Abstract
Sepsis is an inflammatory disease characterized by dysregulation of inflammation. Macrophage-mediated inflammation has been implicated in the pathophysiology of sepsis. Itaconate is a metabolite produced in activated macrophages which has anti-inflammatory activities. In the present study, we investigated the potential effects of a cell-permeable itaconate derivative dimethyl itaconate on inflammation in sepsis. We established a lipopolysaccharide (LPS)-induced septic mouse model and administered dimethyl itaconate to the septic mice. The survival rate, serum level of pro-inflammatory cytokines, and lung pathology were evaluated. We also administered dimethyl itaconate to LPS-treated bone marrow–derived macrophages (BMDMs), and measured the cytokine production and Nrf2 expression. We also evaluated the effects of dimethyl itaconate on Nrf2-deficient mice. Administration of dimethyl itaconate enhanced survival rate, decreased serum level of TNF-α and IL-6, and ameliorated lung injury in septic mice. Dimethyl itaconate also suppressed LPS-induced production of TNF-α, IL-6, and NOS2 in BMDMs. Dimethyl itaconate activated Nrf2 and promoted the expression of Nrf2 and its downstream factor HO-1 and NQO-1. The regulatory activities of dimethyl itaconate on inflammatory cytokine production, mouse survival rate were abolished in septic Nrf2−/− mice. Dimethyl itaconate suppressed the inflammatory responses of macrophages in sepsis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Sepsis, which is characterized by dysregulation of inflammation, is an inflammatory immune response triggered by an infection [1]. Due to the high morbidity and mortality, sepsis affects around 30 million people and causes around 7 million deaths every year, causing great economic burden [2].
The core mechanism underlying sepsis development is immune dysfunction. Excessive inflammatory responses have been identified to be associated with septic patients, which contribute to tissue damage and organ failure. When there is bacterial infection, the innate immune system, which is the first line of defense, is activated through the activation of pattern recognition receptor by pathogen- or damage-associated molecular patterns, resulting in the activation of cytokine-encoded genes. Various immune cells including macrophages, neutrophils, and dendritic cells play critical roles in sepsis [3]. Macrophages play a central role in sepsis pathophysiology. Macrophages are the principal source of pro-inflammatory cytokines including TNF-α, IL-6, and IL-1β in sepsis, which aggravate the inflammatory response. Macrophages also produce a large amount of nitric oxide, which causes hypotension and tissue damage during sepsis [4]. Therefore, targeting macrophage could be used as a potential therapeutic approach to treat sepsis [5, 6].
Itaconate is one of the most abundant metabolites produced in activated macrophages [7]. Several studies have revealed the crucial anti-inflammatory activities of itaconate in mammals, as well as anti-bacterial activities [7]. A recent publication described that a cell-permeable itaconate derivative 4-octyl itaconate (OI) has anti-inflammation activity through activating nuclear factor-erythroid 2–related factor 2 (Nrf2) to suppress inflammation [8]. In the present study, we investigated the potential effects of another cell-permeable itaconate derivative, dimethyl itaconate, on inflammation in sepsis and explored the underlying mechanisms.
MATERIALS AND METHODS
Mice and Treatment
Wild-type C57BL/6J mice 6–8 weeks old and Nrf2−/− mice were purchased from the Nanjing Model Animal Center (Nanjing, China). Mice were kept in a specific pathogen-free environment in accordance with protocols approved by the ethics committee of Xingtai People’s Hospital of Hebei Province.
For sepsis induction, C57BL/6J mice were grouped randomly and injected intraperitoneally with 0.1 μg/mouse lipopolysaccharides (LPS, Sigma, USA) plus 0.5 mg/g d-galactosamine (Sigma, St. Louis, MO, USA). Survival was monitored every hour for 24 h. Blood was obtained from challenged mice at various times for analysis.
For skin infection, C57BL/6J mice were anesthetized with 5% isoflurane in 100% oxygen. After shaving the dorsal hair, 70% (v/v) ethanol was used to clean the skin. Then, 50-μl volume of methicillin-resistant Staphylococcus aureus strain USA300 suspension (106 CFU/ml) in phosphate-buffered saline (PBS) was inoculated subcutaneously to induce skin infection. Lesion progression was monitored 6 days after infection, and images captured using a digital camera. For dimethyl itaconate treatment, dimethyl itaconate (Sigma) was dissolved in PBS and mice were intraperitoneally injected with 50 mg/kg dimethyl itaconate 2 h before induction of sepsis and skin infection. PBS was used as control.
Bone Marrow–Derived Macrophage Generation
Bone marrow cells isolated from C57BL/6J mice were cultured in DMEM contained 10% fetal bovine serum (FBS, Gibco, Grand Island, NY) supplemented with M-CSF (10 ng/ml) for 5 days as described previously [9]. The differentiated bone marrow–derived macrophages (BMDMs) were defined by F4/80 staining. BMDMs were pretreated with 200 μM dimethyl itaconate for 1 h. Then, 100 ng/ml LPS or 1 μg/ml R848 was added to cell culture. Samples were harvested at different time points as indicated for analysis. For certain experiments, BMDMs were treated with different concentrations of dimethyl itaconate.
Flow Cytometry
Bone marrow cells were washed with staining buffer (2% FBS in PBS) once and then stained with anti-CD11b, anti-F4/80, and anti-Gr-1 antibodies conjugated with different colors (Abcam, Shanghai, China) on ice for 30 min. After 3 washes with staining buffer, cells were subjected to analysis in a BD LSRII machine. Results were analyzed using FlowJo software.
Hematoxylin and Eosin Staining
After treatment, lung tissues were harvested and fixed in formalin (Sigma). The fixed tissues were dehydrated and embedded. Then, 5-μm slices were made and stained with hematoxylin and eosin (Abcam, Shanghai, China) following manufacturer’s instructions. The lung injury score was quantified based on criteria described previously [10]. Three slices were randomly selected from the container and examined and scored by independent pathology specialists who were blind to the experimental groups. To determine the extent and severity of the lung tissue injury, a score from 0 to 4 was assigned for the characteristics: Inflammation was scored as follows: 0, no inflammation; 1, perivascular cuff of inflammatory cells; 2, mild inflammation, extending throughout 25% of the lung; 3, moderate inflammation covering 25–50% of the lung; 4, severe inflammation involving over one-half of the lung.
ELISA
Supernatant level of serum and BMDM culture of IL-6 and TNF-α was measured using commercial ELISA kits (Abcam) following manufacturer’s protocols.
RT-PCR
After treatment, the total RNA from BMDMs was extracted using the RNeasy Mini kit (Qiagen, USA). The cDNA was synthesized using the PrimeScript™ RT Reagent Kit (Takara, China). Real-time quantitative PCR were set up in triplicate with TB Green® Advantage® qPCR Premix (Takara, China) and performed using the QuantStudio 3 Real-time PCR System (Applied Biosystems, USA). Sequences of primers for real-time PCR are presented in Table 1.
Western Blot
Nuclear proteins from BMDMs were isolated using Nuclear Extraction Kit (Abcam). Proteins (25 μg) were subjected to SDS-PAGE. After transfer, the PVDF membranes (Bio-Rad, USA) were blocked with 5% non-fat dry milk in PBS for 1 h at room temperature. Then, membranes were incubated with primer antibodies at 4 °C overnight. The next day, membranes were washed with 0.1% PBST three times and then were incubated with horseradish peroxidase–conjugated corresponding secondary antibodies. Immuno-reactive bands were visualized by adding the chemiluminescent substrate (Bio-Rad, Hercules, CA, USA). Primary antibodies used in the study were anti-Nrf2 (Abcam), anti-HO1 (Abcam), anti-NQO1 (Abcam), and anti-Lamin B (Abcam).
Statistical Analysis
Data were expressed as mean ± SEM. The significance of difference in multiple groups was determined by Dunnett’s multiple comparisons test. The Gehan-Breslow-Wilcoxon test was used for survival curve. The significances of differences in two-group comparisons were determined by two-tailed Student’s t test. GraphPad was used to draw the graph and calculate the significance. When p < 0.05, the statistical difference was considered significant.
RESULTS
Dimethyl Itaconate Did Not Affect the Development and Survival of Immune Cells
As dimethyl itaconate (Fig. 1a) has been described as a physiological regulator in inflammation [11], first we evaluated whether administration of dimethyl itaconate to mice would affect macrophage and neutrophil development in mice. We intraperitoneally injected mice with 50 mg/kg dimethyl itaconate or control PBS. Bone marrow was harvested and subjected to staining of macrophages and neutrophil markers. As shown in Fig. 1b, there was a similar percentage of F4/80+ CD11b+ macrophages in bone marrow between PBS-treated and dimethyl itaconate–treated mice. Similarly, there was no difference of percentage of Gr-1+CD11b+ neutrophils in bone marrow between PBS-treated and dimethyl itaconate–treated mice (Fig. 1c). Taken together, our data demonstrated that dimethyl itaconate did not affect the development and survival of macrophages and neutrophils.
Dimethyl itaconate has no effect on the development and survival of various immune cells. a Structure of dimethyl itaconate. b, c Flow cytometry analysis of macrophages (CD11b+F4/80+) (b) and neutrophils (CD11b+Gr-1+) (c) in bone marrow from PBS- or dimethyl itaconate–treated mice. Data in all panels are presented as representative FACS plots (left) and mean ± SEM values based on multiple samples (right) (n = 3). Similar results were obtained in three independent experiments. ns, no significance.
Dimethyl Itaconate Suppressed LPS-Induced Sepsis in Mice
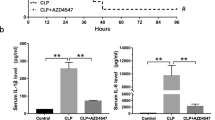
Next, we evaluated the potential effects of dimethyl itaconate on skin infection and LPS-induced sepsis in mice. Administration of dimethyl itaconate obviously ameliorated the skin damage in mice with skin infection (Fig. 2a). As shown in Fig. 2b, LPS-induced sepsis resulted in quick mouse death. In contrast, mice treated with dimethyl itaconate had much less mortality when compared with PBS-treated mice. We also detected significantly decreased serum levels of IL-6 and TNF-α in dimethyl itaconate–treated septic mice (Fig. 2c). Correspondingly, obvious inflammation was detected in the lung of septic mice. In contrast, there was much less inflammation in the lung of dimethyl itaconate–treated septic mice. After quantitation, the lung injury score of dimethyl itaconate–treated septic mice was significantly less than that of PBS-treated septic mice, indicating dimethyl itaconate significantly reduced the lung injury (Fig. 2d). Collectively, our data demonstrated that dimethyl itaconate suppressed LPS-induced sepsis in mice.
Dimethyl itaconate ameliorated LPS-induced sepsis shock. a Images of the mice were captured 6 days after skin infection by the USA300 strain. Mice were administered with 106 CFU USA300 in PBS subcutaneously, and synthetic melittin (100 μg in 80 μl) or sterile PBS was applied to the surface of the skin infection. b WT mice (8 weeks old, n = 10) were injected (i.p.) with LPS plus d-galactosamine. Lethality was monitored every other hour for 24 h. c Mice were bled at the indicated times after injection, and the serum concentration of the indicated cytokines was determined by ELISA (n = 3). d Representative H&E slides of the lung. The scale bar indicates 100 μm (n = 3). Data are presented as mean ± SEM values and representative of at least three independent experiments. Statistical analyses represent variations in experimental replicates. *p < 0.05; **p < 0.01.
Dimethyl Itaconate Prevented LPS-Induced Production of Pro-inflammatory Cytokines in BMDMs
As macrophages played an essential role in the pathophysiology of sepsis by producing various pro-inflammatory cytokines and nitric oxide [4], we continued to explore the effects of dimethyl itaconate on BMDMs after LPS treatment. We pretreated BMDMs with dimethyl itaconate and then treated them with LPS. The mRNA expression of TNF-α, IL-6, and NOS2 in BMDMs was measured. As shown in Fig. 3a, in PBS/LPS-treated BMDMs, increased mRNA of TNF-α and IL-6 was detected at 2 h post LPS treatment, and the mRNA level of TNF-α and IL-6 continued to increase at 6 h post LPS treatment. LPS treatment also resulted in increased mRNA level of NOS2 at 6 h post treatment. In contrast, significantly decreased mRNA level of TNF-α, IL-6, and NOS2 was detected in dimethyl itaconate/LPS-treated BMDMs when compared with mRNA level in PBS/LPS-treated BMDMs at 2 h or 6 h post LPS treatment. Similarly, LPS treatment induced production and secretion of TNF-α and IL-6 in supernatant of BMDM culture (Fig. 3b). Dimethyl itaconate also prevented the LPS-induced production of TNF-α and IL-6, as a significantly decreased protein level of TNF-α and IL-6 was detected in cell culture supernatant of dimethyl itaconate/LPS-treated BMDMs. Interestingly, we detected a significantly decreased mRNA level of TNF-α, IL-6, and NOS2 in TLR7 ligand R848/dimethyl itaconate–treated BMDMs at 6 h post R848 treatment (Fig. 3c), indicating dimethyl itaconate also suppressed R848-induced expression of TNF-α, IL-6, and NOS2. Taken together, our data demonstrated that dimethyl itaconate suppressed LPS-induced production of pro-inflammatory cytokines in BMDMs.
Dimethyl itaconate inhibits LPS-induced production of pro-inflammatory cytokines in BMDMs. a The expression of indicated cytokines in primary BMDMs treated with dimethyl itaconate and LPS (100 ng/ml) was measured by qRT-PCR. b ELISA of the indicated cytokines in the supernatants of BMDMs stimulated with LPS for 24 h. c The expression of indicated cytokines in primary BMDMs treated with dimethyl itaconate and R848 (1 μg/ml) was measured by qRT-PCR. All data are presented as fold relative to the β-actin mRNA level. Data are presented as mean ± SEM values and representative of at least three independent experiments. Statistical analyses represent variations in experimental replicates. *p < 0.05; **p < 0.01.
Dimethyl Itaconate Regulated Nrf2 in BMDMs
It has been described that dimethyl itaconate could activate Nrf2 and its targeting proteins [12]. We next evaluated the effects of dimethyl itaconate on Nrf2 and its targeting proteins HO1 and NQO1. As shown in Fig. 4a, 24-h LPS treatment induced the mRNA expression of Nrf2 targeting proteins HO1 and NQO1. Administration of dimethyl itaconate promoted the expression of HO-1 and NQO1 in LPS-treated BMDMs. Dimethyl itaconate itself was capable of inducing the expression of Nrf2, HO1, and NQO1 in BMDMs. As shown in Fig. 4b, we detected increased protein levels of Nrf2, HO1, and NQO1 in nuclear fraction of BMDMs after dimethyl itaconate treatment in a dose-dependent manner.
NRF2-targeted genes were induced by dimethyl itaconate. a The expression of indicated genes in primary BMDMs treated with dimethyl itaconate plus LPS (20 ng/ml) was measured by qRT-PCR. b IB analysis of the indicated proteins in the nucleus of BMDMs treated with different doses of dimethyl itaconate. All data are presented as fold relative to the Actb mRNA level. Data are presented as mean ± SEM values and representative of at least three independent experiments. Statistical analyses represent variations in experimental replicates. *p < 0.05.
Dimethyl Itaconate–Mediated Suppression of Sepsis Depended on Nrf2
Next, we assessed whether dimethyl itaconate–mediated suppression of sepsis depended on Nrf2. We treated BMDMs from Nrf2−/− mice with dimethyl itaconate and LPS. As shown in Fig. 5a, in BMDMs from wild-type septic mice, 6-h LPS treatment induced the expression of TNF-α, IL-6, and NOS2 while dimethyl itaconate significantly decreased the expression of all three genes. In contrast, the inhibitory effects of dimethyl itaconate were abolished in Nrf2-deficient BMDMs as there was no difference of expression between PBS-treated and dimethyl itaconate–treated BMDMs. Correspondingly, dimethyl itaconate–treated septic Nrf2−/− mice displayed a similar survival rate to PBS-treated septic Nrf2−/− mice (Fig. 5b), indicating dimethyl itaconate did not affect sepsis in Nrf2−/− mice. In addition, we detected similar serum levels of TNF-α and IL-6 in PBS-treated and dimethyl itaconate–treated septic Nrf2−/− mice (Fig. 5c). All these results demonstrated that the effects of dimethyl itaconate were abolished in Nrf2−/− mice, indicating dimethyl itaconate–mediated suppression of sepsis depended on Nrf2.
The negative regulation of sepsis by dimethyl itaconate is dependent on NRF2. WT and Nrf2−/− mice were treated with dimethyl itaconate. a The expression of indicated cytokines in primary dimethyl itaconate–pretreated, LPS-stimulated BMDMs was measured by qRT-PCR. b WT and Nrf2−/− mice (8 weeks old, n = 10) were injected (i.p.) with dimethyl itaconate, and then treated with LPS plus d-galactosamine. Lethality was monitored every other hour for 24 h. c Mice were bled at the indicated times after injection, and the serum concentration of the indicated cytokines was determined by ELISA. All data are presented as fold relative to the Actb mRNA level. Data are presented as mean ± SEM values and representative of at least three independent experiments. Statistical analyses represent variations in experimental replicates. *p < 0.05.
DISCUSSION
In the present study, we evaluate the potential effects of dimethyl itaconate on inflammation using a septic murine model. We demonstrated that dimethyl itaconate ameliorated sepsis, promoted survival rate of septic mice, and decreased the serum level of pro-inflammatory cytokines in septic mice. Dimethyl itaconate also inhibited LPS-induced production of pro-inflammatory cytokines in BMDMs. We further demonstrated that dimethyl itaconate activated Nrf2, which was required for the inhibitory effects of dimethyl itaconate on inflammation. Our finding confirmed the anti-inflammatory activities of itaconate and strongly suggested that itaconate could be used as a potential therapeutic approach to treat inflammation-related diseases, such as sepsis.
Sepsis is caused by the dysregulated innate immune response against infection. The immunopathogenesis of sepsis is complicated, which involves both overactivation and suppression of immune response [13]. The innate immune system quickly responds and initiates an overwhelming inflammatory immune response at the early stage of sepsis [14]. During early sepsis, innate immune response is activated by pathogen-associated molecular patterns (PAMPs) and/or damage-associated molecular patterns (DAMPs). The immediate result of the intracellular signaling process is the cytokine storm, which is essential in initiating and escalating both innate and adaptive immune responses [15]. The excessive inflammatory response results in auto-destruction and followed by tissue damage [16]. As one of the major immune cells, macrophages are activated at the early stage of sepsis and release a massive amount of pro-inflammatory factors including IL-1, IL-6, TNF-α, and nitric oxide synthase (NOS), as well as a large number of chemokines, which can induce T cell–mediated immune response. Using an in vitro macrophage cell model, we also demonstrated that after stimulation with PAMPs LPS or R848, the BMDMs produced IL-6, TNF-α, and NOS2.
After rapidly responding to inflammatory stimuli, macrophages produce cellular metabolites such as itaconate, which could reprogram the cell metabolism. Itaconate, a derivative of the tricarboxylic acid (TCA) cycle, has been reported to have antimicrobial effects through inhibiting isocitrate lyase [17, 18]. Besides the antimicrobial activities, the anti-inflammatory activities of itaconate have been described [8, 19]. Administration of dimethyl itaconate, a cell-permeable derivative of itaconate, reduced the expression levels of pro-inflammatory cytokines in LPS-stimulated BMDMs and ameliorated the injury in ischemia-reperfusion mice [11]. In the present study, we also demonstrated that dimethyl itaconate, a cell-permeable itaconate derivative, suppressed LPS-induced production of TNF-α, IL-6, and NOS2 in BMDMs. Mills and colleagues demonstrated that 4-octyl itaconate (OI) protected mice against LPS-induced death and inhibited pro-inflammatory cytokine production [8]. In the present study, we found that dimethyl itaconate also ameliorated LPS-induced mouse death and prevented pro-inflammatory cytokine production in LPS-treated mice.
Furthermore, we found that the anti-inflammatory activities of itaconate depended on Nrf2. Nrf2 is a transcriptional factor which regulates the expression of antioxidant and cytoprotective genes [20]. The regulatory effects of Nrf2 are critical for inflammation. Using Nrf2-deficient mice, Thimmulappa et al. demonstrated that there was greater inflammation in Nrf2-deficient mice, indicating Nrf2 negatively regulated inflammatory immune response [16]. In LPS-treated mice, the activation of Nrf2 by itaconate was observed [8]. Consistent with this finding, we also found that dimethyl itaconate promoted Nrf2 expression, activated Nrf2 signaling pathway, and induced the expression of Nrf2 targeting gene HO1 and NQO1 in both LPS-treated mice and macrophages. In addition, we found that the protective effects of dimethyl itaconate against sepsis depended on Nrf2 as dimethyl itaconate did not protect septic Nrf2−/− mice. These findings revealed the underlying mechanisms of dimethyl itaconate–mediated protection against inflammation and sepsis.
CONCLUSION
In summary, we demonstrated that dimethyl itaconate prevented the inflammation response in septic mice in a Nrf2-dependent manner.
Abbreviations
- LPS:
-
Lipopolysaccharides
- BMDMs:
-
Bone marrow–derived macrophages
- OI:
-
Octyl itaconate
- Nrf2:
-
Nuclear factor-erythroid 2–related factor 2
- PBS:
-
Phosphate-buffered saline
- FBS:
-
Fetal bovine serum
- H&E:
-
Hematoxylin and eosin
References
Pinsky, M.R. 2004. Dysregulation of the immune response in severe sepsis. The American Journal of the Medical Sciences 328: 220–229.
Fleischmann, C., A. Scherag, N.K. Adhikari, C.S. Hartog, T. Tsaganos, P. Schlattmann, D.C. Angus, K. Reinhart, and International Forum of Acute Care Trialists. 2016. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. American Journal of Respiratory and Critical Care Medicine 193: 259–272.
Qiu, P., Y. Liu, and J. Zhang. 2019. Review: The role and mechanisms of macrophage autophagy in sepsis. Inflammation 42: 6–19.
Evans, T.J. 1996. The role of macrophages in septic shock. Immunobiology 195: 655–659.
Kumar, V. 2018. Targeting macrophage immunometabolism: dawn in the darkness of sepsis. International Immunopharmacology 58: 173–185.
van der Poll, T., F.L. van de Veerdonk, B.P. Scicluna, and M.G. Netea. 2017. The immunopathology of sepsis and potential therapeutic targets. Nature Reviews. Immunology 17: 407–420.
Hooftman, A., and L.A.J. O’Neill. 2019. The immunomodulatory potential of the metabolite itaconate. Trends in Immunology 40: 687–698.
Mills, E.L., D.G. Ryan, H.A. Prag, D. Dikovskaya, D. Menon, Z. Zaslona, M.P. Jedrychowski, A.S.H. Costa, M. Higgins, E. Hams, J. Szpyt, M.C. Runtsch, M.S. King, J.F. McGouran, R. Fischer, B.M. Kessler, A.F. McGettrick, M.M. Hughes, R.G. Carroll, L.M. Booty, E.V. Knatko, P.J. Meakin, M.L.J. Ashford, L.K. Modis, G. Brunori, D.C. Sévin, P.G. Fallon, S.T. Caldwell, E.R.S. Kunji, E.T. Chouchani, C. Frezza, A.T. Dinkova-Kostova, R.C. Hartley, M.P. Murphy, and L.A. O’Neill. 2018. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature 556: 113–117.
Weischenfeldt J., Porse B. 2008. Bone marrow-derived macrophages (BMM): isolation and applications. CSH Protoc 2008:pdb prot5080.
Zhao, Y.F., Y.M. Luo, W. Xiong, W. Ding, Y.R. Li, W. Zhao, H.Z. Zeng, H.C. Gao, and X.L. Wu. 2015. Mesenchymal stem cell-based FGF2 gene therapy for acute lung injury induced by lipopolysaccharide in mice. European Review for Medical and Pharmacological Sciences 19: 857–865.
Lampropoulou, V., A. Sergushichev, M. Bambouskova, S. Nair, E.E. Vincent, E. Loginicheva, L. Cervantes-Barragan, X. Ma, S.C.C. Huang, T. Griss, C.J. Weinheimer, S. Khader, G.J. Randolph, E.J. Pearce, R.G. Jones, A. Diwan, M.S. Diamond, and M.N. Artyomov. 2016. Itaconate links inhibition of succinate dehydrogenase with macrophage metabolic remodeling and regulation of inflammation. Cell Metabolism 24: 158–166.
O’Neill, L.A.J., and M.N. Artyomov. 2019. Itaconate: the poster child of metabolic reprogramming in macrophage function. Nature Reviews. Immunology 19: 273–281.
Iskander, K.N., M.F. Osuchowski, D.J. Stearns-Kurosawa, S. Kurosawa, D. Stepien, C. Valentine, and D.G. Remick. 2013. Sepsis: multiple abnormalities, heterogeneous responses, and evolving understanding. Physiological Reviews 93: 1247–1288.
Huang, X., F. Venet, Y.L. Wang, A. Lepape, Z. Yuan, Y. Chen, R. Swan, H. Kherouf, G. Monneret, C.S. Chung, and A. Ayala. 2009. PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proceedings of the National Academy of Sciences of the United States of America 106: 6303–6308.
Banyer, J.L., N.H. Hamilton, I.A. Ramshaw, and A.J. Ramsay. 2000. Cytokines in innate and adaptive immunity. Reviews in Immunogenetics 2: 359–373.
Thimmulappa, R.K., H. Lee, T. Rangasamy, S.P. Reddy, M. Yamamoto, T.W. Kensler, and S. Biswal. 2006. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. The Journal of Clinical Investigation 116: 984–995.
McFadden, B.A., and S. Purohit. 1977. Itaconate, an isocitrate lyase-directed inhibitor in Pseudomonas indigofera. Journal of Bacteriology 131: 136–144.
Rittenhouse, J.W., and B.A. McFadden. 1974. Inhibition of isocitrate lyase from Pseudomonas indigofera by itaconate. Archives of Biochemistry and Biophysics 163: 79–86.
Bambouskova, M., L. Gorvel, V. Lampropoulou, A. Sergushichev, E. Loginicheva, K. Johnson, D. Korenfeld, M.E. Mathyer, H. Kim, L.H. Huang, D. Duncan, H. Bregman, A. Keskin, A. Santeford, R.S. Apte, R. Sehgal, B. Johnson, G.K. Amarasinghe, M.P. Soares, T. Satoh, S. Akira, T. Hai, C. de Guzman Strong, K. Auclair, T.P. Roddy, S.A. Biller, M. Jovanovic, E. Klechevsky, K.M. Stewart, G.J. Randolph, and M.N. Artyomov. 2018. Electrophilic properties of itaconate and derivatives regulate the IkappaBzeta-ATF3 inflammatory axis. Nature 556: 501–504.
Thimmulappa, R.K., K.H. Mai, S. Srisuma, T.W. Kensler, M. Yamamoto, and S. Biswal. 2002. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Research 62: 5196–5203.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Mice were kept in a specific pathogen-free environment in accordance with protocols approved by the ethics committee of Xingtai People’s Hospital of Hebei Province.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, S., Jiao, Y., Li, C. et al. Dimethyl Itaconate Alleviates the Inflammatory Responses of Macrophages in Sepsis. Inflammation 44, 549–557 (2021). https://doi.org/10.1007/s10753-020-01352-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-020-01352-4