Abstract
Figs are important resources for frugivores, and Ficus is an ideal taxon for evaluating patterns of primate foraging related to food color. Ficus spp. can be classified as conspicuous (color change from greenish to reddish during ripening) or cryptic (green throughout ripening). To investigate the effect on foraging of color vision phenotype variation for these 2 types of figs, we conducted a 20-mo study on 4 groups of white-faced capuchins (Cebus capucinus) in the Santa Rosa Sector of the ACG, Costa Rica between May 2004 and September 2008. We genotyped all individuals and collected behavioral data on feeding rates, acceptance indices, and foraging sequences. We found a significant effect of fig type; feeding rates and acceptance indices were higher for conspicuous figs than for cryptic figs, and subjects sniffed cryptic figs more often than conspicuous figs. We also found that dichromats sniffed more figs and had longer foraging sequences than trichromats, especially for cryptic figs. Among 6 subtypes of dichromats and trichromats, monkeys possessing the trichromat phenotype with the most spectrally separated L-M opsin alleles showed the highest acceptance index for conspicuous figs, though there were no differences in feeding rates among phenotypes. We conclude: 1) conspicuous figs are visually salient not only for trichromats but also for dichromats, 2) olfaction is important for evaluating edibility of cryptic figs, and 3) the reliance on olfaction for selecting fruit is greater in dichromats. These results indicate divergent foraging strategies among color vision phenotypes for assessing food items.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Most species of Neotropical monkeys (Jacobs 1997; Jacobs and Blakeslee 1984; Jacobs and Deegan 2005) and several species of strepsirhines (Tan and Li 1999; Veilleux and Bolnick 2008) have multiple alleles of the long to mid-wavelength sensitive (L-M or red-green) opsin gene on the X chromosome in addition to a single-allelic short-wavelength sensitive (S or blue) opsin gene on an autosome, enabling polymorphic color vision. Heterozygous females on the L-M opsin possess trichromatic vision, whereas males and homozygous females possess dichromatic (red-green color deficient) vision (Jacobs and Blakeslee 1984; Mollon et al. 1984). In platyrrhine monkeys, it is widely supported that polymorphism is maintained by balancing selection (Boissinot et al. 1998; Cropp et al. 2002; Surridge and Mundy 2002), although the mechanisms remain poorly understood.
Color vision seems to be suited for finding food in the forest (Mollon 1989; Surridge et al. 2003), and researchers have linked trichromacy among catarrhine primates to both frugivory (Osorio et al. 2004; Osorio and Vorobyev 1996; Sumner and Mollon 2000a,b) and folivory (Dominy 2004a; Dominy and Lucas 2001; Lucas et al. 1998, 2003). However, a frugivory hypothesis is a more likely explanation for many Neotropical monkeys, e.g., marmosets and capuchins, because they rarely eat young leaves (Caine and Mundy 2000; Fragaszy et al. 2004). Trichromacy could be beneficial for both the detection and selection of desirable fruits because fruits are often conspicuous in color to a trichromat against background leaves (Osorio et al. 2004; Smith et al. 2003; Sumner and Mollon 2000a), and fruit ripening is often associated with green to reddish (reds, oranges, yellows) color changes that in turn are positively associated with desirable nutrients or fleshiness (Gautier-Hion et al. 1985; Riba-Hernandez et al. 2005). If this is true, then trichromacy could confer a fitness advantage selecting for heterozygote females and maintaining the opsin polymorphism. Some evidence has supported the frugivory hypothesis. Several studies using visual models suggest that fruits should be more detectable to trichromats than to dichromats (Hiramatsu et al. 2008; Regan et al. 2001; Sumner and Mollon 2000a). Other studies on captive monkeys (Caine and Mundy 2000; Smith et al. 2003) using naturalistic stimuli have shown that trichromatic monkeys can find and eat conspicuously colored food items faster than dichromats can. In contrast, studies on wild primates have not found that trichromats feed at faster rates or have higher net energy gain than dichromats, even when foraging on fruits that are of colors for which trichromacy is predicted to be advantageous (Hiramatsu et al. 2008; Vogel et al. 2007). Further, many fruits eaten by primates are not conspicuously colored, but instead are green or brown when ripe (Dominy 2004a; Dominy et al. 2003; Janson 1983; Knight and Siegfried 1983) so that the green-red color channel does not always assist trichromatic primates in discriminating edible from inedible fruits or in detecting nutritional rewards (Dominy and Lucas 2004).
Trichromats should not have a foraging advantage for cryptically colored (green or brown) fruit; instead they may be encumbered. For example, trichromatic marmosets had reduced foraging performance when the food items and background were red-green color-camouflaged (Caine et al. 2003). Dichromatic primates may have an advantage over trichromats for detection tasks involving breaking crypsis. This advantage may occur because dichromats may experience less chromatic interference with perception of shape, texture, borders, or luminance (Morgan et al. 1992; Saito et al. 2005), or because the luminance signal is corrupted in trichromats due to the differing spectral inputs (Osorio et al. 2004; Osorio and Vorobyev 1996). The relative abilities of dichromats and trichromats to detect color camouflaged versus conspicuous foods may affect foraging ability (Melin et al. 2007) and has the potential to impact foraging choices (Caine et al. 2003; Melin et al. 2008).
It is possible that dichromats and trichromats adapt behaviorally to their sensory capabilities. Trichromats may be more affected by red-green color camouflage because they have learned to rely on green to red color signals for differentiating food items (Lovell et al. 2005; Mollon 1989). Conversely, dichromats may be more reliant on blue-yellow or achromatic visual cues, such as food luminance, shape, size, or orientation, when foraging because they do not have the option of using red-green color signals. Recent behavioral and spectrometric evidence indicates that achromatic signals can also be useful to foraging primates under some conditions. Dichromatic spider monkeys use fruit luminance cues to perform as efficiently as trichromatic monkeys for short-range fruit foraging in their natural habitat (Hiramatsu et al. 2008).
Color vision is clearly not the only sense available to foraging primates. On the contrary, “detecting and selecting fruits on the basis of cues other than color is a persistent theme in primate evolution” (Dominy 2004b: 295). Although the use of nonvisual senses by primates is receiving attention (Dominy 2004b; Dominy et al. 2006), there remains a dearth of information on how wild primates use their senses of touch, smell, taste, and hearing to select fruit. Next to vision, olfaction is probably the most informative sense to primates for fruit selection (Smith et al. 2003). Ethanol plays an important role in regulating foraging behavior (Dudley 2004) in primates, and their olfactory systems are well suited for detecting even low levels of ethanol (Laska et al. 2000). The concept of enhancing visual acuity and color perception at the cost of olfaction is common in discussions of primate evolution (Ciochon and Fleagle 1987). For example, primates with routine trichromatic color vision may have fewer olfactory genes than other primates do (Gilad et al. 2007; Go and Niimura 2008; Nei et al. 2008), and many vertebrates have either a well-developed sense of smell, e.g., dogs, or of vision, e.g. raptors, indicating a trend toward an evolutionary trade-off. However, more recent evidence suggests that evolutionary relationships among the senses may be more complex than previously believed (Dominy et al. 2004; Laska et al. 2000). We know little about how different species rely on different senses, and how these are integrated during foraging. Recently, Hiramatsu et al. (2009) found that spider monkeys rely increasingly on their sense of smell when visual cues are less informative. It has also been reported that when primates smell fruits they also use digital and dental evaluation of texture in conjunction with sniffing (Dominy 2004b). It is yet uninvestigated whether dichromats and trichromats differ in their use of nonvisual senses while foraging.
To address some of the aforementioned issues, we genotyped and observed the fig foraging behaviors of 4 groups of white-faced capuchins (Cebus capucinus) in the Santa Rosa Sector of the Area de Conservación Guanacaste, Costa Rica. We chose fig foraging because there is a color polymorphism within Ficus. At our study site, like others in Central America (Kalko et al. 1996), several fig species change from green to red as they ripen (conspicuous figs), while others always remain green (cryptic figs). This provides an excellent natural control for fruit-level characteristics that could affect food processing and feeding time, such as type of protective covering, part of fruit eaten, and gross size differences in either the fruits or their seeds. We also selected figs because they are important resources for primates and many other vertebrates in tropical regions (Gautier-Hion et al. 1985; Janzen 1979; Terborgh 1983). Specifically, figs are an important resource to the capuchins at our study site, with ranging patterns of capuchin groups significantly affected by the presence of fruiting figs in their home ranges (Parr et al. 2009).
We investigated the use of figs relative to other fruits in the diet of the capuchins, whether color vision type affects ability to detect and accurately select figs, use of nonvisual senses during foraging, and the extent to which monkeys integrated multiple senses during fruit foraging. Deconstructing foraging sequences in this manner allows us to consider the importance of the individual components, provides us with foraging measures other than simply measuring feeding rate alone, and allows us to detect variation in foraging strategies between dichromats and trichromats. Further, in prior studies, Melin et al. (2008); Rose (1994), and Vogel (2005) found that sex, age, and social dominance have important impacts on the foraging behaviors of capuchin monkeys in prior studies. We therefore include these variables in our analyses to evaluate and, when possible, control for their effects. Assessing the impact of sex, age, and social dominance allows us to assess more accurately the importance of color vision variation in the foraging behavior of capuchins.
If figs are an important resource, then we predict that they will constitute a large percentage (>10%) of the total fruit eaten in an annual cycle. If figs are a preferred resource, then we predict that they will be overselected relative to their abundance. We also predict that foraging behaviors will differ between conspicuous and cryptic types of figs and that monkeys will use nonvisual senses, i.e., biting, sniffing, and touching and longer foraging sequences more often when investigating cryptic figs. Our prediction for trichromatic monkeys is that they will have a foraging advantage for conspicuous, but not cryptic, figs and that when they are in conspicuous fig trees, trichromats will exhibit higher attempt rates, acceptance indices, and feeding rates than dichromats. For dichromatic monkeys, we predict a foraging advantage for cryptic (but not conspicuous) figs, and that when they are in cryptic fig trees dichromats will exhibit higher attempt rates, acceptance indices, and feeding rates than dichromats. Finally, we predict that dichromats and trichromats will differ in their use of nonvisual senses. Dichromats will rely less on their sense of vision, and will bite, sniff, and touch figs more often and exhibit longer foraging sequences than trichromats.
Methods
Study Site and Subjects
We observed 4 groups (Cerco de Piedra [CP], Exclosure [EX], Guanacaste [GN], and Los Valles [LV]) of free-ranging white-faced capuchins inhabiting the Santa Rosa Sector of the Area de Conservación Guanacaste (ACG) in Northwestern Costa Rica. The area consists of tropical dry forest in various stages of regeneration and is highly seasonal for precipitation. We conducted observations over 20 mo between May 2004 and September 2008 for a total of 2708 contact hours. We collected 10 mo of data in each of the seasons, rainy (May–November) and dry (December–April).
Focal individuals were fully habituated and individually recognizable. The sizes of our study groups were as follows: CP (19–25); LV (19–21); EX (8–11); and GN (27–35). Variation in group size was due to births, deaths, immigrations, and emigrations. For the first 7 mo of the study, we followed CP and LV groups. We followed each group for ca. 8 d each month and an average of 10 h each day. For the last 13 mo of the study, we followed all 4 groups with 2–4 full-day follows (dawn to dusk, ca. 13 h/d) per group each month.
We studied 31 dichromat males, 15 dichromat females, and 26 trichromat females (Table I). We performed color vision genotyping from fecal samples using established protocols (Hiramatsu et al. 2005; Surridge et al. 2002) and required 2 identical results from different fecal samples to assign a color vision phenotype. We minimized the chance of mistakenly classifying a heterozygote female as a homozygote due to allelic dropout by requiring that ≥1 fecal sample contained no less than 200 pg of genomic DNA in the polymerase chain reaction (PCR; Hiramatsu et al. 2005; Morin et al. 2001). As in previous studies, we found 3 L-M opsin alleles with peak sensitivities of 532 nm, 543 nm, and 561 nm (Hiramatsu et al. 2005), which we refer to as green-, yellow-, and red-sensitive opsins, respectively. Because all individuals possess the S opsin, we categorized trichromats into 3 subtypes based on their 2 L-M opsin alleles possessed per female individual—green/red, green/yellow, or yellow/red—and dichromats into 3 subtypes based on their single L-M opsin allele: green, red, or yellow.
Behavioral Data Collection
We collected behavioral data using Behavior© on hand-held PSION© Workabout computers. When the group entered a fig tree we conducted consecutive focal animal samples (Altmann 1974) on as many individuals as possible. While the focal individual was visually foraging, i.e., directing its gaze at nearby (<1 m) tree branches, trunks, or foliage, we recorded each time a fig was investigated via touching, biting, sniffing, or close visual inspection behaviors. For each fig fruit investigated, we also recorded the sequence and the total number of the different behavioral events used. An investigation sequence always ended with the focal individual either eating or rejecting the fig. To minimize the chance of missing investigation events, we recorded data only when we had an unobstructed view of the subject’s face and whenever possible, 2 observers (1 caller, 1 recorder) would watch the focal monkey simultaneously from slightly different locations. If we could not clearly see all of the investigation and the eat/reject events, we discarded the sample.
We determined the lengths of our focal individual samples by the visibility of the subject in the fig tree and they varied from 1 to 5 min. Subjects would often move from an area of good visibility, where we would begin our observation, to an area of poor visibility, at which point we would end our sample. We also ended our sample if the focal individual ceased foraging for >30 s. We discarded extremely short focal samples (<60 s). Our choice of focal subjects was also largely determined by the visibility of the monkeys in the fig tree, but we attempted to observe each monkey in the fig tree at least once per group visit to that tree. We compared interobserver reliability frequently (every 3–7 d) to maintain consistency.
For 12 mo (each month of the year represented) from January 2007 to August 2008 we also conducted scan sampling (Altmann 1974). We scanned the group every 30 min during all contact hours to obtain activity and frugivory budgets. During each scan we recorded the behavior (foraging, resting, social, or traveling) for each monkey that we could locate within a 10-min period. For group EX we usually encountered every individual, for groups CP and LV ca. 80% of individuals, and for group GN 70% of individuals. To minimize under-representation of peripheral group members, we started each scan with a different monkey. If the monkey was foraging we recorded the type of food (invertebrate prey, vertebrate prey, vegetative, or other). For vegetative food items we recorded the species of plant with the help of local botanists.
Fig Classification
The capuchins at our study site ate 6 different Ficus spp. that we grouped into 2 categories (Table II) based on the characteristics of their fruit. (Technically a fig is an enclosed infructescence, but for the purposes of this paper we refer to it as a fruit.) We defined conspicuous figs as those that undergo a color change from green to red as they ripen. This change makes ripe figs conspicuous (to trichromatic viewers) relative to leaves and to unripe fruit. The conspicuous fig group also tends to be smaller (ca. 1 cm diameter) and have a slight odor at maturity. The fig species in the cryptic category do not change color as they ripen. Rather, they always remain green and the ripening process is obscure to viewers, rendering these figs camouflaged in color relative to the background leaves. Figs in the cryptic group tended to be slightly larger (ca. 2–3 cm diameter) and have a stronger odor at maturity. We classified fig odors as slight or strong based on subjective assessment by several human observers at the study site. We provide photographs and reflectance plots of sample figs and leaves for conspicuous and cryptic species in Fig. 1.
In Fig. 2 we present chromaticity diagrams of the surface reflectance values for figs of each species, as well as upper and lower leaf surfaces. For conspicuous species, we plot the reflectance values for both ripe and unripe fruit. For cryptic species, we plot only 1 value because ripe and unripe fruit are similar in color. The distance between 2 points along the x-axis represents the extent to which they differ from each other along the red-green chromatic channel, which is available to trichromats only. The distance between points along the y-axis represents variation in the blue-yellow chromatic channel, which is available to both dichromats and trichromats. The 3 separate diagrams in Fig. 2a, b, and c represent the 3 possible trichromatic phenotypes. Variation in the spread of points along the x-axis is due to variation in spectral sensitivity of their long to mid-wavelength sensitive cones.
Chromaticity diagrams showing the variation in surface reflectance values of the fruits and leaves of 6 Ficus spp. (calculations follow Hiramatsu et al. 2008). The x-axis represents the red-green chromatic channel (L/(M + L) and the y-axis the blue-yellow chromatic channel (S/(M + L) for 3 trichromatic subtypes. These have peak opsin sensitivities of a 426 nm (Jacobs and Deegan 2003), 532 nm, and 561 nm (green/red); b 426 nm, 543 nm, and 561 nm (yellow/red); and c 426 nm, 532 nm, and 543 nm (green/yellow). Each plot contains 5 ripe and 5 unripe figs of the 3 conspicuous species (Ficus cotinifolia, F. hondurensis, and F. ovalis) and 5 ever-greenish figs of the cryptic species (F. bulleni, F. moraziana, and F. obtucifolia) together with upper and lower leaf surfaces for each species.
Fruit Availability
To assess the abundance of figs relative to that of other fruit trees, we recorded the presence of all trees with a diameter at breast height (DBH) of >3 cm (the smallest trees we observed individuals to eat from), in 55 botanical transects that were 100 m × 2 m in size and evenly distributed across the home ranges of the 4 capuchin groups (transect total area = 1.06 ha). To assess food availability, we calculated the sum of the DBH (cm)/ha for each species of fruit tree we observed the monkeys to eat (Chapman and Fedigan 1990).
Data Analysis
Dietary Importance of Figs
To evaluate their ecological relevance for our focal individuals, we estimated the dietary importance and selectivity for Ficus spp. (Moraceae). We defined importance as the proportion of the capuchins’ frugivorous time budget that was occupied by fig foraging. We calculated the percentage of all behavioral scans for which we recorded foraging behavior on figs versus fruit of other species. To assess capuchin preference for figs, we calculated time spent foraging in fig trees relative to their availability, i.e., density of Ficus trees (cm DBH/ha) as a percentage of the density of all fruit tree species consumed by the focal individuals. When more time is spent foraging on a type of fruit than expected from its relative availability, we considered that fruit to be a preferred resource.
Definitions of Foraging Measures for Statistical Analysis
We calculated the feeding rate as no. of fruits eaten/min. We then broke this measure down into its 2 components: the attempt rate (no. of fruits investigated/min) and the acceptance index (no. of fruits eaten/no. of fruits investigated). To assess the use of nonvisual senses, we created a touch index, sniff index, bite index, and visual inspect index. For example, the touch index is no. of figs touched/no. of figs investigated. Finally, to assess how often capuchins used multiple types of investigation together to assess a fruit, we created a length index (no. of long foraging sequences/no. of fig investigations). Long foraging sequences involved ≥2 types of sensory evaluation, e.g., touch and sniff, before the fig was eaten or rejected. Short foraging sequences were those with 1 type of evaluation, e.g., touch only, or if a fig was directly eaten from the branch without any observable prior evaluation.
Statistical Analyses
We used general linear mixed models (LMMs) to analyze our data. We performed separate models to test the effect of fig type alongside 4 other predictor variables: 1) color vision/sex group, 2) color vision subtype, 3) age group, and 4) dominance group, which we modeled against the 8 foraging measures as dependent variables (32 models). For each model, fixed effects were fig type (conspicuous or cryptic), one of the aforementioned predictor variables, and the fig type * predictor variable interaction. We also set fig type as a repeated measure, because the same monkey had foraging values both for cryptic and conspicuous figs. We included individual ID as a random effect in all models. Our method of estimation was maximum likelihood with diagonal covariance structure. We used type III fixed effects (F and t) and set statistical significance of p-values as < 0.05. We removed nonsignificant variables from the model in a stepwise manner. We performed analyses in SPSS 16.0.
Sex and Color Vision Effects
Because color vision and sex group are linked, we split individuals into 3 groups: dichromatic males, dichromatic females, and trichromatic females. To test for a sex effect we compared the values of males to dichromatic females. If there was no significant difference, we compared the combined values of all dichromats to trichromatic females. If a significant sex effect was found, we compared only dichromatic females to trichromatic females. To test for differences among the 6 color vision subtypes we performed a LMM with individuals grouped based on their opsin sensitivities: green, red, yellow, green/red, green/yellow, red/yellow. For these analyses, we included conspicuous figs only and we did not split groups by sex given sample size constraints. To compare the foraging performance among subtypes, we used the estimates of fixed effects to run pairwise comparisons for each of the color vision subtypes relative to the reference subtype, which in SPSS is always the last one listed in the model. Subtypes were then removed from the model sequentially until we compared each subtype with all others.
Age Effects
To quantify the effect of age on foraging, we classified monkeys into 3 groups as: adult (females 6 yr of age or older, males 10 yr of age or older); subadult (males 6–9 yr of age) or juvenile (all individuals <6 years of age; Fedigan et al. 1996). If we detected a significant age effect, we repeated the sex/color vision analysis including adults only.
Dominance Effects
For our social dominance analysis we included only adult females to minimize confounding the effects of sex and age. We classified them as high-, mid-, or low-ranking based on outcomes of dyadic agonistic interactions recorded in our own observations and in a separate study investigating social dominance in these monkey groups (Bergstrom 2009). Reversals in dominance are infrequent in this species and our focal subjects did not change dominance category during the study period. Fortunately, there was a relatively even distribution of dichromats and trichromats among females of different dominance classes (Table I), which minimized the impact of color vision type on our dominance analysis. However, we could not account for any potential interaction effect between dominance rank and color vision phenotype.
Results
Dietary Importance of Figs
In total, we recorded 31,974 scans over 12 mo, which encompassed a full annual cycle. From these data, we calculated activity and frugivory time budgets (Fig. 3). We found that the monkeys spent 15% of their total activity budget searching for and consuming 76 different species of fruit. Of this fruit foraging time, 31% was spent on Ficus spp., and of the fig foraging time the majority (87%) was spent on conspicuous fig species. The cumulative DBH of all fruit tree species in our focal groups’ habitat was 12,460 cm DBH/ha; we found 2 species of Ficus in our transects, F. ovalis and F. cotinifolia, which are both classified as conspicuous figs. They occupied a cumulative density of 196 cm DBH/ha. This is ca.1.6% of the total fruit tree area.
Fig Type Effects
The type of fig, conspicuous versus cryptic, had an impact on several of the foraging measures. All monkeys fed at a faster rate on conspicuous figs (Table III), eating ca. 9 figs more per minute of foraging time (Fig. 4a). We also found that fig type affected the acceptance index; individuals ate significantly more conspicuous figs (>0.9) than cryptic figs (<0.4) subsequent to investigating them (Table III; Fig. 4b). Finally, individuals had a higher attempt rate for conspicuous figs than for cryptic figs. This difference is relatively small (14.29 ± 0.65 vs. 12.16 ± 1.11; mean ± SE) but statistically significant (Table III).
There is a significant main effect of fig type for the sniff, bite, and sequence length indices. Capuchins used nonvisual senses more often when foraging on cryptic figs than on conspicuous figs. They sniffed and bit cryptic figs significantly more often before ingestion or rejection, and they used long foraging sequences (≥2 different types of sensory evaluation) significantly more often when foraging on cryptic versus conspicuous figs (Table III).
Sex and Color Vision Effects
We found 1 significant effect of sex in our analyses. Males bit figs during fruit evaluation significantly more often than females (bite index: F = 3.485, df = 1, 46.638, p = 0.042). Because of this effect, we compared only dichromatic females to trichromatic females to assess the impact of color vision type on bite index (Table III, denoted with a superscript a). For all other analyses, we compared trichromatic females with all dichromats.
We found significant differences between dichromats and trichromats in 4 of our 8 foraging measures: acceptance index, attempt rate, sniff index, and foraging sequence length index (Table III). Trichromatic monkeys had a significantly higher acceptance index. Though the interaction effect of color vision and fig type is not significant, the effect of color vision phenotype was driven by results for conspicuous figs because trichromat females actually had a slightly lower acceptance index than dichromat males did for cryptic figs (Table III). Dichromats had a higher attempt rate, sniffed more figs, and had longer foraging sequences than trichromats did, with all of these effects being especially evident for cryptic fig foraging, and with significant interaction effects between color vision type and fig type (Table III; Fig. 5).
Mean proportion (± SE) of conspicuous and cryptic figs. a Sniffed during evaluation. b Included in long investigation sequences by dichromatic males, dichromatic females, and trichromatic females. Note that in each case 2 scales are provided for each graph, one for conspicuous figs (on the left) and the other for cryptic figs (on the right).
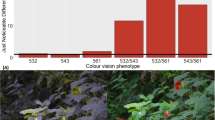
We found a significant main effect of color vision subtype for 1 foraging measure, the acceptance index (F = 3.464, df = 5, 72, p = 0.007). Trichromatic females with the green/red phenotype had the highest acceptance index among the 6 phenotypes (Fig. 6), being significantly higher than both red dichromats (t = 3.811, df = 55, p < 0.001) and yellow dichromats (t = 2.430, df = 65, p = 0.018). Green/red trichromats also had a higher acceptance index than green/yellow trichromats (t = 2.061, df = 27, p = 0.049). Finally, we also found a significant difference among dichromats; green dichromats had a higher acceptance index than red dichromats did (t = 2.390, df = 55, p = 0.020; Fig. 6).
Age Effects
Age has a significant effect on several of the foraging measures. There is a main effect of age on both feeding rate (F = 4.858, df = 2, 80.966, p = 0.010) and attempt rate (F = 4.835, df = 2, 78.909, p = 0.010). Estimates of fixed effects revealed that subadult males had significantly higher rates than both adults (feeding rate, t = 2.022, df = 73.357, p = 0.047; attempt rate, t = 2.076, df = 72.834, p = 0.047) and juveniles (feeding rate, t = 2.870, df = 73.612, p = 0.005; attempt rate t = 2.916, df = 73.003, p = 0.005), whereas adults and juveniles did not significantly differ from each other. Because of these significant age effects, we repeated our color vision analyses including only adult monkeys. Our results did not differ qualitatively when we included adult monkeys only from those obtained when we included all individuals.
Dominance Effects
We found no significant main effect of dominance, nor a significant interaction term, for any of the foraging measures (all p > 0.1).
Discussion
Dietary Importance of Figs
Our findings support the predictions that figs are both an important and a preferred fruit source. We found that figs constituted nearly one-third of the capuchin’s annual frugivory budget and that they were overselected relative to their abundance. The density of fig trees was 196 cm DBH/ha, which falls within the range documented in an earlier study (Chapman and Fedigan 1990). At these densities, figs represent <3% of the fruit tree biomass yet account for >30% of capuchin fruit foraging time. Further, Lemmon and Melin (unpubl. data) found that 75% of all fig trees at the field site were conspicuous species (N = 130 trees), and in the current study we find that 87% of the total fig foraging time was devoted to conspicuous figs (27% of the 31% devoted to all figs). Because the percentage of the time budget devoted to conspicuous versus cryptic figs was higher than expected based on the availability of conspicuous figs, we suggest conspicuous figs are preferred. This may be related to higher feeding rates and foraging accuracy, though it would be interesting also to compare nutritional profiles and energy intake rates for the different fig types.
Different resources can exert different selection pressures on consumer populations. For example, fallback foods, i.e., those consumed during periods when preferred foods are not available, are thought to exert strong selection pressures on morphological adaptations for food processing, such as dentition. Likewise, researchers have suggested that preferred resources exert strong selection pressures on harvesting adaptations, such as resource detection (Marshall and Wrangham 2007). Because researchers have suggested that color vision is an adaptation for both long (Sumner and Mollon 2000a) and short (Parraga et al. 2002) distance fruit detection, its expression may be especially influenced by foraging for preferred resources. If, as we suggest, figs are a preferred resources for the capuchins at our study site, then it is feasible that this resource may affect the expression of trichromacy in the subjects.
Fig Type Effects
In accordance with our prediction, the type of fig did have a marked effect on foraging. Capuchins ate conspicuous figs 3 times faster than they ate cryptic figs, which was attributable predominantly to the higher acceptance rate of the former. When the monkeys were foraging in conspicuous species of fig tree we observed them eating yellow (mid-ripe) and red (ripe) figs, although green, unripe fruits were always present in the tree, usually in greater quantities than riper figs. These results imply that vision may inform foraging attempts on conspicuous figs, and that capuchins may have been visually discriminating among figs before making a foraging attempt. In addition, given that conspicuous figs are more common, and are fed on more often, capuchins may have had a better search image for the individual fruits and as a result, higher foraging accuracy. The high rejection rate of cryptic figs indicates that their edibility was more difficult to discern without direct contact or close-distance evaluation (touching, sniffing, and biting).
We found support for several components of our prediction that capuchins will increase their use of nonvisual senses to investigate the cryptic figs. We found that the monkeys used mechanical, and potentially gustatory, testing with the teeth and jaws through biting, olfactory assessment by sniffing, and they combined ≥2 types of investigation before accepting or rejecting cryptic figs. Therefore, we may conclude that vision alone is insufficient for assessing ripeness in cryptic species and accordingly. Our results here are in accordance with those of Hiramatsu et al. (2009), who found that spider monkeys have lower acceptance indices and sniff fruits more often when the fruits have cryptic ripeness.
Monkeys may have sniffed cryptic figs more often, not only because vision was less useful, but also because odor was an important ripeness cue for figs of these species. Seed dispersal of large, cryptic figs is thought to be primarily by bats (Kalko et al. 1996). Smell is a much more useful attractant to bats, which forage in the dark, than is color, and the stronger odor in ripe cryptic figs was detectable by human researchers during our study. Although fruit characters (such as color, size, odor, and flesh type) are often interrelated traits (Fischer and Chapman 1993; Janson 1983; Regan et al. 2001), it is possible that some cryptic fruit are not odoriferous, and capuchins may not sniff those fruit as often.
Our prediction that cryptic figs would be touched more often than conspicuous figs was not supported. There were no differences in how often monkeys touched conspicuous versus cryptic figs. While touching is indisputably a mode of mechanical investigation (and many figs were rejected after only being touched), using the hands to grasp fruit is also the predominant way that capuchins transport foods to their mouths. Unlike spider monkeys and howlers, capuchins rarely eat figs directly from branches (A. Melin, pers. obs.). Thus, this method of food transportation may mask the importance of fingers as mechanical testers (Dominy 2004b). We also did not find a difference in our visual inspect index between fig types. However, this is the most difficult type of sensory evaluation to measure. We recorded visual inspections only after the fruit was handled, because we could not say with certainty if individual figs were inspected visually before this. We consequently had few observations of this behavior.
Sex and Color Vision Effects
Our results support one of our predictions for a trichromatic advantage. Trichromats have a higher acceptance index than dichromats do, which suggests that they have a superior ability for selecting edible (riper) figs. Further, the results from our analyses comparing the different dichromatic and trichromatic subtypes for conspicuous figs indicate that the primary advantage is to green/red trichromats. This is in accordance with the information presented in the chromaticity diagrams (Fig. 2), which show that for conspicuous Ficus spp., the chromatic difference between ripe and unripe figs is most salient for the phenotype with the widest spectral separation in opsin sensitivities, i.e., the green/red subtype (Fig. 2a). This supports the hypothesis that larger separations in cone sensitivities are beneficial to trichromats (Rowe and Jacobs 2004). Red-green chromatic contrasts are also more salient to birds than humans because of the larger spectral separation between the L-M cones in the former (Lovell et al. 2005). If L-M cone separation affects the acceptance index, this may potentially explain why trichromatic spider monkeys, which have a smaller spectral separation in opsins than capuchins, do not seem to accept more fruit than dichromats (Hiramatsu et al. 2008).
The higher acceptance index scores could represent a foraging advantage to trichromats, especially the green-red subtype; however, we did not find any significant differences in feeding rates attributable to color vision. The reason is that dichromatic monkeys investigated more figs per unit time, indicating that both red-green contrast and brightness or blue-yellow contrasts were useful for detecting fruit from foliage, as Hiramatsu et al. (2008) have demonstrated for spider monkeys. That dichromats and trichromats did not differ in feeding rates is consistent with other studies published on free-ranging monkeys with polymorphic color vision (Hiramatsu et al. 2008; Vogel et al. 2007). The biological relevance of higher foraging accuracy of trichromats is unclear because it does not translate into eating more fruits per minute of foraging, though it may conserve effort.
Our results support one of our predictions for dichromat advantage. Dichromats had a higher attempt rate for cryptic figs than trichromats did, which may indicate that dichromats were able to detect more figs. However, dichromats did not eat cryptic figs at a faster rate or have a higher acceptance index than trichromats and thus we cannot argue for an overall advantage in cryptic fruit foraging. Previously, Melin et al. (2007) found that dichromats have a foraging advantage for catching surface-dwelling insects However, the insect rejection rates were very low in that study, whereas the cryptic fruit rejection rates were well over 50% for the fig species in our study. A dichromat advantage for fruit foraging may be restricted to cryptic species that have high acceptance indices, perhaps for those for which size or shape provide good ripeness cues.
We found that dichromatic monkeys sniffed both conspicuous and cryptic figs more often than trichromatic monkeys did, which supports our prediction that dichromats use nonvisual senses more often than trichromats do. This may be especially prevalent when the monkeys are foraging on odoriferous fruit, such as the large, green figs at our study site. Likewise, increased use of nonvisual senses explains why dichromats used longer, more diverse foraging sequences when assessing cryptic food items. Interestingly, the use of longer evaluations for figs did not decrease the feeding rate of dichromats relative to trichromats. An explanation for this could be that evaluation events—touching, sniffing, and biting—do not take long to complete and that feeding rates for cryptic figs are limited by factors other than food assessment time, such as time spent searching for, processing, or consuming figs. We cannot yet discount the idea that observed difference in sniff and length indices between trichromats and dichromats may reflect the amount of chromatic and achromatic visual information available to monkeys with different phenotypes. Further study is necessary to substantiate this possibility by analyzing colorimetric properties of figs and other fruits in depth.
Taken together, our results indicate that trichromats may have an advantage in discriminating between ripe and unripe figs, and that dichromats may compensate for their chromatic deficiency by increasing foraging effort, i.e. attempt rates, and by using their other senses more to achieve the same net feeding rate as trichromats. It is possible that the improved discrimination ability of trichromats may translate into higher feeding rates, and more relevantly higher energy intake rates, for trichromats foraging on other types of fruit. In species in which fewer fruit are present in the tree, where resources might deplete more quickly, increased intragroup feeding competition may manifest trichromat advantages. This idea requires substantiation from other field studies, ideally on several different primate species.
Age Effects
Age affected foraging, with subadult males unexpectedly having higher feeding and investigation rates than adults and juvenile monkeys. We did not anticipate this result because young primates, being less experienced, often have lower foraging success than adults (Janson and Van Schaik 1993). A possible explanation may be that subadult male capuchin forage quickly so that they can allocate more time to high-energy activities such as vigilance for and deterrence of neighboring monkey groups and predators, or that subadults are still growing, with large body mass important in male-male competition, or finally that they may be supplanted by the higher-ranking adults which may engender fast foraging (Fragaszy et al. 2004; Jack and Fedigan 2006).
Dominance Effects
Researchers have found that high social dominance rank increases feeding and energy intake rates in white-faced capuchins and in other primate species (Koenig 2000; Saito 1996; Vogel 2005). However, our results differ from studies that find a link between dominance rank and feeding advantages. Most of the fig trees capuchins visited were extremely large, having trunks greater than 3 m in circumference. The fruit crops of these trees were abundant and may not be easily monopolized by dominant individuals. Further, in large fruit trees individuals may satiate and leave the resource available to others. It is possible that dominance was still manifested around figs, e.g., in priority of access to the tree or access to preferred foraging locations (Janson 1990) such as lower branches, where aerial predation risk is lower (Miller 2002).
Conclusions
Fig trees are an important resource to capuchins at our field site and in all likelihood throughout Costa Rica. When foraging in fig trees, dichromatic and trichromatic monkeys used divergent foraging strategies to achieve the same net fig intake. Dichromatic capuchins sniffed figs more often and used longer, more diverse foraging sequences than trichromatic monkeys did. We also found that trichromats in general, and especially red/green trichromatic subtypes, had a higher acceptance index for conspicuous figs than dichromats did, indicating that such trichromats can make more accurate initial visual assessments. However, these differences did not translate into higher feeding rates, so we cannot argue that trichromats have a clear foraging advantage over dichromats. It is plausible that trichromats may benefit from their higher accuracy via lower overall foraging effort, or that they may have higher feeding rates in tree species where ripe fruit is less available or quickly depleted. However, these ideas require substantiation in future research efforts.
References
Altmann, J. (1974). Observational study of behaviour: sampling methods. Behaviour, 49, 227–265.
Bergstrom, M. L. (2009). Dominance among female white-faced capuchins at Santa Rosa National Park, Costa Rica. M.A. Thesis, University of Calgary.
Boissinot, S., Tan, Y., Shyue, S.-K., Schneider, H., Sampaio, I., Neiswanger, K., et al. (1998). Origins and antiquity of x-linked triallelic color vision systems in New World monkeys. Proceedings of the National Academy of Sciences, USA, 95, 13749–13754.
Caine, N. G., & Mundy, N. I. (2000). Demonstration of a foraging advantage for trichromatic marmosets (Callithrix geoffroyi) dependant on food colour. Proceedings of the Royal Society of London Series B Biological Sciences, 267, 439–444.
Caine, N. G., Surridge, A. K., & Mundy, N. I. (2003). Dichromatic and trichromatic Geoffrey’s marmosets (Callithrix geoffrey) differ in relative foraging ability for red-green color-camouflaged and non-camouflaged food. International Journal of Primatology, 1163–1175.
Chapman, C., & Fedigan, L. (1990). Dietary differences between neighbouring Cebus capucinus groups: Local traditions, food availability or responses to food profitability? Folia Primatologica, 54, 177–186.
Ciochon, R., & Fleagle, J., (Eds.) (1987). Primate evolution and human origins. Hawthorne, New York: Aldine de Gruyter. 395 p.
Cropp, S., Boinski, S., & Li, W.-H. (2002). Allelic variation in the squirrel monkey x-linked color vision gene: biogeographical and behavioral correlates. Journal of Molecular Evolution, 54, 734–745.
Dominy, N. J. (2004a). Color as an indicator of food quality to anthropoid primates: Ecological evidence and an evolutionary scenario. In C. Ross & R. F. Kay (Eds.), Anthropoid origins: New visions. New York: Kluwer Academic.
Dominy, N. J. (2004b). Fruits, fingers and fermentation: the sensory cues available to foraging primates. Integrative and Comparative Biology, 44, 295–303.
Dominy, N. J., & Lucas, P. W. (2001). Ecological importance of trichromatic vision to primates. Nature, 410(6826), 363–366.
Dominy, N. J., & Lucas, P. W. (2004). Significance of color, calories and climate to the visual ecology of catarrhines. American Journal of Primatology, 62(3), 189–207.
Dominy, N. J., Svenning, J.-C., & Li, W.-H. (2003). Historical contingency in the evolution of primate color vision. Journal of Human Evolution, 44, 25–45.
Dominy, N., Ross, C., & Smith, T. (2004). Evolution of the special senses in primates: past, present and future. Anatomical record, 281A, 1078–1082.
Dominy, N., Lucas, P. W., & Supardi, N. N. (2006). Primate sensory systems and foraging behavior. In G. Hohmann, M. Robbins & C. Boesch (Eds.), Feeding ecology in apes and other primates: Ecological, physiological and behavioural aspects (pp. 489–509). Cambridge: Cambridge University Press.
Dudley, R. (2004). Ethanol, fruit ripening, and the historical origins of human alcoholism in primate frugivory. Integrative and Comparative Biology, 44, 315–323.
Fedigan, L. M., Rose, L. M., & Morera, R. A. (1996). See how they grow. Track capuchin monkey populations in a regenerating Costa Rican dry forest. In M. A. Norconk, A. L. Rosenberger & P. Garber (Eds.), Adaptive radiations of neotropical primates (pp. 289–307). New York: Plenum Press.
Fischer, K. E., & Chapman, C. A. (1993). Frugivores and fruit syndromes: differences in patterns at the genus and species level. Oikos, 66, 472–482.
Fragaszy, D., Visalberghi, E., & Fedigan, L. M. (2004). The complete capuchin monkey. Cambridge, UK: Cambridge University Press.
Gautier-Hion, A., Duplantier, J.-M., Quris, F. F., Sourd, C., Decoux, J.-P., Dubost, G., et al. (1985). Fruit characters as a basis of fruit choice and seed dispersal in a tropical forest vertebrate community. Oecologia, 65, 324–337.
Gilad, Y., Wiebe, V., Przeworski, M., Lancet, D., & Paabo, S. (2007). Correction: loss of olfactory receptor genes coincides with the acquisition of full trichromatic vision in primates. PLoS Biology, 5, e148.
Go, Y., & Niimura, Y. (2008). Similar numbers but different repertoires of olfactory receptor genes in humans and chimpanzees. Molecular Biology and Evolution, 25, 1897–1907.
Hiramatsu, C., Tsutsui, T., Matsumoto, Y., Aureli, F., Fedigan, L. M., & Kawamura, S. (2005). Color vision polymorphism in wild capuchins (Cebus capucinus) and spider monkeys (Ateles geoffroyi) in Costa Rica. American Journal of Primatology, 67(4), 447–461.
Hiramatsu, C., Melin, A. D., Aureli, F., Schaffner, C. M., Vorobyev, M., Matsumoto, Y., et al. (2008). Importance of achromatic contrast in short-range fruit foraging of primates. PLoS One, 3(10), 1–12.
Hiramatsu, C., Melin, A. D., Aureli, F., Schaffner, C. M., Vorobyev, M., & Kawamura, S. (2009). Interplay of olfaction and vision in fruit foraging of spider monkeys. Animal Behaviour, 77, 1421–1426.
Jack, K. M., & Fedigan, L. M. (2006). Why be alpha male? Dominance and reproductive success in wild white-faced capuchins (Cebus capucinus). In A. Estrada, P. A. Garber, M. S. M. Pavelka & L. Luecke (Eds.), New perspectives in the study of mesoamerican primates. Chicago: Springer US.
Jacobs, G. H. (1997). Color vision polymorphisms in New World monkeys: Implications for the evolution of primate trichromacy. In W. G. Kinzey (Ed.), New World primates: Ecology, evolution and behaviour (pp. 45–74). New York: Walter de Gruyter, Inc.
Jacobs, G., & Blakeslee, B. (1984). Individual variation in color vision among squirrel monkeys (Samiri sciureus) of different geographical origins. Journal of Comparative Psychology, 98(4), 347–357.
Jacobs, G. H., & Deegan, I. J. F. (2003). Cone pigment variations in four genera of New World monkeys. Vision Research, 43, 227–236.
Jacobs, G., & Deegan, J. (2005). Polymorphic New World monkeys with more than three M/L cones. Journal of the Optical Society of America A, 22(10), 2072–2079.
Janson, C. (1983). Adaptation of fruit morphology to dispersal agents in a neotropical forest. Science, 219, 187–189.
Janson, C. H. (1990). Ecological consequences of individual spatial choice in foraging groups of brown capuchin monkeys, Cebus apella. Animal Behaviour, 40, 922–934.
Janson, C. H., & Van Schaik, C. P. (1993). Ecological risk aversion in juvenile primates: Slow and steady wins the race. In M. E. Pereira & L. A. Fairbanks (Eds.), Juvenile primates life history, development and behavior (p. 428). New York: Oxford University Press.
Janzen, D. H. (1979). How to be a fig. Annual Review of Ecology Systematics, 10, 13–51.
Kalko, E. K. V., Herre, E. A., & Handley, C. O., Jr. (1996). Relation of fig fruit characteristics to fruit-eating bats in the New and Old World tropics. Journal of Biogeography, 23(4), 565–576.
Knight, R. S., & Siegfried, W. R. (1983). Inter-relationships between type, size and colour of fruits and dispersal in southern African trees. Oecologia, 56, 405–412.
Koenig, A. (2000). Competitive regimes in forest-dwelling Hanuman langur females (Semnopithecus entellus). Behavioral ecology and sociobiology, 48, 93–109.
Laska, M. S., Seibt, A., & Weber, A. (2000). “Microsmatic” primates revisited: olfactory sensitivity in the squirrel monkey. Chemical Senses, 25, 47–53.
Lovell, P. G., Tolhurst, D. J., Parraga, C. A., Baddeley, R., Leonards, U., Troscianko, J., et al. (2005). Stability of the color-opponent signals under changes of illuminant in natural scenes. Journal of the Optical Society of America A, 22(10), 2060–2071.
Lucas, P. W., Darvelle, B. W., Lee, P. K. D., Yuen, T. D. B., & Choong, M. F. (1998). Colour cues for leaf food selection by long-tailed macaques (Macaca fascicularis) with a new suggestion for the evolution of trichromatic colour vision. Folia Primatologica, 69, 139–152.
Lucas, P. W., Dominy, N. J., Riba-Hernandez, P., Stoner, K., Yamashita, N., Loria-Calderon, E., et al. (2003). Evolution and function of routine trichromatic vision in primates. Evolution, 57(11), 2636–2643.
Marshall, A., & Wrangham, R. (2007). Evolutionary consequences of fallback foods. International Journal of Primatology, 28(6), 1219–1235.
Melin, A., Fedigan, L., Hiramatsu, C., Sendall, C., & Kawamura, S. (2007). Effects of colour vision phenotype on insect capture by a free-ranging population of white-faced capuchins (Cebus capucinus). Animal Behaviour, 73(1), 205–214.
Melin, A. D., Fedigan, L. M., Hiramatsu, C., & Kawamura, S. (2008). Polymorphic color vision in white-faced capuchins (Cebus capucinus): is there foraging niche divergence among phenotypes? Behavioral Ecology and Sociobiology, 62, 659–670.
Miller, L. (Ed.) (2002). Eat or be eaten. Cambridge: Cambridge University Press, 297 pp.
Mollon, J. D. (1989). “Tho’ she kneel’d in that place where they grew...” The uses and origins of primate color vision. Journal of Experimental Biology, 146, 21–38.
Mollon, J. D., Bowmaker, J. K., & Jacobs, G. H. (1984). Variations of color vision in a New World primate can be explained by polymorphism of retinal photopigments. Proceedings of the Royal Society of London Series B: Biological Sciences, 222, 373–399.
Morgan, M. J., Adam, A., & Mollon, J. D. (1992). Dichromats detect colour-camouflaged objects that are not detected by trichromats. Proceedings of the Royal Society of London Series B: Biological Sciences, 248, 291–295.
Morin, P. A., Chambers, K. E., Boesch, C., & Vigilant, L. (2001). Quantitative polymerase chain reaction analysis of DNA from noninvasive samples for accurate microsatellite genotyping of wild chimpanzees (Pan troglodytes verus). Molecular Ecology, 10, 1835–1844.
Nei, M., Niimura, Y., & Nozawa, M. (2008). The evolution of animal chemosensory receptor gene repertoires: roles of chance and necessity. Nature Reviews Genetics, 9, 951–963.
Osorio, D., & Vorobyev, M. (1996). Colour vision as an adaptation to frugivory in primates. Proceedings of the Royal Society of London Series B: Biological Sciences, 263, 593–599.
Osorio, D., Smith, A. C., Vorobyev, M., & Buchanan-Smith, H. M. (2004). Detection of fruit and the selection of primate visual pigments for color vision. American Naturalist, 164(6), 696–708.
Parr, N., Melin, A. D., & Fedigan, L. (2009). How fruiting fig trees affect the ranging behavior of wild white-faced capuchins (Cebus capucinus) in Santa Rosa National Park, Costa Rica. 32nd Meeting of the American Society of Primatologists San Diego, California.
Parraga, C. A., Troscianko, T., & Tolhurst, D. J. (2002). Spatiochromatic properties of natural images and human vision. Current Biology, 12, 483–487.
Regan, B. C., Julliot, C., Simmen, B., Vienot, F., Charles-Dominique, P., & Mollon, J. D. (2001). Fruits, foliage and the evolution of primate colour vision. Philosophical Transactions of the Royal Society of London Series B Biological Sciences, 356, 229–283.
Riba-Hernandez, P., Stoner, K. E., & Lucas, P. W. (2005). Sugar concentration of fruits and their detection via color in the Central American spider monkey. American Journal of Primatology, 67(4), 411–423.
Rose, L. M. (1994). Sex differences in diet and foraging behaviour in white-faced capuchins (Cebus capucinus). International Journal of Primatology, 15(1), 95–114.
Rowe, M. P., & Jacobs, G. H. (2004). Cone pigment polymorphism in New World Monkeys: are all pigments created equal? Visual Neuroscience, 21, 217–222.
Saito, A., Kawamura, S., Mikami, A., Ueno, Y., Hiramatsu, C., Koida, K., et al. (2005). Demonstration of genotype-phenotype correlation in polymorphic color vision of a non-callitricine New World monkey, capuchin Cebus apella. American Journal of Primatology, 67(4), 471–485.
Saito, C. (1996). Dominance and feeding success in female Japanese macaques, Macaca fuscata: effects of food patch size and interpatch distance. Animal Behaviour, 51, 967–980.
Smith, A. C., Buchanan-Smith, H. M., Surridge, A. K., Osorio, D., & Mundy, N. I. (2003). The effect of color vision on the detection and selection of fruits by tamarins (Saguinus spp.). Journal of Experimental Biology, 206, 3159–3165.
Sumner, P., & Mollon, J. D. (2000a). Catarrhine photopigments are optimized for detecting targets against a foliage background. Journal of Experimental Biology, 203, 1963–1986.
Sumner, P., & Mollon, J. D. (2000b). Chromacy as a signal of ripeness in fruits taken by primates. Journal of Experimental Biology, 203, 1987–2000.
Surridge, A. K., & Mundy, N. I. (2002). Trans-specific evolution of opsin alleles and the maintenance of trichromatic colour vision in Callitrichine primates. Molecular Ecology, 11, 2157–2169.
Surridge, A. K., Smith, A. C., Buchanan-Smith, H. M., & Mundy, N. I. (2002). Single-copy nuclear DNA sequences obtained from noninvasively collected primate feces. American Journal of Primatology, 56, 185–190.
Surridge, A. K., Osorio, D., & Mundy, N. I. (2003). Evolution and selection of trichromatic vision in primates. Trends in Ecology & Evolution, 51, 198–205.
Tan, Y., & Li, W.-H. (1999). Trichromatic vision in prosimians. Nature, 402, 36.
Terborgh, J. (1983). Five New World primates: A study in comparative ecology. Princeton, NJ: Princeton University Press.
Veilleux, C., & Bolnick, D. (2008). Opsin gene polymorphism predicts trichromacy in a cathemeral lemur. American Journal of Primatology, 70, 1–5.
Vogel, E., Neitz, M., & Dominy, N. (2007). Effect of color vision phenotype in the foraging of white-faced capuchins, Cebus capucinus. Behavioral Ecology, 18, 292–297.
Vogel, E. R. (2005). Rank differences in energy intake rates in white-faced capuchin monkeys, Cebus capucinus: the effects of contest competition. Behavioral ecology and sociobiology, 58, 333–344.
Acknowledgments
We thank James Higham for the invitation to contribute to this issue. We thank R. Blanco Segura, M. M. Chavarria, and other staff of the Area de Conservación Guanacaste for local support and the Ministerio de Ambiente y Energía (MINAE) of Costa Rica for giving us permission to conduct this study in the Santa Rosa Sector of the ACG. We thank Adrian Guadamuz for assistance with tree species identification; Michael Lemmon for data on availability of Ficus; and Adrienne Blauel, Brandon Klug, Courtney Sendall, and Laura Weckman for their assistance in the field. We thank John Addicott and Tak Fung for helpful advice on statistical analyses, and James Higham and 2 anonymous reviewers for helpful suggestions on previous versions of the manuscript. This study was supported by grants from the Leakey Foundation, the Alberta Ingenuity Fund, the Animal Behavior Society, and the National Sciences and Engineering Research Council of Canada (NSERC) to A. D. Melin; NSERC and the Canada Research Chairs Program to L. M. Fedigan; the Grant-in-Aid for the Japan Society for the Promotion of Science (JSPS) Fellows (15-11926) to C. Hiramatsu; and the Grants-in-Aid for Scientific Research (B) (16405015) and (A) (19207018) from JSPS to S. Kawamura. We also thank the British Ecological Society for funding the attendance of AM to present these results at the XXII Congress of the International Primatological Society. All research protocols abide by national law and were approved by the Animal Care Committee (LESARC) of the University of Calgary.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Melin, A.D., Fedigan, L.M., Hiramatsu, C. et al. Fig Foraging by Dichromatic and Trichromatic Cebus capucinus in a Tropical Dry Forest. Int J Primatol 30, 753–775 (2009). https://doi.org/10.1007/s10764-009-9383-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-009-9383-9