Abstract
We demonstrate a method for non-destructive and contactless measurements of the water content of plants, e.g. agricultural crops. The measurement is based on the absorption of microwave radiation at 35 GHz inside the plant and additionally takes scattering on the surface of the plant into account.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Over the last decades methods for genetic analysis of plants have become a common tool for plant biologists. Standardized Systems can quickly analyze huge numbers of samples. On the other hand the resulting behavior of a plant with a certain genome still needs to be determined in separate experiments, which are much more time consuming. This discrepancy is known as the “phenotyping bottleneck”. To overcome this bottleneck, methods for the continuous and simultaneous observation of many different properties of large numbers of plants are needed. Tools which aim to fulfill this purpose are summarized under the term “phenomics” [1]. Here we present a measurement setup which is capable to determine the water content of a plant in a non-destructive and contactless way. The possibility to use radiation in the microwave range for water status determination has been demonstrated before [2, 3] and is often used in the field of remote sensing, especially on forests [4–7]. The setup presented here aims at the use in a laboratory or phenotyping facility. For this purpose it is desirable to perform measurements on living plants instead of detached leaves [8]. Compared with methods which use radiation in the THz range [9–12], radiation in the microwave range allows for measurements on a whole plant instead of just a single leaf. This can be an advantage in cases where a high spatial resolution is not needed, but where the goal is rather an assessment of the overall health of the plant. The measurement procedure can be fully automated and thus it can be integrated into a high-throughput phenotyping facility.
2 Methods
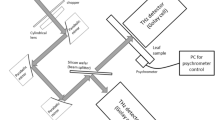
The basic idea behind this method is to make use of the fact that microwave radiation is much more absorbed by water than by dry biological tissue. So the absorption of the radiation by a plant allows for the determination its water content. But as a plant is not a standardized sample, its rather complicated and individual geometry needs to be considered. Especially scattering on the surface of the plants leaves needs to be taken into account. If a plant is just placed in a collimated beam of microwave radiation and the intensity on the other side of the plant is recorded, there is no possibility to know how much of the radiation is absorbed and how much is scattered away in a direction that is not covered by the detector. Since the geometry of the leaves differs from plant to plant, no fixed ratio between absorption and scattering can be assumed. A simple, yet effective way to compensate for this issue in the measurement is to scan around the plant with the detector as shown in Fig. 1.
In our setup microwave radiation from a 35 GHz Gunn-oscillator is collimated by a HDPE lens with a focal length of 22 cm and a diameter of 19.5cm. The resulting beam has a FWHM of 3.4 cm. Symmetrical to the emitter side, the radiation is focused onto the detector by another HDPE lens. The plant is placed in the middle of this setup. While the emitter is kept in a fixed position, the detector can be moved around the plant within an angular range of 270° on a motorized arm. An example for the typical distribution of the transmitted and scattered radiation from a barley plant is shown in Fig. 2.
a) While the biggest part of the radiation can be captured in the forward direction, there is still a significant amount of radiation which would be missed if only the forward direction was observed. b) Comparison of the amplitude of the signal, which is transmitted straight trough the plant, and the integrated signal including the scattered parts for all datasets captured in the experiment. The non-linearity of the relationship shows that omitting the scattered parts would distort the results of the measurements.
Varying environmental temperatures and other influences can cause fluctuations of the performance of the microwave components. To compensate for this, each measurement on a plant is accompanied by a measurement without a plant to characterize the current state of the measurement system.
After integrating over the values from the different angles, the result of the measurement with the plant is compared to the value from the empty setup. From these two values the amount of radiation which has been absorbed in the plant can be easily calculated.
In a typical drought stress experiment plants are deprived of water and their water content is measured repeatedly. To test our measurement setup, barley plants are grown in 22 pots under good watering conditions. Fig. 3 shows one of the plants before the start of the measurements. From then on measurements on all pots are carried out on a regular basis. At least once in two days the plants are put in the measurement setup by hand one after another (with the exception of a distance of 3 days between the measurements on day 26 and day 29). From the beginning of the measurements on, one half of the pots is irrigated as normal and the other half is deprived.
3 Results
The extinction values draw a clear picture of the dynamics of the plants’ drought response. While the extinction values for the control group continuously stay on a high level, the values of the deprived plants show a steep decrease at the time when the plants are no longer able to maintain the water supply of the leaves.
For obtaining the actual water content of the plants, the correlation between the absoption and the water content can be used. This means that while the actual measurement series is non-destructive, destructive measurements are needed for calibration. After completing the measurement series, the water content of the plants is determined by measuring the fresh and dry weight of the plants. Of course, it is important to perform these destructive measurements at the very end of the measurement series. As the control group was irrigated until the end of the experiment, plants with sufficiently different water content were still available at this point. A linear fit between the amounts of water which were determined by the destructive gravimetric measurements and the extinction values from the non-destructive microwave measurements resulted in a proportionality factor of (0.89 ± 0.14) g-1 with an offset of (0.34 ±0.10) g-1 (Pearson correlation coefficient r2=0.83230). The physical origin of the offset is absorption not only by the water but also by the dry biomass of the plants and the bamboo sticks, which are used to support the plants. The water content, which was calculated through this correlation, is plotted in Fig. 4.
Absolute water content of the examined part of the plants. Stressed (circles) and unstressed (crosses) plants can be clearly distinguished, but using a linear correlation between extinction and water content leads to implausible values for plants with very low water content. The inset shows the data which was used for calculating the linear fit. The errorbars show the error of the water content with respect to the uncertainty of the correlation between extinction and water content.
An alternative approach using an effective medium theory (EMT) is used to extract the percentage of water in the plant. The EMT model of Landau, Lifschitz and Looyenga describes the effective permittivity of a composite material. This model is especially appropriate here, because it does not rely on any assumptions about the inner structure of the mixture. In this case, we describe the effective permittivity of a plant as [10, 13]:
where {a i } are the volume fraction of water (W), solid plant material (S) and air (A) and {ε i } are the corresponding permittivities. The role of the plant as an absorbing sample in the beam path can be described by the law of Lambert-Beer:
Our goal is to solve this equation for a W after substituting ε L into it. For solving the equation, we need to make assumptions for some of the unknown values. The volume fraction of dry plant material a S is calculated from the gravimetric measurements, which were performed at the end of the measurement series. For the thickness of the plant, an estimated value d = 2cm is assumed. For practical reasons, the equation is solved numerically. From the volume fractions the according mass fractions can be easily calculated.
The results in terms of the water mass fraction over the whole time span can be seen in Fig. 5. The sample group shows a clear drop in the water mass fraction towards the end, while the reference group's water mass fraction stays relatively constant. Notably, the sample group never falls below 30%. This is probably due to the plants never drying out completely under normal ambient conditions, so that a certain fraction of water always remains. Spikes in the decreasing trend of the sample group are very likely attributable to changes of geometry of the plants within the beam, since they required to be restaked multiple times.
4 Conclusion and outlook
We have demonstrated a method for water status measurements based on the absorption of microwave radiation at 35 GHz by the plant. As no destructive measurements are needed throughout the measurement series, continuous monitoring of the water content of plants over several days becomes possible. The plant is just placed in the middle of the setup without physical contact to its components. With regard to Fig. 2 b) it might also be possible to simplify the measurement setup even more by creating a mathematical model for the nonlinearity of the scattered signal. In either case the design of the measurement setup facilitates the integration of the method in high throughput phenotyping facilities where the plants are moved by an automated transportation system. In such phenotyping facilities other sensors like camera setups for 3d-reconstruction are quite common. When incorporating such additional data, invasive measurements could be completely avoided.
References
R. T. Furbank, M. Tester, “Phenomics – technologies to relieve the phenotyping bottleneck”, Trends in Plant Science, 16, p. 635-544 (2011)
K. Y. Chuah and T. W. Lau, “Dielectric constants of rubber and palm leaf samples at X-band”, IEEE Trans. on Geoscience Remote Sensing, 33(1), p.221-223 (1995).
C. Mätzler, „Microwave (1-100 GHz) dielectric model of leaves“, IEEE Trans. Geoscience Remote Sensing, 32(5), p. 947-949 (1994).
J. C. Calvet, et al., “Plant water content and temperature of the Amazon forest from satellite microwave radiometry”, IEEE Transactions on Geoscience and Remote Sensing, 32(2), p. 397-408 (1994)
P. Ferrazzoli and L. Guerriero, „Passive microwave remote sensing of forests: a model investigation“, IEEE Geoscience Trans. Remote Sensing, 34(2), p. 433-443 (2002)
E. R. Hunt, Jr. et al., “Comparison of vegetation water contents derived from shortwave-infrared and passive-microwave sensors over central Iowa”, Remote Sensing of Environment, 115(9), p. 2376-2383, (2011)
G. Macelloni et al., “Microwave radiometric measurements of soil moisture in Italy”, Hydrology and Earth Syst. Science, 7(6), p. 937-948 (2003).
D. Sancho-Knapik et al., „Microwave l-band (1730 MHz) accurately estimates the relative water content in poplar leaves. A comparison with a near infrared water index (R1300/R1450)“, Agricultural and Forest Meteorology, 151(7), p. 827-832 (2011)
D. M. Mittleman, R. H. Jacobsen, M. C. Nuss, “T-ray imaging”,Selected Topics in Quantum Electronics, IEEE Journal of, 2, p. 679-692 (1996)
C. Jördens, M. Scheller, B. Breitenstein, D. Selmar, M. Koch, “Evaluation of leaf water status by means of permittivity at terahertz frequencies”, Journal of Biological Physics, Springer Netherlands, 35, p. 255-264 (2009)
E. Castro-Camus, M. Palomar, A. Covarrubias, “Leaf water dynamics of Arabidopsis thaliana monitored in-vivo using terahertz time-domain spectroscopy”, Scientific reports, Nature Publishing Group, 3, p. 1-5 (2013)
N. Born, D. Behringer, S. Liepelt, S. Beyer, M. Schwerdtfeger, B. Ziegenhagen, M. Koch, “Monitoring plant drought stress response using terahertz time-domain spectroscopy”, Plant physiology, 164(4), p. 1571-1577 (2014)
R. Gente, N. Born, N. Voß, W. Sannemann, J. Léon, M. Koch, E. Castro-Camus, “Determination of Leaf Water Content from Terahertz Time-Domain Spectroscopic Data” Journal of Infrared, Millimeter, and Terahertz Waves, 34, p. 316-323 (2013)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gente, R., Rehn, A. & Koch, M. Contactless Water Status Measurements on Plants at 35 GHz. J Infrared Milli Terahz Waves 36, 312–317 (2015). https://doi.org/10.1007/s10762-014-0127-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10762-014-0127-3