety of research sites (Poulsen and Clark 2007; Wallis 1979; Waser 1977). In Kibale National Park, male and female gray-cheeked mangabeys spent 8% and 1% of the observed time on the ground, respectively. Females spent 29% traveling, 43% foraging, 14% drinking, and 14% drinking and foraging. Males used the ground more for travel and spent 59% of the sampling intervals traveling, 39% foraging, 1% drinking, and 1% grooming (K. Janmaat and R. Chancellor, unpubl. data). Males and females from different groups were observed to forage on the ground for ants, dirt, animal matter inside logs, fruit, seeds, and algae and other water plants (K. Janmaat and R. Chancellor, unpubl. data; G. Arlet, pers. comm.). Due to the thick ground and understory vegetation in the Kanyawara study area (Struhsaker 1997), terrestrial areas safe and suitable for feeding, drinking, and traveling are not easily discovered (K. Janmaat and R. Chancellor, pers. obs.). Therefore, we expected that the lack of knowledge of suitable areas for terrestrial behavior (areas with little ground vegetation, or drinking/foraging sites) would result in a decrease in the proportion of days mangabeys descended to the ground in the new area.
Third, we investigated the effect of the move into a new area on the efficiency with which individuals found fruit. The majority of the gray-cheeked mangabey’s diet consists of fruit (59% of foraging time; Olupot 1998). We focused our analyses of the mangabeys’ localization efficiency on a highly preferred food source, fig trees (Ficus spp.). Mangabeys, though using a wide variety of food types and species, tend to specialize on species represented by small numbers of large, scattered fruiting trees, such as figs. Large figs are thought to influence strongly the mangabeys’ ranging behavior (Barrett 1995; Waser 1974). In particular, recent studies have shown that gray-cheeked mangabeys remember the fruiting histories of individual fig trees of Ficus sansibarica, a species that produces fruit throughout the year (Janmaat 2006; Janmaat et al. 2006b). This fruit is highly preferred by the mangabeys and profitable to exploit in terms of energy gain (rank 3, 5, and 3 by Ivlev’s electivity index, which incorporates percentages of feeding time and relative tree density (Barrett 1995; Krebs 1988), from 3 study periods (Wallis 1979 in Barrett 1995; Waser 1977; R. Chancellor, unpubl. data). Janmaat et al. (2006) found that when the focal group was within a 100 m radius (about 1/10th of their average daily travel distance) of a tree of Ficus sansibarica that was fed in during their previous visit, the group reentered it 65% of the time. Proportions of revisits stayed high throughout the year (2003: April: 0.6, August: 0.5, September: 0.7, 2004: January: 0.7, February: 0.8, March: 0.6, April: 0.6, but May 2003: 0.4). The density of trees of Ficus sansibarica is low, and fruit does not show obvious visual signs of edibility, such as a certain color or size, complicating the discovery of fruit by sight (1.7 trees/ha; Chapman et al. 1999; Janmaat et al. 2006a; N. J. Dominy, P. W. Lucas, R. W. Wrangham, and L. Ramsden, unpubl. data). In sum, accounting for the potential differences in food availability in both areas, we expected that individual males and females within our focal group would have a lower efficiency in finding the fruit of Ficus sansibarica in the new vs. the old area.
Methods
Study Group and Site
We studied the Butanzi group of gray-cheeked mangabeys (Olupot 2000) in the Kanyawara study area in Kibale National Park, Uganda (0°34′N, 30° 21′W; Chapman et al. 1997; Struhsaker 1997). During the first year of our study period (April 2003–April 2004), the group consisted of 8 adult females, 4–10 adult males, 1 subadult female, 3 juvenile females, 4 juvenile males, and 0–2 infants (n = 20–28). All individuals were well habituated to human observers on foot, allowing observation as close as 2 m. We could individually identify all reproductively active females (n = 8) and a total of 9 males, including 2 resident males that were radio-collared in 1997 by Olupot (1999).
Before the start of this study, various researchers had studied the Butanzi group intensively for a total of 6 yr: W. Olupot (July 1997–January 2001), G. Arlet (February–June 2001), J. Lambert (June 2001–June 2002), and R. Chancellor (July–September 2002). Within this time period, the group had never been observed south of the 0º 32′ 42′′ latitude (W. Olupot, G. Arlet, J. Rusoke [field assistant of Olupot, Arlet, and Lambert] and R. Chancellor, pers. comm. and unpubl. data; Janmaat et al. 2009). Five and a half months after the start of this study (September 17, 2003), the group crossed this latitude and continued to travel >1.5 km further south (Fig. 1a.). Based on the observations of previous researchers, we believe that it was the first time that the group moved this far south. Though the group had experienced an influx of new males, some of which may have been familiar with the area south of 0º 32′ 42′′ latitude, it is unlikely that most members of the group had used this area before, so we refer to this area as new and to the area north of this latitude as old.
Group ranging in old and new areas. (a) Group locations and home range estimates of the parent group before (April 1, 2003–September 16, 2004) and after (September 17, 2004–April 14, 2004) the exploration of the new area. (b) Group locations and home range estimates of daughter groups I and II (April 24, 2004–July 30, 2005). We recorded locations every half hour for 314 d and at least twice a day for 154 additional days.
Seven months after it had moved into the new area, the group split into 2 daughter groups. The first split occurred on April 15, 2004, after which the group rejoined. The final split occurred on April 23, 2004. After the split, daughter group I returned to range in the old area, while daughter group II continued to range in the new area and was observed to move between the old and new area again only in February 2005 (Fig. 1b). After the split, the daughter groups were not observed to meet until February 23, 2005. On this date both groups were ranging in the old area and were involved in a fight that included aggressive attacks by males and females. The nature of the fight differed from that of intragroup aggression as individuals from one group formed a coalition to chase individuals from the other group: a species typical behavior for the rare occasions that groups do not avoid each other (Waser 1976; R. Chancellor, unpubl. data). We did not observe the daughter groups to rejoin again within the study period. Daughter groups I and II initially consisted of 4 adult females, 3 adult males, 1 subadult female, 1 juvenile female, 1 juvenile male, and 1 infant (n = 11), and 4 adult females, 3 adult males, 2 juvenile females, and 3 juvenile males (n = 12), respectively.
Data Collection
We conducted the study from April 2003 until July 2005 and based it on data collected within 2 separate studies planned for different purposes. We describe both methods of data collection. Owing to differences in methodology, we compare only data from the same observer. Observation periods of daily travel distance, group spread, terrestrial behavior, and fruit finding efficiency are provided in Table I.
Daily Travel Distance (DTD)
We followed the groups with a total of 3 teams consisting of Janmaat, Chancellor, field assistant R. Mijer (all experienced in focal animal sampling), and 1 local assistant each. We recorded the groups’ geographic location via a 12XL Garmin G.P.S. in combination with a detailed map of the Kanyawara trail system with an extended trail system for the newly explored area (Janmaat 2006). The G.P.S. had an average error of 7.7 m (n = 1497 locations). The location of the groups’ center of mass was recorded every half hour, by standing at a location in the group where the observer could spot a similar number of individuals in each cardinal direction. We analyzed daily group travel distance for full observation days only. For the parent group, a full day consisted of continuous half-hour locations from 0730 h until 1800 h (10.5 h), which coincided with times that mangabeys depart from their sleeping sites in the morning and when the first individuals in the group positioned themselves to go to sleep in the evening, respectively. The time of sunrise changed by only 18 min over the entire study period and was earlier after the first exploration in the new area than before. After the split, we calculated full days for continuous half-hour locations from 0800 h until 1700 h because daughter group II had moved far away from camp. We calculated daily travel distances by summing straight-line distances between consecutive half-hour locations. We analyzed daily travel distance of the daughter groups for the periods: April 2004–July 2004 and November 2004–July 2005. Within these periods we followed both daughter groups on alternating days. We defined full days that were spent in both areas (n = 70) as spent in the old area when the majority of half-hour locations were in that area and vice versa. We defined daily increments in home range size using the locations of full days and the minimum convex polygon technique (MCP) in the Animal Movement extension in Arc View 3.3 (Altmann and Altmann 1970; Newton-Fisher 2003).
Group Spread
Chancellor determined group spread at the end of every 30 min in which she followed a female by pacing between the 2 farthest points of the group or, if this was not possible, by calculating the distance between the 2 farthest points using a G.P.S. We trained 3 local field assistants to pace the group spread, and used intraobserver tests (25 per assistant) to calibrate their pace lengths with the actual distances.
Terrestrial Behavior
We noted all occurrences of terrestrial behavior, defined as an observation of ≥1 group member drinking, foraging, or traveling on the ground, during focal sampling. Janmaat mapped the start and end locations of terrestrial travel using a G.P.S. and trail maps. The distances that individuals traveled on the ground were calculated as the straight-line distances between start and end locations.
Fruit Finding Efficiency
We collected data on fruit finding efficiency during focal samples of individual males and females. For males, every minute Janmaat (or Mijer) recorded the distance traveled (in steps), food items fed on, and the number of mangabey food trees entered. We chose one-zero sampling of the above variables, as other measurements not related to this study had to be taken simultaneously (Martin and Bateson 1986). The observer’s step lengths were calibrated over a stretch of 500 m within the forest habitat with varying elevation levels. For females, Chancellor estimated the distance traveled (in m), and recorded feeding duration and the number and species of trees fed in during continuous focal sampling. Feeding was defined as the consumption of food items only. There was a high degree of reliability between the assistant’s estimates and the actual distances measured by a tape measure (Spearman’s p = 0.98, p < 0.001, n = 330; Martin and Bateson 1986).
In the parent group, Janmaat followed 7 individually identifiable males, and Chancellor, 8 individually identifiable females. In the daughter groups, Janmaat followed 3 males in each group and 1 male that was observed in both groups, while Chancellor followed 4 females in each group. We conducted observations within the old and new areas on alternating days. Two of the 7 males that were followed in the parent group disappeared after the split (Ha and Em). After the split, Janmaat therefore followed 2 new males, 1 that had entered the parent group in August 03 (Pl) and 1 that entered daughter group I after the split (Mg).
For both males and females, data collection started by identifying and locating one of the selected individuals. We followed a male selected from a randomly ordered list for a distance of 300–400 m. If the male was lost or the next male on the list could not be found within 1 h, the next male was followed. If later during the day the lost male was located, he was followed again for a total of 300–400 m. Janmaat stopped following the male when calculations of the sum of our step lengths showed that we had followed him for a distance of ≥300 m. The males’ routes were marked with brightly colored flagging. For females, Chancellor sampled individuals in the order that she encountered them. We sampled all females in the group before another round of sampling began. We conducted one 30-min sample on each female in the group being followed over each round. If a female went out of view during a focal sample, an attempt to locate her lasted for 20 min. If she was found again within 5 min, the sampling continued, and we recorded her estimated straight-line distance from the time she was last seen to the time she was found. If she was found after 5 min, we discounted the time and distance covered while out of sight and we followed her for a total of 30 focal min.
For males and females, we analyzed the number of food trees that were 1) entered and fed in per 100 m traveling for each 300–400 m distance sample and 2) fed in per 30-min time sample, respectively. In addition, we analyzed the percentage of time or the proportion of 1-0 samples that females and males ate fruit in each time and distance sample, respectively. Samples of each individual were separated in time by ≥1 d.
Fig Fruit and Patch Size Densities
To investigate the possibility that differences in fig finding efficiency between the old and new area resulted from differences in fig availability, we measured fig densities in close proximity to the males’ routes. Each day after the males were followed, Janmaat (or Mijer) walked a transect route parallel to and 15 m away from the male’s route. The distance from the male’s route was estimated by sight, as the route was marked with flagging. We chose 15 m because it was the farthest distance at which the flagging could reliably be spotted and because it was still close enough to give a good representation of the area in which the male had traveled. The observers mapped each Ficus sansibarica if the trunk was ≤5 m of the transect route, and determined its fruiting state (full or empty). Ten meters is the standard width of transects walked in the Kanyawara research area (Chapman et al. 1999). Thereafter, the same observers walked the male’s actual route of the previous day to collect the same measurements. This time-efficient method enabled us to finish both the 300–400 m transect and route within the same day and minimized influences of fruit consumption by other frugivores within our measuring period. For the analyses, we calculated the number of trees per 100 m traveling on both the route and the transect, as both were not always exactly of equal length.
To assess whether the males fig finding efficiency was influenced by the availability of figs in their ranging area, we calculated the male’s relative approach efficiency (RAE).
We added the value of 1 to the denominator to enable the use of a larger number of transects (some did not contain figs).
To investigate the possibility that differences in exploration rate, i.e., area traversed per day, were caused by differences in the size of food patches, we estimated patch size in the old and new areas. For this we relied on behavioral observations. We defined a patch size as the ratio of the time spent feeding on fruit within a focal sample, divided by the number of fruit trees fed on within the same sample.
Statistical Analyses
We tested whether daily travel distance or group spread differed between new and old areas via analyses of covariance in which we corrected for the covariates rainfall and average daily temperature. For the group spread data of the daughter groups, the homogeneity of variance assumption was violated and we used the separate variance t-test to calculate Welch’s approximate t′ using SPSS. To test whether familiarity influenced the proportion of days in which mangabeys were observed to descend to the ground we used nonparametric χ 2 tests (Sokal and Rohlf 1981). To analyze the nonnormally distributed fruit finding efficiency and patch size data, we compared the performance of each sex in old and new areas via nonparametric statistics. To compare fruit finding efficiency of the males in the parent group, we conducted Wilcoxon signed rank matched-pairs tests. To compare fruit finding efficiency of the males in the daughter groups, we conducted an exact permutation test, enumerating all possible arrangements of the data. We conducted permutations such that for the 6 males, which provided only 1 data point each, we combined all possible selections of 3 and 3 out of them with both possible assignments of male LB that provided data in both areas. As a test statistic, we chose the U-value as measured in the Mann-Whitney U test. We determined the p-value as the proportion of U-values in the sampling distribution, as derived by permutation, being at least as far away from their mean as the U-value of the original data. The script for this test was written in R by Roger Mundry at K. Janmaat’s institute. We compared the fruit finding efficiency of the females in the daughter group via a standardized permutation test (Mann-Whitney U test). We assumed that measurements recorded per day, such as daily travel distance, were independent of each other. In each case we assessed evidence about specific hypotheses, so we did not adjust significance criteria using the Bonferroni method (Perneger 1998). All tests were 2-tailed.
Results
Did Mangabeys Travel Farther in the New Area?
The parent group traveled significantly longer distances each day in the new than in the old area (X new ± SE = 1260 ± 50 m and X old ± SE = 1120 ± 33 m, F 1,195 = 5.86, p = 0.016, n new = 64, n old = 133). However, temperature and rainfall are known to influence mangabey daily travel distances (Janmaat 2006), so we performed a further analysis to control for factors other than familiarity that might influence rate of movement. First, we confined our comparisons of daily travel distances to pairs of consecutive days during which the parent group shifted from old to new areas and vice versa. Second, we controlled statistically for the covariates rainfall and temperature in the ANCOVA. Again, daily travel distance was significantly longer in the new compared to the old area (X new ± SE = 1478 ± 203 m and X old ± SE = 1149 ± 238 m; F 1,15 = 14.29, p = 0.002, n new = 9, n old = 8).
Results were similar when we compared daily travel distance for the 2 daughter groups in the old and new areas. Daughter group II, in the new area, moved significantly further than daughter group I, in the old area (X new ± SE = 1089 ± 64 m and X old ± SE = 904 ± 36 m; F 1,100 = 7.62, p = 0.007 (controlled for covariates), n new = 35, n old = 69). We did not conduct intragroup comparisons of daughter group II because the group moved back to the old area only in February 2005, long after the initial shift.
A plot of cumulative home range size provides another illustration of the different rates and patterns of movement in new and old areas (Fig. 2). When we plotted cumulative minimum convex polygon (MCP) areas used in the new and old areas as a function of the number of days we had observed the parent group using those areas, we found that cumulative MCP increased at a higher rate in the new area. In addition, cumulative home range size calculated from a similar number of observation days (n = 64) was higher for days spent in the new (516 ha) than in the old (313 ha) area. Similarly, the cumulative MCP used by daughter group II using the new area increased faster than the cumulative area of daughter group I using the old area. The cumulative range size calculated for a similar number of days (n = 35) was also higher for daughter group II while traveling in the new area (421 ha) than for daughter group I traveling in the old area (200 ha). These findings suggest that the group not only traveled farther but also explored more area per day in the new than in the old area.
Exploration rate in old and new areas. Increase in observed MCP home range size with time for (a) the parent group in old and new areas and (b) daughter group I in old area and daughter group II in the new area. Open and closed circles represent the daily home range size increments in the new and old area, respectively.
Was Group Spread Larger in the New Area?
Group spread in the parent group was significantly larger in the new than in the old area (X new ± SE = 107 ± 6.8 m and X old ± SE = 88 ± 5.7 m; F 1,116 = 5.936, p = 0.016 [corrected for covariates], n new = 62, n old = 58). Group spread in daughter group II in the new area was significantly larger than that of daughter group I in the old area, (t′ 96 = 2.7, p = 0.008; X new ± SE = 62 ± 4.4 m and X old ± SE = 49 ± 1.6 m, n new = 147, n old = 66). For this last subset of data we could not correct for the effect of covariates because the data violated the assumption of equal variances.
Were Mangabeys Less Terrestrial in the New Area?
After >6 yr of habituation, ≥1 individual in our study group was observed on the ground in 72% of all observation days in the old area. When mangabeys descended to the ground, they drank or fed (most often on ants, dirt, animal matter inside logs, sometimes on fruit of Blighia unijugata, Myriantha cloa, or Strombosia scheffleri, seeds of Diospyros abyssinica, and algae from ponds or creeks). In addition, they traveled on the ground in logged, primary and secondary forest areas. However, descent to the ground was initially confined to just a few sites in the new area (Fig. 3).
Terrestrial behavior in the parent group. Locations and trajectories in which we observed ≥1group member to forage, drink, or travel on the ground. The polygons represent the area surrounding all sightings in either the old or new area, within the period during which we collected data on terrestrial behavior.
We found that in the parent group, the proportion of days in which ≥1 mangabey was observed on the ground was significantly higher in the old compared to the new area (χ 21 = 34.58, p < 0.001, 31 out of 44 d in old, 4 out of 44 d in new area). Similarly, we sighted members of daughter group I in the old area on the ground during a larger proportion of days than members of daughter group II traveling in the new area during the first 3 mo after the split (χ 21 = 3.820, p = 0.05, 11 out of 26 d in old, 7 out of 36 d in new area).
In addition, we conducted the same analysis with a subset of the data and asked whether familiarity, i.e. being in the old or new area, also influenced terrestrial travel, as opposed to other terrestrial activities. In the parent group, we found that the number of days in which ≥1 mangabey was observed to travel >20 m on the ground was also significantly associated with their familiarity with the area. The proportion of days in which individual(s) were observed to travel on the ground was higher in the old than in the new area (χ 21 = 36.81, p < 0.001; Fig. 4). A similar association was found for the days that daughter group I traveled in the old area and daughter group II traveled in the new area ≤3 mo after the split (χ 21 = 4.407, p = 0.036; Fig. 4).
Terrestrial travel (>20 m) in the old and new areas. Each circle represents the proportion of days that we observed ≥1 individual to travel >20 m on the ground in the old or the new area in the parent group and daughter groups. White numerals in black segments: number of days that we observed individual(s) to travel on the ground. Black numerals in white segments: number of days in which we observed no individuals to travel on the ground.
To investigate whether the change in terrestrial behavior in the new area was indeed a consequence of a lack of spatial knowledge of suitable areas for terrestrial foraging and traveling or whether it was simply a result of fewer such areas being available, we observed how terrestrial behavior developed during familiarization. Did the mangabeys become more terrestrial when the individuals had spent more time in the new area?
At the start of the data collection on terrestrial behavior, the group had already been ranging within the new area for some time, meaning that we were too late to follow the process of familiarization. However, on February 26, 2004 the group moved again farther south and passed another latitude, 0º 32′ 24′′, which gave us a new opportunity to follow the process of familiarization. Within this new area (south of 0º 32′ 24′′), it was 3.5 mo later that the first individual—in daughter group II—was observed on the ground. The frequency with which ≥1 individual was observed on the ground in daughter group II was significantly influenced by the amount of time the group had spent in the new area (April 23, 2004–July 27, 2004 vs. November 26, 2004–July 27, 2005; χ 21 = 6.728, p = 0.009, 7 out of 36 d in first, 17 out of 35 d in second period). The proportion of days in which individual(s) were observed on the ground was higher in the second period, in which more time had been spent in the new area.
Were Mangabeys Less Efficient in Food Finding in the New Area?
Fruits in General
We investigated the efficiency with which individual mangabeys found edible fruit in the new area. We compared the number of trees in which males and females fed on fruit per focal sample and the percentage of time that males and females ate fruit in the old vs. new area.
We investigated the fruit finding efficiency of 9 males (no. of trees/100 m) and 8 females (no. of trees/30 min; Table II). In the parent group, neither males nor females entered significantly more trees with edible fruit per focal sample in the old than in the new areas (matched-pairs, males: T + = 20, n = 7, p = 0.38, females: T + = 12.5, n = 8, p = 0.48, Fig. 5). The proportion of sampling intervals during which males, and the percentage of time in which females spent feeding on these fruits within each focal sample, did not differ significantly between areas either (matched-pairs, males: T + = 23, n = 7, p = 0.16, females: T + = 26, n = 8, p = 0.31, Table III). Males and females in daughter group I, which traveled in the old area, did not enter significantly more trees with edible fruit per focal distance or period than the males and females in daughter group II, which traveled in the new area (males, permutation test: U = 6, n old = 3, n new = 3, n old&new = 1, p = 0.55; females, Mann-Whitney U: U = 5, n old = 4, n new = 4, p = 0.49, Table III). Finally, we found no difference between the proportion of sampling intervals during which males and the percentage of time in which females of daughter group I, spent feeding on these fruits vs. males or females in daughter group II (males, permutation test: U = 5, n old = 3, n new = 3, n old&new = 1, p = 0.40; females, MWU: U = 8, n old = 4, n new = 4, p = 1.0, Table III).
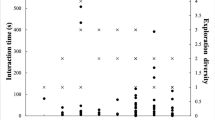
Fruit finding efficiency in old and new areas. (a) Fruit finding efficiency for individual males and females (top left and right, respectively). (b) Fig finding efficiency for individual males and females (bottom left and right, respectively). Open and closed circles represent the average numbers of trees fed in per focal sample per individual in new and old areas, respectively.
These results suggest that familiarity with an area did not influence the efficiency with which individual monkeys found edible fruit in general. However, it is difficult to explain the results, as no independent information is available on the overall fruit availability in both areas. Therefore, we analyzed the monkeys’ behavior toward trees that carry highly preferred fruit of Ficus sansibarica, for which we have fruit density data available in both areas. Monkeys were expected to approach these trees irrespective of the overall food availability in both areas.
In the parent group, males entered significantly more Ficus sansibarica with edible fruit per focal distance (100 m) in the old vs. the new area (matched-pairs: T + = 21, n = 6 [1 tie], p = 0.03, Fig. 5). Females also entered more Ficus sansibarica with edible fruit per 30 min focal period, but the difference was not significant (matched-pairs: T + = 6, n = 3 [5 ties], p = 0.25, Fig. 5). In addition, the proportion of sampling intervals during which males were feeding on these figs, was higher in the old vs. the new area (matched-pairs: T + = 21, n = 6 [1 tie], p = 0.03, Table III). The percentage of time in which females fed on figs in the old area was also higher, but the difference again was not significant (matched-pairs: T + = 6, n = 3 [5 ties], p = 0.25, Table III). The females and males in daughter group I, in the old area, entered more Ficus sansibarica with edible fruit per focal period or distance than those in daughter group II, in the new area. This time the difference was significant only for the females (females: MWU: U = 0, n old = 4, n new = 4, p = 0.029; males, permutation test: U = 5, n old = 3, n new = 3, n old&new = 1, p = 0.55, Table III). The proportion of sampling intervals during which males and the percentage of time in which females spent feeding on figs was higher in daughter group I vs. males or females in daughter group II. However, the difference was significant only for the females (females: MWU U = 0, n old = 4, n new = 4, p = 0.03; males: permutation test: U = 7, n old = 3, n new = 3, n old&new = 1, p = 0.90, Table III).
Could Habitat Differences Explain Changes in Foraging and Ranging Behavior?
We used data on the density of fig-bearing Ficus sansibarica in the transect routes to test whether the mangabeys’ fig finding efficiency simply reflected tree densities in the 2 areas. We found that the relative approach efficiency (RAE) of fruit-bearing fig trees of males in the parent group tended to be higher in the new than in the old area (T + = 15, n = 5 [2 ties], p = 0.06). This suggests the possibility that the males were more actively approaching fruit-bearing fig trees in the old than in the new area, irrespective of the distribution of fruit-bearing figs in their direct ranging area.
We further investigated whether differences in food patch sizes in the old and new areas could explain the higher increase in cumulative MCP in the new area. To estimate food patch size, we calculated the ratio of the proportion of sampling intervals during which males, and the percentage of time in which females spent feeding on fruit, and the number of trees they fed in, for each focal sample. We found that ratios did not differ significantly between the old and new areas, suggesting that the time spent in individual fruit trees did not differ (parent group, males: T + = 24, n = 7, p = 0.11, females: T + = 21, n = 8, p = 0.74 ; daughter group, males: permutation test: U = 3, n old = 3, n new = 3, n old&new = 1, p = 0.25, females: MWU: U = 6, n old = 4, n new = 4, p = 0.69).
Discussion
We had a unique opportunity to observe a group of rain forest primates explore an area known not to have been used during at least the preceding 6 yr, followed by an even-sized group split 7 mo later. We took advantage of this opportunity to measure the behavioral consequences of the exploration of a new area in which the monkeys had little or no spatial memory.
We found that mangabeys in both the parent group and daughter groups moved significantly longer daily travel distances, had a quicker increase in cumulative range size, and had larger group spreads in the new area vs. the old area. These differences were unlikely to have been a result of climatic differences between the different observation periods because we controlled for the effect of temperature and rainfall. In addition, mangabeys were less often observed on the ground in the new vs. the old area; as time progressed and the monkeys presumably became more familiar with the new area, this difference decreased. We did not find a difference in the efficiency with which mangabeys found fruit-bearing trees overall; however, the results indicate that individual mangabeys were less efficient at finding fruit-bearing trees of the preferred Ficus sansibarica in the new vs. the old area.
Underlying Explanations for Behavioral Change
The greater daily travel distance and quicker increase in cumulative range size in the new area suggest that the groups increased daily area covered. In addition, the larger group spread in the new area suggests that the groups traveled and foraged in a more dispersed fashion. These 2 behavioral changes increased individual search swath and decreased the overlap between the search swaths of group members. This likely increased the mangabeys’ chances of discovering unknown food sources by individual search and local enhancement (Crook 1965). It is tempting, therefore, to interpret the increase in daily travel distance and group spread as a behavioral adaptation to counteract the lack of knowledge of food locations in the new area. The results from our analyses, which suggest that both males and females were equally efficient in finding fruit-bearing trees, and spent an equal amount of time eating fruit in the old and new areas, are consistent with this idea. However, in the parent group, individual males found Ficus sansibarica with edible fruits at lower rates in the new area. In addition, males spent less time feeding on these figs in the new vs. the old area. Females also found fewer trees with edible fruit and spent less time feeding on these figs, though we found no significant difference, as the rate with which females encountered figs in both areas was very low. Females in daughter group II, which ranged in the new area, were significantly less efficient at finding edible figs of Ficus sansibarica than the females in daughter group I, which ranged in the old area, and in addition spent less time feeding on this fruit as the group continued to explore new areas. Results for the males were similar but no significant difference was found. The median values of fig finding efficiency and fig feeding duration were higher in the old area vs. the new area, and a difference might have appeared if the sample size for males in the daughter group had been larger (Table III). However, it is also possible that we found no significant difference because 1 out of 4 males had recently entered daughter group I after the split. This male may have been as unfamiliar with the locations of fig fruit in the old area as the males in daughter group II were of the locations of fig fruit in the new area.
Results on fig finding efficiency of the males in the parent group were equally difficult to interpret, as only 2 out of 7 males in the parent group were collared and confirmed resident males (Ma and Bg). It is possible that the decrease in fig finding efficiency was unrelated to the males’ lack of spatial knowledge, but simply reflected differences in fig availability. We therefore conducted an additional analysis for the males in the parent group using a relative measure of fig finding efficiency (RAE) and concluded that potential differences in fig availability could not explain the observed decrease in fig finding efficiency in the new area. Neither do we think that differences in the availability of other food sources provide an adequate explanation, as Ficus sansibarica scores higher on the preference list than most other mangabey food sources. In addition, the monkeys are known to approach the fig trees from considerable distances (1/10th of their daily travel route) in a majority of cases, provided that they have fed in the tree on previous visits (Janmaat 2006). Proportions of revisits remained high throughout the different months of observation, suggesting that the temporal availability of surrounding food sources did not influence the approach probability of fig trees (K. Janmaat, unpubl. data). We infer instead that the monkeys had trouble finding trees that carried edible fruit. A fig crop does not emerge and disappear from one day to the next. Instead, fig fruit in the Kanyawara study area can take up to 69 d to ripen, and the focal group revisited trees up to 15 times over periods of up to 66 d (K. Janmaat, unpubl. data). Hence, the lack of memory regarding the previous fruiting states of individual fig trees at the start of their explorations must have made it difficult for the mangabeys to efficiently time their visits to those trees in the new area.
The increase in daily travel distance in the new area could be explained by temporal differences in the availability of food items. For example, if the overall availability of fruit was lower at the time in which the parent group started exploring the new area, the monkeys could have been forced to travel longer distances to find sufficient food than before the exploration. This is difficult to test because we do not have data on the overall fruit availability in the old and new areas. However, when we focused on the parent group’s behavior within short time periods, wherein the compared days in old and new areas were consecutive, we still found that the parent group traveled longer distances in the new area than in the old area.
In addition, it is possible that differences in other habitat parameters such as the size of food patches influenced the monkeys’ increase in group spread and cumulative range size. The new area, e.g., contained a higher density of fruits of Uvariopsis congensis (Janmaat et al. submitted; Zuberbühler and Janmaat 2010), which are small fruit trees. Feeding in these small food patches could have caused the monkeys to travel more often from one feeding tree to the next, resulting in the coverage of a larger area and more dispersed travel. However, we did not find a difference in the time that individuals spent feeding in each fruit tree, suggesting that the overall size of food patches was similar in both areas.
An obvious explanation for a decrease in terrestrial behavior in the new area is that it had fewer suitable places for terrestrial travel, foraging, or drinking. Perhaps foraging and drinking places were rarer or more risky owing to a higher density of understory vegetation. However, the finding that individuals in daughter group II were observed on the ground more often after they had spent more time in the new area suggests that it was the initial absence and subsequent acquisition of information acquired by memory that influenced the terrestrial behavior of the mangabeys.
Considering the results of these analyses, we argue that the best explanation for the changes in the mangabeys’ daily travel distance, cumulative range increase, group spread, fig finding efficiency, and terrestrial behavior was their limited spatial knowledge of the newly explored area.
Predation Risk vs. Foraging Efficiency
The finding that group spread, daily travel distance, and area covered increased in the new area may seem surprising when we consider theories on predation risk. Metzgar (1967) argued that predation risk is expected to be higher in less familiar areas because animals have more difficulty in detecting danger and are less effective in escaping predators due to a lack of knowledge of the terrain (Brown 2001 [escape response]; Isbell et al. 1990; Manzer and Bell 2004; Windberg 1996). Considering this reasoning, we could have made the opposite prediction that the monkeys would travel more slowly in the new area to increase the chances of detecting predators (Janson and Di Bitetti 1997), and that as a result daily travel distances would decrease. In addition, we might have predicted that group spread would decrease, as the mangabeys’ main predator, the crowned eagle (Stephanoaetus coronatus), is an ambush predator, and more cohesive foraging would put more individuals between a group member and the predator, potentially decreasing a group member’s predation risk (Forsman et al. 1998; Hamilton 1971; Shultz et al. 2003). Struhsaker and Leakey (1990) showed that eagle predation has a major impact on the mangbey population of Kibale: ≥3.8% of males and 2.2% of females are killed in this way each year.
However, travel distances, daily area covered, and group spread increased. Hence, our results raise the interesting possibility that the need to increase foraging efficiency in a new area was more important than the need to decrease predation risk.
Costs of Exploring New Areas
An increase in daily travel distance clearly increased the daily costs of travel in the new area (Muruthi et al. 1991; Steudel 2000). In addition, we speculate that the increase in cumulative range size increased travel costs even further. While entering new areas, monkeys are expected to be less updated on the location of efficient travel routes (Di Fiore and Suarez 2007). Apart from this cost, we propose a third type of travel cost, i.e., one that is related to the monkey’s type of locomotion when traveling terrestrially. Walking is suggested to require less energy than bridging or climbing (often after leaping; Aronsen 2004; Mermier et al. 1997; Steudel 2000). The idea that mangabeys saved energy by terrestrial travel is further supported by anecdotal observations of repeated terrestrial travel at specific locations in primary forest that occurred only in uphill directions (Janmaat 2006).In addition, we suggest that decreased terrestrial foraging in newly explored areas and reduced access to terrestrial food sources such as dirt or algae or other water plants that are known to contain important vitamins and minerals (Knezevich 1998; Oates 1978) could have had a negative effect on the monkeys’ health state. This, however, is a topic for future research.
Conclusion
In summary, we conclude that the mangabeys experienced an increase in daily travel costs, via increased distances traveled or a decrease of efficient terrestrial (quadrupedal) locomotion, and a lower efficiency in the finding of fruit-bearing fig trees or locations for terrestrial behavior while exploring a new area. The increase in daily search swath, by travel distance and group spread, appears to compensate for the lack of knowledge of food locations as localization efficiency of fruit trees in general did not differ between both areas. However, the mangabeys were less efficient at finding preferred fruit from trees with large fruit crops that show no visual signs of edibility, such as figs. Although we cannot fully exclude alternative explanations, our results are consistent with the hypothesis that an absence of knowledge of fruiting states and locations suitable for terrestrial behavior acquired via long-term memory decreased the localization efficiency of fig fruit and terrestrial foraging locations; increased travel costs, by distance and type of locomotion; and as a result, decreased the monkeys’ overall foraging efficiency.
The results of this study may provide insight into other natural observations of primates that have exhibited site fidelity even when prime feeding locations changed. For example, a case study of a deposed α-chimpanzee male found that he tended to remain in the same area despite changes in his dominance status and the potential to move into areas with higher fruit availability (Murray et al. 2008). Singleton and van Schaik (2001) observed a similar example in orangutans (Pongo pygmaeus), with individuals remaining in the same areas despite clear foraging advantages elsewhere due to fruit masting. In an extreme case, Nilgiri langurs (Presbytis johnii) remained in their home range until the last trees were cut (Poirier 1968). Cases like these suggest the importance of spatial information per se. Our study is one of the first to attempt to quantify the importance of spatial memory on foraging efficiency. We encourage other primatologists to pay attention to the rare occurrence of exploration and to realize the need for detailed monitoring of such events for understanding the adaptive values of memory.
References
Altmann, S. A., & Altmann, J. (1970). Baboon ecology: African field research. Basel: Karger.
Aronsen, G. P. (2004). Locomotor energetics and forest canopy variation: implications for conservation and management. Folia Primatologica Supplement, 1, 233.
Balda, R. P. & Kamil, A. C. (1998). The ecology and evolution of spatial memory in corvids of the Southwestern USA: The perplexing pinyon jay. In R. O. Balda, I. M. Pepperberg & A. C. Kamil (Eds.), Animal cognition in nature: The convergence of psychology and biology in laboratory and field (pp. 29–64). San Diego, CA: Academic Press.
Barrett, L. (1995). Foraging strategies, ranging patterns and territoriality among gray-cheeked mangabeys in Kibale forest, Western Uganda. Ph.D. thesis, University College London.
Bronikowski, M., & Altmann, J. (1996). Foraging in a variable environment: weather patterns and the behavioral ecology of baboons. Behavioral Ecology and Sociobiology, 39, 11–25.
Brown, C. (2001). Familiarity with the test environment improves escape responses in the crimson spotted rainbowfish, Melanotaenia duboulayi. Animal Cognition, 4, 109–113.
Chalmers, N. R. (1968). Group composition, ecology and daily activities of free living mangabeys in Uganda. Folia Primatologica, 8, 247–262.
Chapman, C. A., Chapman, L. J., Wrangham, R., Isabirye-Basuta, G., & Ben-David, K. (1997). Spatial and temporal variability in the structure of a tropical forest. African Journal of Ecology, 35, 341–436.
Chapman, C. A., Wrangham, R. W., Chapman, L. J., Kennard, D. K., & Zanne, A. E. (1999). Fruit and flower phenology at two sites in Kibale National Park, Uganda. Journal of Tropical Ecology, 15, 189–211.
Chepko-Sade, B. D., & Sade, D. S. (1979). Patterns of group splitting within matrilineal kinship groups: a study of social group structure in Macaca mulatta (Cercopithecine: Primates). Behavioral Ecology and Sociobiology, 5, 67–87.
Cords, M., & Rowell, T. E. (1986). Group fission in blue monkeys of the Kakamega Forest, Kenya. Folia Primatologica, 46, 70–82.
Crook, J. H. (1965). The adaptive significance of avian social organization. Symposia of the Zoological Society of London, 14, 181–218.
Di Fiore, A., & Suarez, S. A. (2007). Route-based travel and shared routes in sympatric spider and woolly monkeys: cognitive and evolutionary implications. Animal Cognition, 10, 317–329.
Doolan, S. P., & MacDonald, D. W. (1996). Dispersal and extra-territorial prospecting by slender tailed meerkats (Suricata suricatta) in the south-western Kalahari. Journal of Zoology, 240, 59–73.
Emery, N. J., & Clayton, N. S. (2004). Comparing the complex cognition of birds and primates. In L. J. Rogers & G. Kaplan (Eds.), Comparative vertebrate cognition (pp. 3–55). The Hague: Kluwer Academic Press.
Forsman, J. T., Mönkkönen, M., Inkeröinen, J., & Reunanen, P. (1998). Aggregate dispersion of birds after encountering a predator: experimental evidence. Journal of Avian Biology, 29, 44–48.
Garber, P. A. (1989). Role of spatial memory in primate foraging patterns Saguinus mystax and Saguinus fuscicollis. American Journal of Primatology, 19, 203–216.
Girvan, J. R. & Braithwaite, V. A. (1997). Orientation mechanisms in different populations of the three spined stickleback. In Orientation and Navigation – birds, humans and other animals. Oxford: Royal institute of Navigation.
Hamilton, W. D. (1971). Geometry for the selfish herd. Journal of Theoretical Biology, 31, 295–311.
Isbell, L. A., & van Vuren, D. (1996). Differential cost of locational and social dispersal and their consequences for female group-living primates. Behaviour, 133, 1–36.
Isbell, L. A., Cheney, D. L., & Seyfarth, R. M. (1990). Costs and benefits of home range shifts among vervet monkeys (Cercopithecus aethiops) in Amboseli National Park, Kenya. Behavioral Ecology and Sociobiology, 27, 351–358.
Janmaat, K. R. L. (2006). Fruits of enlightenment: Fruit finding strategies in wild mangabey monkeys. Ph.D. thesis, University of St. Andrews.
Janmaat, K. R. L., Byrne, R. W., & Zuberbühler, K. (2006a). Evidence for spatial memory of fruiting states of rain forest fruit in wild ranging mangabeys. Animal Behaviour, 71, 797–807.
Janmaat, K. R. L., Byrne, R. W., & Zuberbühler, K. (2006b). Primates take weather into account when searching for fruit. Current Biology, 16, 1232–1237.
Janmaat, K. R. L., Olupot, W., Chancellor, R. L., Arlet, M. E., & Waser, P. M. (2009). Long-term site fidelity and individual home range shifts in Lophocebus albigena. International Journal of Primatology, 30, 443–466.
Janmaat, K. R. L., Mijer, R., Chapman, C. A., & Zuberbuhler, K. (submitted). The use of fruiting synchrony in foraging mangabeys. Animal Cognition
Janson, C. H. (1998). Experimental evidence for spatial memory in foraging wild capuchin monkeys Cebus apella. Animal Behaviour, 55, 1229–1243.
Janson, C. H., & Di Bitetti, M. S. (1997). Experimental analysis of food detection in capuchin monkeys: effects of distance, travel speed, and resource size. Behavioral Ecology and Sociobiology, 41, 17–24.
Knezevich, M. (1998). Geophagy as a therapeutic mediator of endoparasitism in a free-ranging group of rhesus macaques (Macaca mulatta). American Journal of Primatology, 44, 71–82.
Krebs, C. J. (1988). Population ecology of individuals (pp. 1059–1060). Princeton: Princeton University Press.
Larsen, K. W., & Boutin, S. (1994). Movements, survival and settlement of red squirrel (Tamiasciurus hudsonicus) offspring. Ecology, 70, 214–223.
Manzer, M. B., & Bell, M. B. (2004). Spatial representation of shelter locations in meerkats, Suricata suricatta. Animal Behaviour, 68, 151–157.
Martin, P., & Bateson, P. (1986). Measuring behavior. Cambridge, UK: Cambridge University Press.
Menzel, E. W. (1973). Chimpanzee spatial memory organization. Science, 182, 943–945.
Mermier, C. M., Robergs, R. A., McMinn, S. M., & Heyward, V. H. (1997). Energy expenditure and physiological responses during indoor rock climbing. British Journal of Sports Medicine, 31, 224–228.
Metzgar, L. H. (1967). An experimental comparison of screech owl predation on resident and transient white-footed mice Peromyscus leucopus. Journal of Mammalogy, 48, 387–391.
Milton, K. (1988). Foraging behaviour and the evolution of primate intelligence. In R.W. Byrne and A. Whiten (Eds.), Machiavellian Intelligence: Social expertise and the evolution of intellect in monkeys, apes and humans (pp. 285–305). Oxford: Clarendon Press.
Murray, C. M., Gilby, I. C., Mane, S. V., & Pusey, A. E. (2008). Adult male chimpanzees inherit maternal ranging patterns. Current Biology, 18, 20–24.
Muruthi, P., Altmann, J., & Altmann, S. (1991). Resource base, parity, and reproductive condition affect females’ feeding time and nutrient intake within and between groups of a baboon population. Oecologia, 87, 467–472.
Newton-Fisher, N. E. (2003). The home range of the Sonso community of chimpanzees from the Budongo Forest, Uganda. African Journal of Ecology, 41, 150–156.
Nilsson, J. A. (1989). Causes and consequences of natal dispersal in marsh tit, Parus palustris. Journal of Animal Ecology, 58, 619–636.
Oates, J. F. (1978). Water-plant and soil consumption by guereza monkeys (Colobus guereza): a relationship with minerals and toxins in the diet? Biotropica, 10, 241–253.
Olupot, W. (1998). Long-term variation in mangabey (Cercocebus albigena johnstoni Lydekker) feeding in Kibale National Park, Uganda. African Journal of Ecology, 36, 96–101.
Olupot, W. (1999). Mangabey dispersal and conservation in Kibale National Park, Uganda. Ph.D. thesis, Purdue University.
Olupot, W. (2000). Body mass differences among male mangabeys inhabiting logged and unlogged forest compartments. Conservation Biology, 14, 833–843.
Perneger, T. V. (1998). What’s wrong with Bonferroni adjustments? British Medical Journal, 316, 1236–1238.
Poirier, F. E. (1968). Analysis of a Nilgiri langur (Presbytis johnii) home range change. Primates, 9, 29–43.
Poulsen, J. R., & Clark, C. J. (2007). Predation on mammals by the grey-cheeked mangabey Lophocebus albigena. Primates, 42, 391–394.
Reed, J. M., Boulinier, T., Danchin, E., & Oring, L. W. (1999). Prospecting by birds for breeding sites. Current Ornithology, 15, 189–259.
Roper, T. J., Ostler, J. R., & Conradt, L. (2003). The process of dispersal in badgers Meles meles. Mammal Review, 33, 314–318.
Scheumann, M., & Call, J. (2006). Sumatran orangutans and a yellow-cheeked crested gibbon know what is where. International Journal of Primatology, 27, 575–601.
Shultz, S., Faurie, C., & Noe, R. (2003). Behavioral responses of Diana monkeys to male long-distance calls: changes in ranging, association patterns and activity. Behavioral Ecology and Sociobiology, 53, 238–245.
Singleton, I., & van Schaik, C. P. (2001). Orangutan home range size and its determinants in a Sumatran swamp forest. International Journal of Primatology, 22, 877–911.
Sokal, R. R., & Rohlf, F. J. (1981). Biometry: The principles and practice of statistics in biological research. San Francisco: W. H. Freeman.
Steudel, K. (2000). The physiology and energetics of movement effects on individual and groups. In S. Boinski & P. A. Garber (Eds.), On the move: How and why animals travel in groups (pp. 9–23). Chicago: The University of Chicago Press.
Stevens, J., Rosati, A., Ross, K., & Hauser, M. (2005). Will travel for food: spatial discounting in two new world monkeys. Current Biology, 15, 1855–1860.
Struhsaker, T. T. (1997). Ecology of an African rainforest. Gainseville, FL: University Press of Florida.
Struhsaker, T. T., & Leakey, M. (1990). Prey selectivity by crowned hawk-eagles on monkeys in Kibale Forest, Uganda. Behavioral Ecology and Sociobiology, 26, 435–443.
Struhsaker, T. T., & Leland, L. (1988). Group fission in redtail monkeys (Cercopithecus ascanius) in the Kibale forest, Uganda. In A. Gautier-Hion, F. Bourlière, J. Gautier, & J. Kingdon (Eds.), A primate radiation: Evolutionary biology of the African guenons (pp. 364–388). New York: Cambridge University Press.
Sugiyama, Y. (1960). On the division of a natural troop of Japanese monkeys at Takasakiyama. Primates, 2, 109–148.
Tolman, E. C. (1948). Cognitive maps in rats and men. The Psychological Review 55, 189–208.
Tinbergen, N. (1972). The animal in its world. Cambridge: Harvard Press.
Wallis, S. J. (1979). The socioecology of Cercocebus albigena johnstonii (Lyddeker): An arboreal rainforest monkey. Ph.D. thesis, University of London.
Waser, P. M. (1974). Intergroup interactions in a forest monkey: The mangabey Cercocebus albigena. Ph.D. thesis, the Rockefeller University.
Waser, P. M. (1976). Cercocebus albigena: site attachment, avoidance, and intergroup spacing. American Naturalist, 11, 91–933.
Waser, P. M. (1977). Individual recognition, intragroup cohesion, and intergroup spacing: evidence for sound playback to forest monkeys. Behaviour, 60, 28–74.
Waser, P. M. (1985). Spatial structure in mangabey groups. International Journal of Primatology, 6, 569–580.
Waser, P. M., & Floody, O. (1974). Ranging patterns of the mangabey Cercocebus albigena, in the Kibale Forest, Uganda. Zeitschrift fur Tierpsychologie, 35, 2–101.
Waser, P. M., & Jones, W. T. (1983). Natal philopatry among solitary mammals. Quarterly Review of Biology, 58, 355–390.
Windberg, L. A. (1996). Coyote responses to visual and olfactory stimuli related to familiarity with an area. Canadian Journal of Zoology, 74, 2248–2253.
Zuberbühler K, Janmaat KRL (2010). Foraging cognition in non-human primates. In: Platt ML & Ghazanfar AA (Eds.), Primate Neuroethology (pp. 64–83). Oxford: Oxford University Press.
Acknowledgments
The Wenner-Gren and Leakey Foundation, the University of St. Andrews’ School of Psychology, the Schure-Bijerinck-Popping Foundation of the KNAW, the Stichting Kronendak, the Dobberke Stichting voor Vergelijkende Psychology, the Lucie Burger Stichting, and the Foundation Doctor Catharine van Tussenbroek provided funding to K. R. L. Janmaat. The Leakey Foundation and the University of California, Davis Department of Anthropology provided funding to R. L. Chancellor. We thank the Office of the President, the Uganda National Council for Science and Technology, the Uganda Wildlife Authority, the Makerere University Biological Field Station, and the Kibale Fish and Monkey Project, C. A. Chapman in particular, for logistic support, permission to conduct research in Kibale National Park, and the rainfall and temperature data needed to conduct our analyses. We thank W. Olupot and G. Arlet for sending us their ranging data and for training our assistants. We thank R. Meijer, L. B. Prevot, D. C. M. Wright, J. Rusoke, P. Irumba, R. Kaserengenyu, S. Katusabe, and R. Sabiiti for invaluable assistance in the field. We thank L. A. Isbell, K. Zuberbühler, C. H. Janson, G. Brown, 3 anonymous reviewers, and P. M. Waser in particular for comments and suggestions that significantly improved earlier drafts of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(JPEG 3555 kb)
Rights and permissions
About this article
Cite this article
Janmaat, K.R.L., Chancellor, R.L. Exploring New Areas: How Important is Long-Term Spatial Memory for Mangabey (Lophocebus albigena johnstonii) Foraging Efficiency?. Int J Primatol 31, 863–886 (2010). https://doi.org/10.1007/s10764-010-9433-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-010-9433-3