Abstract
Osteoarthritis (OA), which is characterized as a common degenerative joint disease, is presently the most prevalent chronic degenerative joint disease. Accumulating evidence has shown a biological function for Garcinol in a variety of diseases; however, whether it could be used to treat OA remains unclear. In this study, we explored the protective effects of garcinol on the progression of OA and explored the underlying mechanism. In vitro, garcinol reduced the expression of pro-inflammatory cytokines, such as IL-6 and tumor necrosis factor alpha (TNF-α). It also decreased the expression of inducible nitric oxide synthase (iNOS), as well as cyclooxygenase-2 (COX-2). Furthermore, garcinol inhibited the expression of thrombospondin motifs 5(ADAMTS5) and metalloproteinase (MMPs), both of which regulate extracellular matrix degradation. These changes could be attributed to garcinol-related suppression of the IL-1β-induced NF-κB signaling pathway. Moreover, we investigated the protective effects of garcinol on the surgical destabilization of the medial meniscus (DMM) of the mouse, an in vivo model of OA. Taken together, our data suggest garcinol as a potential future agent for the treatment of OA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Osteoarthritis (OA) is now the most prevalent chronic degenerative joint disease, affecting tens of millions of people [7]. Articular cartilage erosion, synovitis and articular hypertrophy, and subchondral bone remodeling are regarded as the main characteristics of OA development [16], resulting in severe joint pain and dysfunction. Currently, pharmaceutical agents such as non-steroidal anti-inflammatory drugs (NSAIDS) and bisphosphonates only alleviate clinical symptoms, and carry their own severe side effects [10]. Therefore, safe and effective drugs that delay the progression of OA represent a critical unmet need.
Although the underlying reason for the initiation of OA is multi-factorial, cartilage destruction seems to be the major cause, and this destruction is a consequence of uncontrolled proteolytic extracellular matrix destruction. Chondrocytes, the sole cell type in articular cartilage, maintain a delicate extracellular matrix (ECM) balance between synthesis and degradation, and play an important role in the OA disease processes. Inflammation is reported to trigger the catabolic abilities of chondrocytes, subsequently leading to the degradation of extracellular matrix (ECM). Interleukin (IL)-1β, a pro-inflammatory cytokine, is reported to be produced and secreted by activated chondrocyte metabolism during the process of OA, and contributing to the development of OA [1, 9]. Studies confirmed that IL-1β could induce chondrocytes to release multiple proteolytic enzymes, including matrix metalloproteinases (MMPs) [3, 5], which can upregulate anabolism in chondrocytes via promoting ECM degradation. In addition, aggrecan, one of the major ECM components of cartilage, could increase the ability of cartilage to resist compressive forces. Accumulating evidence demonstrates the role of IL-1β in the degradation of aggrecan [22], and aggrecan is cleaved at specific “aggrecanase” sites during the process of OA [35]. This cleavage can be attributed to several members of the ADAMTS family of metalloproteases, such as ADAMTS4 and ADAMTS5 [23, 30, 32, 38]. Stimulation by IL-1β highly facilitates the expression of ADAMTS, thereby increasing the degradation of aggrecan [34, 39, 43].
Collagen II has been revealed to be the main component of ECM, and is responsible for supporting cartilaginous structures. Treatment with IL-1β contributes to the degradation of collagen II, resulting in the degeneration of articular cartilage. On the contrary, collagen X was considered a standard marker of hypertrophic cartilage, and high expression of collage X can result in ossification [41, 47]. Stimulation with IL-1β promotes the expression of collagen X, and may thus accelerate the ossification of cartilage, resulting in dysfunction.
In addition, chondrocytes obtained from patients suffering from OA were potent producers of nitric oxide (NO), IL-1β, TNF-α, IL-6, and IL-8, and elevated levels of tumor necrosis factor-α (TNF-α) and IL-1β have been found in the synovial fluid and cartilage tissue of OA patients [4, 13]. Therefore, drugs targeting IL-1β-induced inflammation are considered an effective method for treating OA.
Studies have demonstrated that garcinol (a polyisoprenylated benzophenone extracted from the fruit of Garcinia indica exhibits a wide range of biological activities including antioxidizing, anti-inflammatory, neuroprotective, antimicrobial, and antineoplastic effects [2, 6, 14, 19, 20, 24, 36, 44]. Garcinol has also been explored for having a protective effect in various inflammatory situations induced by IL-1β [18, 42]. We thus hypothesized that it could inhibit IL-1β-induced inflammatory reactions.
In this study, we demonstrated the inhibitory effects of garcinol on IL-1β-induced inflammation via the NF-κB signal pathway, and its protective effects on the destabilized medial meniscus (DMM) of mice in an in vivo model for OA. Our study demonstrated that garcinol has potential as a novel drug for the treatment of OA.
MATERIALS AND METHODS
Reagents
Garcinol (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in dimethylsulfoxide (DMSO) for storage at a concentration of 50 mM, then further diluted in cell culture medium until DMSO comprised < 0.1% of the total volume. Recombinant human IL-1β (Peprotech) was dissolved in water. Dulbecco’s Modified Eagle Medium (DMEM/F12), fetal bovine serum (FBS), penicillin, and streptomycin were purchased from Gibco (Rockville, MD, USA). Type II collagenase was purchased from Sigma-Aldrich (St Louis, MO, USA). 0.25% trypsin–EDTA was obtained from Gibco (Rockville, MD, USA). Safranin-O and Fast Green were purchased from Solarbio (Beijing, China). MTT kit and BCA were bought from the Beyotime Institute of Biotechnology (Shanghai, China).
Primary Mice Chondrocytes Culture
Chondrocytes were isolated from the knee joints and costal cartilage of C57BL/6 mice (2-day old). The costal cartilage and articular cartilage were extracted carefully under aseptic conditions and treated with 0.25%type II collagenase for 6 h at 37 °C, with 5% CO2. Later, the digested cartilage tissues were washed twice by PBS, and then seeded into tissue culture flasks for culture in DMEM/F12 supplemented with 10% FBS, 100 U/mL penicillin, and 100 mg/mL streptomycin in an incubator maintained at 37 °C with 5% CO2. The medium was changed after 24 h of incubation. When chondrocytes in culture demonstrated 70–90% confluency, we harvested the cells by using 0.25% trypsin–EDTA (Gibco, Invitrogen).
Cytotoxicity Assays
Mouse chondrocytes were seeded in 96-well plates at a density of 8000 cells/well. After culturing for 24 h, the medium was changed with fresh culture medium containing various concentrations of garcinol (0, 2, 4, 8, 16, 32, and 64 μM) according to the time until cell adherence. Each garcinol concentration was repeated in 6 wells. At each experimental end point, 10 μl MTT solution was added to each well, and chondrocytes were further incubated for 4 h at 37 °C with 5% CO2. Later, the medium was changed to 200 μl DMSO, allowed to dissolve for 10 min, and then measured using the Enzyme standard instrument at OD 570 nm (Thermo Scientific, Multiskan GO, Waltham, MA, USA).
Quantitative Real-time PCR
Total RNA was extracted from the chondrocytes in 6-cm culture plates using TRIzol reagent. Chondrocytes at a density of 2 × 105 cells/well were seeded onto a six-well plate and treated with IL-1β, with or without various concentrations of garcinol, and an untreated group was used as a control. At the end of the study, total RNA was extracted from each well using RNAiso Plus (Takara Bio, Otsu, Japan). First-strand cDNA was synthesized using PrimerScript RT cDNA Synthesis kit (Takara Bio) and 1 μg of extracted RNA template. qPCR was then carried out using the SYBR Green–based Real-time PCR Master Mix (Takara Bio) with the following cycling parameters: 42 cycles of 94 °C for 20 s; 60 °C for 20 s; and 72 °C for 30 s. The results were normalized to β-actin, an internal housekeeping gene. The specific primers (based on the mouse sequences) used are shown in Table 1.
Western Blot Analysis
Cellular proteins were treated with the indicated conditions in an incubator maintained at 37 °C with 5% CO2. At the end of the experiment, cells were washed by PBS twice, and later collected by cell lysis buffer. After exposure to cell lysis buffer for 30 min on ice, the samples were then centrifuged at 15000g for 10 min at 4 °C. The protein-containing supernatant was collected, and we measured protein concentration using a bicinchoninic acid (BCA) protein assay kit. After normalizing the protein concentration, the sample was mixed with sodium dodecyl sulfate–sampling buffer, followed by boiling at 95 °C for 5 min. To continue, protein (40 μg) was loaded onto 10–12% SDS-PAGE gels and subsequently transferred to a 0.22-μm PVDF membrane. The PVDF membranes were later incubated with blocking buffer for 1 h. The blocked membranes were then incubated with primary antibodies against collagen X (1:1000), collagen II (1:1000), INOS (1:1000), COX-2 (1:1000), Aggrecan (1:1000), ADAMT5(1:1000), MMP-13(1:1000), MMP-3(1:1000), p65 (1:1000), P-p65 (1:1000), IκBα (1:1000), and actin (1:1000) overnight at 4 °C, then incubated with the secondary antibody for 1 h. Finally, the bands were detected via ECL Western Blotting Substrate and visualized by the Bio-Rad ChemiDoc system.
Immunofluorescence Microscopy
Chondrocytes (5 × 105 cells/mL) were seeded on a 6-well plate with glass coverslips in advance and incubated for 24 h. At the end of the experiment, the glass coverslips were washed three times in PBS. Later, chondrocytes were fixed with 4% paraformaldehyde for 30 min and permeabilized by treatment with Triton X-100 for 10 min at room temperature. Following blocking by BSA for 1 h, cells were incubated with antibody against Aggrecan (1:500), P65 (1:500), and collagen II (1:500) overnight. Afterwards, the cells were incubated with secondary antibody for 1 h and cell nuclei were incubated with DAPI for 5 min. Finally, images were observed using an Olympus FV1200 microscope (Olympus, Japan) and a Nikon ECLIPSE Ti microscope (Nikon, Japan).
Animal Experiment
Thirty 6–8-week-old C57BL/6 female mice were obtained from the Animal Center of the Chinese Academy of Sciences (Shanghai, China). All animal care and experimental procedures were approved by the Zhejiang University Institutional Animal Care and Use Committee (No.15253). Surgical destabilization of the medial meniscus (DMM) was used as an osteoarthritis model as previously described [15]. During the experiment, mice were randomly divided into three groups (10 mice each group), such as a sham group (Sham), DMM group (destabilization of the DMM), and GAR-treated group (DMM + GAR). The mice were anesthetized by pentobarbital (40 mg/kg), with surgery beginning after the mice slept. We opened the knee joints, and then carefully resected the medial meniscuses. To close, the skin was sutured carefully, and penicillin was intramuscularly injected for 3 days following the procedure. One week after induction of DMM, the DMM + GAR group was administered garcinol at 10 mg/kg/day subcutaneously until the mice were sacrificed. The concentration of 10 mg/kg of garcinol was selected, based on the literatures [12, 17, 33, 37, 40, 46], and our previous publication [21]. Garcinol was dissolved in 5% DMSO (dimethylsulfoxide) in PBS. Mice in the sham and DMM groups were administered 5% DMSO (dimethylsulfoxide) in PBS without garcinol in a similar fashion.
Histological Analysis
Each group of knee joints was collected and fixed in 4% formaldehyde, then each group of knee joints for 14 days in 10% EDTA (pH 7.4) was used to decalcify the tissues. Next, after dehydration, samples were embedded in paraffin. Paraffin blocks which contain knee joints were coronally sectioned, then Safranin-O and hematoxylin and eosin (H & E) staining were used to measure cartilage destruction. Immunofluorescence was performed to measure the expression of collagen II in cartilage.
Statistical Analysis
Experiments were performed independently at least 3 times. All data is shown as mean ± SD. One-way analysis of variance (ANOVA) followed by a Tukey’s post hoc test was used to assess statistical differences between different experimental groups. P values < 0.05 were considered significant.
RESULTS
Effects of Garcinol on Chondrocyte Viability
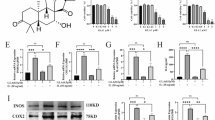
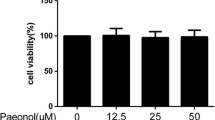
The chemical structure of garcinol is shown in Fig. 1 a, and representative images of the cell morphology of chondrocytes are displayed in Fig. 1 b. In order to estimate the cytotoxicity of garcinol, chondrocytes were treated with or without garcinol at different concentrations (0, 0.5, 1, 2, 4, 8, 16, and 32 μM) for 24 h and 48 h, and the cellular viability was ultimately observed by MTT assay. Treatment with garcinol at the indicated concentrations, ranging from 0 to 8 μM, at 24 h and 48 h showed no significant variation in cell viability (Fig. 1c). Therefore, we chose to use garcinol (2, 4, or 8 μM) in all subsequent experiments.
The cytotoxicity of garcinol on chondrocytes. a The chemical structure of garcinol. b The morphology of primary chondrocytes. c Cytotoxicity of garcinol on chondrocytes was explored at different concentrations (0, 0.5, 1, 2, 4, 8, 16, and 32) for 24 h and 48 h using an MTT assay. Data are presented as mean ± SD. Significant differences among groups are indicated as #P < 0.01 vs. control group; *P < 0.05, **P < 0.01, ***P < 0.001 vs. IL-1β alone treatment group, n = 3.
Protective Effect of Garcinol on IL-1β-Induced Inflammation in Mouse Chondrocytes
In order to investigate the protective effect of garcinol in chondrocytes under inflammatory condition induced by IL-1β, mouse chondrocytes were treated with IL-1β at various concentrations of garcinol for 24 h, then RT-PCR and western blot analyses were performed to explore the expression levels of RNA and protein. The results demonstrated that IL-1β upregulated the expression of inflammation-related maker genes: TNF-α, IL-6, and INOS, while treatment with garcinol restored them to baseline (Fig. 2a). In addition, at the protein level, garcinol decreased IL-1β-induced INOS and COX-2 in a dose-dependent manner (Fig. 2b).
Protective effects of garcinol on IL-1β-induced inflammation in mouse chondrocytes. a Garcinol dose-dependently suppressed IL-1β-induced inflammation–related marker genes. The expression of TNF-α, IL-6, and INOS in chondrocytes was assessed by real-time PCR. The expression of target genes was normalized to β-actin, and then expressed as fold change relative to control group. b Garcinol dose-dependently suppressed IL-1β-induced expression of INOS and COX-2. Total cellular extracts from chondrocytes treated with or without IL-1β in media containing garcinol for 24 h were subject to western blot analyses using specific antibodies against INOS and COX-2. β-actin served as an internal loading control. Data are presented as mean ± SD. Significant differences among groups are indicated as #P < 0.01 vs. control group; *P < 0.05, **P < 0.01, ***P < 0.001 vs. IL-1β alone treatment group, n = 3.
Garcinol Alleviated IL-1β-Induced ECM Degradation in Mouse Chondrocytes
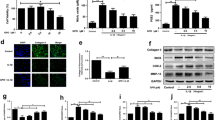
To evaluate the function of garcinol on IL-1β-induced degradation of the ECM, we investigated the effect of garcinol on ECM components in chondrocytes by using qPCR and western blot analysis. As the figure shows, IL-1β increased the gene expression of MMP3, MMP9, and MMP13, while garcinol prevented this expression in a dose-dependent manner (Fig. 3a, b). We also demonstrated that stimulation with IL-1β (10 ng/mL) led to the acceleration of ADAMTS-5 (Fig. 3b), while garcinol inhibited this expression. As ADAMTS-5 has been demonstrated to act in cleaving aggrecan, we next investigated the effect of garcinol on IL-1β-induced degradation of aggrecan, and the data revealed a protect role for garcinol (Fig. 3b). Additionally, the results of our immunofluorescence staining for aggrecan (Fig. 3c, d) confirmed the protective effect of garcinol on IL-1β-induced ECM degradation.
Garcinol alleviated IL-1β-induced ECM degradation in mouse chondrocytes. a, b IL-1β increased the expression of MMP3, MMP9, and MMP13, while treatment with garcinol attenuated the expression at both the gene and protein levels. Expression of target genes was normalized to β-actin, and then expressed as fold change relative to controls group. b Treatment with IL-1β degraded aggrecan and increased the expression of ADAMTS-5, while treatment with garcinol reversed this effect. c Representative aggrecan was detected by immunofluorescent and DAPI staining. d The fluorescence intensity of aggrecan was analyzed by image J. Data are presented as mean ± SD. Significant differences among groups are indicated as #P < 0.01 vs. control group; *P < 0.05, **P < 0.01, ***P < 0.001 vs. IL-1β alone treatment group, n = 3.
Garcinol Protected against IL-1β-Induced Hypertrophic Conversion
As for articular cartilage, collagen II was revealed to be the main component of ECM, responsible for supporting cartilaginous structure and tensile strength, while collagen X is a marker of hypertrophic cartilage, and high expression of collagen X can result in ossification [41, 47]. We thus explored the effect of garcinol on collagen II and X. Our results showed that IL-1β led to the upregulation of collagen X (Fig. 4a), the primary maker of hypertrophic conversion; however, treatment with garcinol inhibited this acceleration. Conversely, IL-1β induced the degradation of collagen II while garcinol reversed this effect (Fig.4a, c).
Garcinol protected against IL-1β-induced hypertrophic conversion. a IL-1β-induced collagen X was decreased in a dose-dependent manner, with an opposite effect on collagen II as shown by western blot. b The expression of collagen X and collagen II relative to actin were determined by ImageJ. c Representative collagen II was explored by immunofluorescent and DAPI staining. d The fluorescence intensity of collagen II was analyzed using image J. Data are presented as mean ± SD. Significant differences among groups are indicated as #P < 0.01 vs. control group; *P < 0.05, **P < 0.01, ***P < 0.001 vs. IL-1β alone treatment group, n = 3.
Garcinol Inhibited IL-1β-Induced Activation of the NF-κB Pathway in Mouse Chondrocytes
The NF-κB signaling pathway is reported to be a vital signaling pathway in the development of OA, due to its ability to elicit matrix degradation and anti-inflammation, and inhibit ECM synthesis [31]. To investigate the deep molecular mechanisms of garcinol in IL-1β-induced inflammation, we further explored its role on the NF-κB signaling pathway. As Fig. 5 a shows, phosphorylation of p65 was markedly upregulated in the IL-1β group compared with the control group. However, garcinol decreased this acceleration in a dose-dependent manner. Moreover, IL-1β significantly stimulated IκBα degradation, while garcinol attenuated this degradation. Confocal microscopy also indicated that IL-1β induced the translocation of p65 from the cytoplasm to the nucleus, while treatment with garcinol suppressed translocation (Fig. 5c).
Garcinol inhibited IL-1β-induced activation of the NF-κB signaling pathway in mouse chondrocytes. a IL-1β promoted IκBα degradation and p65 phosphorylation, while garcinol alleviated both in a dose-dependent manner. b The expression of P-p65 relative to total p65 and Iκβα relative to actin were determined by ImageJ. c IL-1β promoted p65 nuclear translocation and localization, while garcinol reversed this effect. Representative immunofluorescence images of p65 localization (green) from chondrocytes. Nuclei were counterstained with DAPI (blue). d The fluorescence intensity of p65 was analyzed using image J. Data are presented as mean ± SD. Significant differences among groups are indicated as #P < 0.01 vs. control group; *P < 0.05, **P < 0.01, ***P < 0.001 vs. IL-1β alone treatment group, n = 3.
Garcinol Ameliorated Cartilage Degeneration of OA in Mice Models
Combined with the in vitro data, we also established a DMM-induced OA model in mice to investigate whether garcinol has preventive effects against osteoarthritis in vivo. The treatment group received an intraperitoneal injection of garcinol (10 mg/kg), while the sham and DMM groups received 5% DMSO via intraperitoneal injection every other day in the 6 weeks following surgery. As shown in Fig. 6 a, the cartilage surface was smooth and stained red by Safranin-O staining in the sham control group, while the OA group showed severe cartilage erosion and cartilage destruction. However, the treatment with garcinol rescued this destruction. The OARSI score was higher in the DMM groups, while the garcinol treatment group received lower OARSI scores (Fig. 6b). To verify whether the protective effect of garcinol in vivo is related to collagen II degradation, we performed immunofluorescent staining for collagen II. Our results showed that the fluorescence intensity of collagen II was significantly decreased in the DMM group as compared to sham; however, the treatment with garcinol reversed the degradation (Fig. 7a). In conclusion, our data suggest that garcinol had the ability to attenuate the progression of OA in mice DMM models.
Garcinol alleviates cartilage degeneration in the mouse DMM model. a Safranin-O staining of the cartilage and synovitis from different experimental groups. b Representative images of H&E. c Diagrams show the cartilage OARSI scores. Data are presented as mean ± SD. Significant differences among groups are indicated as #P < 0.01 vs. control group; *P < 0.05, **P < 0.01, ***P < 0.001 vs. IL-1β alone treatment group, n = 3.
DISCUSSION
Osteoarthritis (OA) is a chronic degenerative disease with features including joint dysfunction, cartilage degradation, and pain [26, 29], and is now highly prevalent among older individuals. Accumulating evidence indicates the important role of inflammation in the initiation and development of OA [28]. However, existing drugs for OA, such as NSAIDs, do not effectively ameliorate symptoms while displaying several side effects [25, 45]. Therefore, development of a safe agent which can inhibit cartilage degradation in OA is urgent. Recently, studies have demonstrated that plant-derived compounds are potent in the treatment of inflammation-related diseases. For instance, garcinol, the main active component derived from Garcinia indica (G. indica), has displayed antioxidant and anti-inflammatory effects in various diseases [8, 27]. In this study, we explored the effects of garcinol on inflammation which was induced by IL-1β. Our data showed that garcinol dramatically inhibited IL-1β-induced inflammation in chondrocytes. The primary mechanism contributing to the garcinol suppression of IL-1β-induced events was NF-κB activation in chondrocytes.
The extracellular matrix (ECM), which consists of type II collagen and aggrecan, primarily maintains the structure of cartilage [11].Uncontrolled proteolytic extracellular matrix destruction results in cartilage destruction, regarded as the material cause of OA. In addition, as the only cell type in cartilage, chondrocytes maintains the balance of ECM between synthesis and degradation in normal joints. However, inflammatory factors such as IL-1β break this homeostasis. Inflammatory reactions lead to the upregulation of matrix metalloproteinases (MMPs), such as MMP3 and MMP13, resulting in the degradation of ECM. In addition, IL-1β also degrades aggrecan, the major ECM component of cartilage, and this degradation contributes to the acceleration of ADAMTS. Our study demonstrated that garcinol promoted the synthesis of the main components of ECM, which were decreased by IL-1β. Furthermore, treatment with garcinol also reduced factors which could promote the catabolism of ECM, such as MMPs and ADAMTS-5.
Collagen II is the main component of the ECM and supports cartilaginous structure, while collagen X is considered as a standard marker of hypertrophic cartilage and high expression of collagen X can result in ossification [41, 47]. Our data demonstrated that garcinol ameliorated IL-1β-induced degradation of collagen II and decreased the expression of collagen X, which was induced by IL-1β, recovering inflammation-induced joint dysfunction.
The NF-κB signaling pathway is reported to be a vital signaling pathway in the process of OA due to its ability to elicit matrix degradation, contribute to inflammation, and inhibit ECM synthesis [31]. NF-κB is normally located in the cytoplasm and is bound to IκBα, but stimulation with IL-1β frees NF-κB and promotes its translocation from the cytoplasm to the nucleus, subsequently facilitating the expression of inflammatory genes such as MMPs, iNOS, COX2, and IL-6 [31]. Our data revealed that garcinol inhibited p65 phosphorylation and nuclear translocation, demonstrating a suppressive role of garcinol on NF-κB in chondrocytes.
The surgical destabilization of the medial meniscus (DMM) was confirmed as an instability model to investigate OA [15]. Mice developed calcification of the cartilage surface, osteophyte formation, and cartilage erosion after receiving the surgical DMM. However, our data showed that treatment with garcinol recovered the destruction induced by DMM.
In summary, our study demonstrated that garcinol inhibited chondrocyte inflammation in vitro and relieved DMM-induced cartilage degeneration in vivo. The primary mechanism contributing to the garcinol suppression of IL-1β-induced events was NF-κB activation in chondrocytes. These findings demonstrate that garcinol has potential as a novel drug for the treatment of OA.
Change history
15 June 2020
The original version of this article contained mistakes, and the authors would like to correct them.
References
Abramson, S.B., and Y. Yazici. 2006. Biologics in development for rheumatoid arthritis: Relevance to osteoarthritis. Advanced Drug Delivery Reviews 58 (2): 212–225. https://doi.org/10.1016/j.addr.2006.01.008.
Balasubramanyam, K., M. Altaf, R.A. Varier, V. Swaminathan, A. Ravindran, P.P. Sadhale, and T.K. Kundu. 2004. Polyisoprenylated benzophenone, garcinol, a natural histone acetyltransferase inhibitor, represses chromatin transcription and alters global gene expression. The Journal of Biological Chemistry 279 (32): 33716–33726. https://doi.org/10.1074/jbc.M402839200.
Chabane, N., N. Zayed, H. Afif, L. Mfuna-Endam, M. Benderdour, C. Boileau, J. Martel-Pelletier, J.P. Pelletier, N. Duval, and H. Fahmi. 2008. Histone deacetylase inhibitors suppress interleukin-1β-induced nitric oxide and prostaglandin E2 production in human chondrocytes. Osteoarthritis and Cartilage 16 (10): 1267–1274. https://doi.org/10.1016/j.joca.2008.03.009.
Chaganti, R.K., E. Purdue, T.P. Sculco, and L.A. Mandl. 2014. Elevation of serum tumor necrosis factor α in patients with periprosthetic osteolysis: A case-control study. Clinical Orthopaedics and Related Research® 472 (2): 584–589. https://doi.org/10.1007/s11999-013-3235-9.
Chao, P.-Z., M.-S. Hsieh, C.-W. Cheng, Y.-F. Lin, and C.-H. Chen. 2011. Regulation of MMP-3 expression and secretion by the chemokine eotaxin-1 in human chondrocytes. Journal of Biomedical Science 18 (1): 86. https://doi.org/10.1186/1423-0127-18-86.
Chatterjee, A., T. Yasmin, D. Bagchi, and S.J. Stohs. 2003. The bactericidal effects of Lactobacillus acidophilus, garcinol and Protykin® compared to clarithromycin, on Helicobacter pylori. Molecular and Cellular Biochemistry 243 (1): 29–35. https://doi.org/10.1023/A:1021649427988.
Chen, D., J. Shen, W. Zhao, T. Wang, L. Han, J.L. Hamilton, and H.J. Im. 2017. Osteoarthritis: Toward a comprehensive understanding of pathological mechanism. Bone Res 5: 16044. https://doi.org/10.1038/boneres.2016.44.
Chen, J., Y.-T. Gu, J.-J. Xie, C.-C. Wu, J. Xuan, W.-J. Guo, Y.Z. Yan, L. Chen, Y.S. Wu, X.L. Zhang, J. Xiao, and X.-Y. Wang. 2018. Gastrodin reduces IL-1β-induced apoptosis, inflammation, and matrix catabolism in osteoarthritis chondrocytes and attenuates rat cartilage degeneration in vivo. Biomedicine & Pharmacotherapy 97: 642–651. https://doi.org/10.1016/j.biopha.2017.10.067.
Chevalier, X., T. Conrozier, and P. Richette. 2011. Desperately looking for the right target in osteoarthritis: The anti-IL-1 strategy. Arthritis Research & Therapy 13 (4): 124. https://doi.org/10.1186/ar3436.
da Costa, B.R., S. Reichenbach, N. Keller, L. Nartey, S. Wandel, P. Jüni, and S. Trelle. 2017. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: A network meta-analysis. The Lancet 390 (10090): e21–e33. https://doi.org/10.1016/S0140-6736(17)31744-0.
Dai, L., X. Zhang, X. Hu, C. Zhou, and Y. Ao. 2012. Silencing of microRNA-101 prevents IL-1β-induced extracellular matrix degradation in chondrocytes. Arthritis Research & Therapy 14 (6): R268. https://doi.org/10.1186/ar4114.
de Jong, R.C.M., M.M. Ewing, M.R. de Vries, J.C. Karper, A.J.N.M. Bastiaansen, H.A.B. Peters, F. Baghana, P.J. van den Elsen, C. Gongora, J.W. Jukema, and P.H.A. Quax. 2017. The epigenetic factor PCAF regulates vascular inflammation and is essential for intimal hyperplasia development. PLoS One 12 (10): e0185820.
Eymard, F., A. Pigenet, D. Citadelle, C.-H. Flouzat-Lachaniette, A. Poignard, C. Benelli, F. Berenbaum, X. Chevalier, and X. Houard. 2014. Induction of an inflammatory and prodegradative phenotype in autologous fibroblast-like synoviocytes by the infrapatellar fat pad from patients with knee osteoarthritis. Arthritis & Rheumatology 66 (8): 2165–2174. https://doi.org/10.1002/art.38657.
Gaonkar, R.H., S. Ganguly, S. Dewanjee, S. Sinha, A. Gupta, S. Ganguly, D. Chattopadhyay, and M. Chatterjee Debnath. 2017. Garcinol loaded vitamin E TPGS emulsified PLGA nanoparticles: Preparation, physicochemical characterization, in vitro and in vivo studies. Scientific Reports 7 (1): 530. https://doi.org/10.1038/s41598-017-00696-6.
Glasson, S.S., T.J. Blanchet, and E.A. Morris. 2007. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis and Cartilage 15 (9): 1061–1069. https://doi.org/10.1016/j.joca.2007.03.006.
Glyn-Jones, S., A.J.R. Palmer, R. Agricola, A.J. Price, T.L. Vincent, H. Weinans, and A.J. Carr. 2015. Osteoarthritis. The Lancet 386 (9991): 376–387. https://doi.org/10.1016/S0140-6736(14)60802-3.
Wang, Y., M.L. Tsai, L.Y. Chiou, C.T. Ho, and M.H. Pan. 2015. Antitumor activity of garcinol in human prostate cancer cells and xenograft mice. Journal of Agricultural and Food Chemistry 63 (41): 9047–9052.
Hsu, C.-L., Y.-J. Lin, C.-T. Ho, and G.-C. Yen. 2013. The inhibitory effect of pterostilbene on inflammatory responses during the interaction of 3T3-L1 adipocytes and RAW 264.7 macrophages. Journal of Agricultural and Food Chemistry 61 (3): 602–610. https://doi.org/10.1021/jf304487v.
Huang, W.-C., K.-T. Kuo, B.O. Adebayo, C.-H. Wang, Y.-J. Chen, K. Jin, T.H. Tsai, and C.-T. Yeh. 2018. Garcinol inhibits cancer stem cell-like phenotype via suppression of the Wnt/β-catenin/STAT3 axis signalling pathway in human non-small cell lung carcinomas. The Journal of Nutritional Biochemistry 54: 140–150. https://doi.org/10.1016/j.jnutbio.2017.12.008.
Jia, Y., J. Jiang, X. Lu, T. Zhang, K. Zhao, W. Han, W. Yang, and Y. Qian. 2018. Garcinol suppresses RANKL-induced osteoclastogenesis and its underlying mechanism. Journal of Cellular Physiology 0 (0). https://doi.org/10.1002/jcp.27511.
Jia, Y., J. Jiang, X. Lu, T. Zhang, K. Zhao, W. Han, W. Yang, and Y. Qian. 2019. Garcinol suppresses RANKL-induced osteoclastogenesis and its underlying mechanism. Journal of Cellular Physiology 234 (5): 7498–7509.
Jianru, W., M. Dessislava, D. Greg Anderson, Zhaomin Zheng, Irving M. Shapiro, and Makarand V. Risbud. 2011. TNF-α and IL-1β promote a disintegrin-like and metalloprotease with thrombospondin type I motif-5-mediated aggrecan degradation through syndecan-4 in intervertebral disc. Journal of Biological Chemistry 286 (46): 39738–39749.
Kuno, K., Okada, Y., Kawashima, H., Nakamura, H., Miyasaka, M., Ohno, H., and Matsushima, K. ADAMTS-1 cleaves a cartilage proteoglycan, aggrecan. FEBS Letters 478 (3): 241–245. https://doi.org/10.1016/s0014-5793(00)01854-8.
Liao, C.-H., C.-T. Ho, and J.-K. Lin. 2005. Effects of garcinol on free radical generation and NO production in embryonic rat cortical neurons and astrocytes. Biochemical and Biophysical Research Communications 329 (4): 1306–1314. https://doi.org/10.1016/j.bbrc.2005.02.110.
Lichtenberger, L.M., Y. Zhou, E.J. Dial, and R.M. Raphael. 2010. NSAID injury to the gastrointestinal tract: Evidence that NSAIDs interact with phospholipids to weaken the hydrophobic surface barrier and induce the formation of unstable pores in membranes. Journal of Pharmacy and Pharmacology 58 (11): 1421–1428. https://doi.org/10.1211/jpp.58.10.0001.
Loeser, R.F. 2009. Aging and osteoarthritis: The role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthritis and Cartilage 17 (8): 971–979. https://doi.org/10.1016/j.joca.2009.03.002.
Lu, C., Y. Li, S. Hu, Y. Cai, Z. Yang, and K. Peng. 2018. Scoparone prevents IL-1β-induced inflammatory response in human osteoarthritis chondrocytes through the PI3K/Akt/NF-κB pathway. Biomedicine & Pharmacotherapy 106: 1169–1174. https://doi.org/10.1016/j.biopha.2018.07.062.
Moos, V., M. Rudwaleit, V. Herzog, K. Höhlig, J. Sieper, and B. Müller. 2001. Association of genotypes affecting the expression of interleukin-1β or interleukin-1 receptor antagonist with osteoarthritis. Arthritis and Rheumatism 43 (11): 2417–2422. https://doi.org/10.1002/1529-0131(200011)43:11<2417::AID-ANR7>3.0.CO;2-R.
Nasi, S., Ea, H.-K., So, A., & Busso, N. (2017). Revisiting the role of interleukin-1 pathway in osteoarthritis: Interleukin-1α and -1β, and NLRP3 inflammasome are not involved in the pathological features of the murine Menisectomy model of osteoarthritis. 8(282). https://doi.org/10.3389/fphar.2017.00282.
Priyanka, V., and D. Krishna. 2011. ADAMTS-4 and ADAMTS-5: Key enzymes in osteoarthritis. Journal of Cellular Biochemistry 112 (12): 3507–3514.
Roman-Blas, J.A., and S.A. Jimenez. 2006. NF-κB as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthritis and Cartilage 14 (9): 839–848. https://doi.org/10.1016/j.joca.2006.04.008.
Ruo-Hua, S., M.D. Tortorella, M. Anne-Marie, J.T. Alston, Y. Zhiyong, E.C. Arner, and D.W. Griggs. 2014. Aggrecan degradation in human articular cartilage explants is mediated by both ADAMTS-4 and ADAMTS-5. Arthritis & Rheumatology 56 (2): 575–585.
Ryu, Y. K., Park, H. Y., Go, J., Kim, Y. H., Hwang, J. H., Choi, D. H., . . . Lee, C. H. J. J. o. N. T. (2018). Effects of histone acetyltransferase inhibitors on l -DOPA-induced dyskinesia in a murine model of Parkinson’s disease. 125(2), 1–13.
Sandell, L.J., X. Xing, and C. Franz. 2008. Exuberant expression of chemokine genes by adult human articular chondrocytes in response to IL-1β. Osteoarthritis and Cartilage 16 (12): 1560–1571.
Sandy, J.D., C.R. Flannery, P.J. Neame, and L.S. Lohmander. 1992. The structure of aggrecan fragments in human synovial fluid. Evidence for the involvement in osteoarthritis of a novel proteinase which cleaves the Glu 373-Ala 374 bond of the interglobular domain. Journal of Clinical Investigation 36 (9): 1512–1516.
Sang, S., M.-H. Pan, X. Cheng, N. Bai, R.E. Stark, R.T. Rosen, S.Y. Lin-Shiau, J.K. Lin, and C.-T. Ho. 2001. Chemical studies on antioxidant mechanism of garcinol: Analysis of radical reaction products of garcinol and their antitumor activities. Tetrahedron 57 (50): 9931–9938. https://doi.org/10.1016/S0040-4020(01)01015-8.
Sethi, G., Chatterjee, S., Rajendran, P., Li, F., Shanmugam, M. K., Wong, K. F., . . . Kundu, T. K. J. M. C. (2014). Inhibition of STAT3 dimerization and acetylation by garcinol suppresses the growth of human hepatocellular carcinoma in vitro and in vivo. 13(1), 66. https://doi.org/10.1186/1476-4598-13-66
Tortorella, M.D., T.C. Burn, M.A. Pratta, I. Abbaszade, J.M. Hollis, R. Liu, et al. 1999. Purification and cloning of aggrecanase-1: A member of the ADAMTS family of proteins. Science 284 (5420): 1664–1666.
Tsuzaki, M., G. Guyton, W. Garrett, J.M. Archambault, W. Herzog, L. Almekinders, D. Bynum, X. Yang, and A.J. Banes. 2003. IL-1β induces COX2, MMP-1, −3 and −13, ADAMTS-4, IL-1β and IL-6 in human tendon cells. Journal of Orthopaedic Research 21 (2): 256–264. https://doi.org/10.1016/S0736-0266(02)00141-9.
Tu, S.H., Y.S. Chiou, N. Kalyanam, C.T. Ho, L.C. Chen, and M.H. Pan. 2017. Garcinol sensitizes breast cancer cells to taxol through the suppression of caspase-3/iPLA and NF-κB/Twist1 signaling pathways in a mouse 4T1 breast tumor model. Food & Function 8 (3): 1067–1079.
van der Kraan, P.M., and W.B. van den Berg. 2012. Chondrocyte hypertrophy and osteoarthritis: Role in initiation and progression of cartilage degeneration? Osteoarthritis and Cartilage 20 (3): 223–232. https://doi.org/10.1016/j.joca.2011.12.003.
Wang, B., J. Chen, F.S. Santiago, M. Janes, M.M. Kavurma, B.H. Chong, J.E. Pimanda, and L.M. Khachigian. 2010. Phosphorylation and acetylation of histone H3 and autoregulation by early growth response 1 mediate interleukin 1β induction of early growth response 1 transcription. Arteriosclerosis, Thrombosis, and Vascular Biology 30 (3): 536–545. https://doi.org/10.1161/atvbaha.109.193821.
Wang, J., D. Markova, D.G. Anderson, Z. Zheng, M.S. Irving, and V.R. Makarand. 2011. TNF-α and IL-1β promote a disintegrin-like and metalloprotease with thrombospondin type I motif-5-mediated aggrecan degradation through syndecan-4 in intervertebral disc. Journal of Biological Chemistry 286 (46): 39738–39749.
Wang, Y.-W., X. Zhang, C.-L. Chen, Q.-Z. Liu, J.-W. Xu, Q.-Q. Qian, et al. 2017. Protective effects of Garcinol against neuropathic pain – Evidence from in vivo and in vitro studies. Neuroscience Letters 647: 85–90. https://doi.org/10.1016/j.neulet.2017.03.015.
Wielage, R.C., J.A. Myers, R.W. Klein, and M. Happich. 2013. Cost-effectiveness analyses of osteoarthritis oral therapies: A systematic review. Applied Health Economics and Health Policy 11 (6): 593–618. https://doi.org/10.1007/s40258-013-0061-x.
Zhao, J., T. Yang, J. Ji, C. Li, and Z. Li. 2018. Garcinol exerts anti-cancer effect in human cervical cancer cells through upregulation of T-cadherin. Biomedicine Pharmacotherapie 107 (undefined): 957–966.
Zhong, L., X. Huang, M. Karperien, and N.J. Post. 2016. Correlation between gene expression and osteoarthritis progression in human. International Journal of Molecular Sciences 17 (7). https://doi.org/10.3390/ijms17071126.
Funding
This study was funded by the National Natural Science Foundation of China (grant nos. 81572126 and 81871801) and the Natural Science Foundation of Zhejiang Province (grant nos. LY15H060005 and LQ16H160013).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jia, Y., Pang, C., Zhao, K. et al. Garcinol Suppresses IL-1β-Induced Chondrocyte Inflammation and Osteoarthritis via Inhibition of the NF-κB Signaling Pathway. Inflammation 42, 1754–1766 (2019). https://doi.org/10.1007/s10753-019-01037-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-019-01037-7