Abstract
C1q/tumor necrosis factor-related protein-3 (CTRP3) is a novel, certified, adipokine that beneficially regulates metabolism and inflammation in the cardiovascular system. Atherosclerotic plaque rupturing and secondary thrombosis cause vascular disorders, such as myocardial infarction and unstable angina. However, the underlying role of CTRP3 in atherosclerosis remains unclear. In this study, we aimed to elucidate whether and how CTRP3 ameliorates inflammation and endothelial dysfunction caused by oxidized low-density lipoprotein (ox-LDL). We first confirmed that CTRP3 expression was inhibited in ApoE−/− mice, compared to normal mice. Then, pcDNA-CTRP3 and siCTRP3 were transfected into mouse aortic endothelial cells after ox-LDL stimulation, and we observed that enhanced CTRP3 remarkably downregulated CRP, TNF-α, IL-6, CD40, and CD40L. We also observed that overexpression of CTRP3 elevated cell activity and decreased lactated hydrogenase release, accompanied by a marked reduction in cell apoptosis induced by ox-LDL. Meanwhile, overexpressed CTRP3 caused a decrease in Ang II, ICAM-1, and VCAM-1 expression, and it restored the balance between ET-1 and NO. Mechanism analysis confirmed that incremental CTRP3 upregulated p-PI3K, p-Akt, and p-eNOS expression, indicating that CTRP3 facilitated activation of the PI3K/Akt/eNOS pathway. On the contrary, siCTRP3 exerted the opposite effect to this activation. Blocking these pathways using LY294002 or L-NAME attenuated the protective role of CTRP3. Overall, these results suggest that CTRP3 can efficiently inhibit the inflammatory response and endothelial dysfunction induced by ox-LDL in mouse aortic endothelial cells, perhaps by activating the PI3K/Akt/eNOS pathway, indicating a promising strategy against atherosclerosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Atherosclerosis is a major cause of cardio-cerebro-vascular diseases, which are the leading causes of mortality and disability worldwide [1]. Plaque rupture and secondary thrombus formation are the main causes of death and disability in cardiovascular disease [2]. The inflammatory response and endothelial dysfunction are highly associated with the stability of atherosclerotic plaques [3]. Thus, most research about atherosclerosis treatment targets these two areas. Although widely applied in atherosclerosis treatment, medical and surgical therapies have been ineffective [4]. Therefore, the molecular base and mechanism of atherosclerosis urgently require elucidation.
C1q/tumor necrosis factor–regulated proteins (CTRPs) belong to a highly conserved family of adiponectin paralogs [5]. The CTRP superfamily (CTRP1–15) contains an N-terminal collagenous region and a C-terminal globular head domain [6]. CTRPs have various physiological functions, including metabolism regulation, endothelial injury protection, and angiogenesis [7, 8]. CTRP3 is expressed in cartilage and has been found in murine and human adipose tissue, as well as monocytic and osteosarcoma cells [9]. CTRP3 is reported to suppress chemokine and cytokine release in adipocytes and monocytes in vitro [10]. Most importantly, CTRP3 can stimulate endothelial cell proliferation and migration and regulate the inflammatory response [11]. Evidence suggests that inflammation has vital roles in different stages of atherosclerosis [12]. However, the potential effect of CTRP3 on inflammatory response and endothelial dysfunction during atherosclerosis remains unclear.
Serine/threonine kinase (Akt) is a downstream effector of phosphatidylinositol-3-kinase (PI3K). Akt regulates cell survival and triggers apoptosis and angiogenesis in response to many death stimuli [13, 14]. PI3K causes the activation of Akt and its phosphorylation on threonine 308 and on serine 473 [15]. Akt also activates endothelial nitric oxide synthase (eNOS), which produces NO, mediating endothelial cell survival [16]. The PI3K/Akt/eNOS pathway likely ameliorates vascular endothelium dysfunction and atherosclerosis [17]. Activation of this pathway also could inhibit thrombosis [18]. CRTP3 is closely related to adiponectin, which is a direct activator of eNOS that may reverse endothelial dysfunction [19]. Low-density lipoproteins (LDL) can be trapped into arterial walls and oxidized in the course of atherosclerosis [20]. Oxidized low-density lipoprotein (ox-LDL), a key inducer in endothelial injury, stimulates endothelial cell inflammation, oxidative stress, and apoptosis and contributes to endothelial dysfunction and plaque destabilization, playing a major role in the development and progression of atherosclerosis [21, 22]. Given this background, we explored whether CTRP3 could alleviate inflammation and endothelial dysfunction in mouse aortic endothelial cells (MAECs) exposed to ox-LDL and the mechanisms involved in the PI3K/Akt/eNOS pathway.
MATERIALS AND METHODS
Cell Culture
Male homozygous mice that were deficient in apolipoprotein E (ApoE) were used for this study. The mice (n = 35) were randomly divided into two groups: an atherosclerosis (ApoE−/−) group and a control (ApoE) group. ApoE−/− mice (n = 25) weighing 30 ± 0.5 g and aged 12 weeks were obtained from the Jackson Laboratory (Bar Harbor, Maine, USA). They consumed an atherogenic diet of semi-purified chow containing 1.25% cholesterol and 0% cholate (Research Diets, New Brunswick, NJ, USA) for 12 weeks. Age-matched ApoE wild-type C57BL/6J mice (n = 10) weighing 29 ± 0.6 g were obtained from CEBIO/Instituto de Ciências Biológicas (UFMG, Belo Horizonte, MG, Brazil). All mice were kept in a controlled room (22 ± 3 °C, 45 ± 10% humidity) with a 12-h light/dark cycle in cages that were appropriate for their size. They were given sterile water. The mice were deprived of food starting 24 h before the experiment but allowed free access to water. All mice were sacrificed under anesthesia, and tissues were collected. All animal care and experimental procedures complied with guidelines for the humane use of laboratory animals in strict accordance with the Chinese Animal Welfare Legislation and were approved by the animal ethics committee of the Gansu Provincial Hospital of TCM.
Cell Culture and Administration
Mouse aortic endothelial cells (MAECs; ATCC, Manassas, VA, USA) were cultured in DMEM/F12 with 10% fetal bovine serum in a humidified incubator at 37 °C and 5% CO2. Cells were then exposed to 100 μg/mL of ox-LDL for 48 h. MAECs were seeded into 6-well plates with serum-free media and incubated at 37 °C. MAECs were randomly divided into six groups: control (MAECs), ox-LDL (100 μg/mL), pcDNA-CTRP3 (10 μg), siCTRP3 (5 μg), pcDNA-CTRP3 + LY294002, and pcDNA-CTRP3 + L-NAME. Ox-LDL was obtained from the Beijing Xiesheng Biotechnology (Beijing, China). The PI3K/Akt agonist LY294002 (10 μM) and the eNOS inhibitor L-NAME (3 mM) were obtained from Sigma (St. Louis, MO, USA). MAECs were stimulated with ox-LDL for 48 h. LY294002 and L-NAME, when used, were added 30 min prior to adding ox-LDL.
Small Interfering RNA (siRNA) and Overexpression Transfection
To silence CTRP3, siRNA-targeting CTRP3 was designed, synthesized, and verified by Sigma-Aldrich (St. Louis, Missouri, USA) (siCTRP3 group). A scrambled sequence was used as a control for knockdown analysis. To assess the overexpression of CTRP3, pcDNA3.1(+)-CTRP3 was constructed as previously described [23]. Briefly, total RNA was extracted from MAECs using TRIZol (Invitrogen, Carlsbad, CA, USA) and reverse-transcribed to cDNA using the SuperScript II First-Strand Synthesis System (Invitrogen). The full-length CTRT3 cDNA was amplified using two primers [24]: sense primer 5′-GCCCCCGTATCAGGTGTGTATTT-3′ and antisense primer 5′-TGAAGACTGTGTTGCCGTTGTGC-3′. Subsequently, the full-length sequences of CTRP3 were subcloned into the pcDNA3.1(+) vector to generate the recombinant plasmids of CTRP3-pcDNA3.1(+). An empty vector served as the negative control. For transfection, the recombinant plasmids of pcDNA3.1(+)-CTRP3 (pcDNA-CTRP3 group) or siCTRP3 were transfected into MAECs, respectively, using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol.
qRT-PCR Assay
Total RNA was extracted from the mice aorta tissues and MAECs using TRIzol reagent (Takara Bio, Inc., Otsu, Japan) and reverse-transcribed into cDNA using the PrimeScript™ RT reagent kit (Takara Bio, Inc.) according to the manufacturer’s instructions. Real-time quantitative polymerase chain reaction (RT-qPCR) was performed using an Applied Biosystems StepOnePlus Real-Time PCR system and SYBR®-Green PCR master mix (both from Applied Biosystems, Foster City, CA, USA). The mRNA expression levels of CTRP3, CRP, TNF-α, IL-6, CD40, and CD40L were measured via RT-qPCR with an ICycler IQ Real-Time PCR detection system (Bio-Rad Laboratories Inc., Hercules, CA, USA). Each measurement was repeated in triplicate. The relative mRNA expression of each molecule was calculated using the 2-ΔΔCt method and normalized to GADPH. The primer sequences are as follows. CTRP3 forward 5′-ATGGAGGTGAGCAGAAGAGC-3′ and reverse 5′-CACAGTCCCCGTTTTAGCAT-3′, CRP forward 5′-CTCGGACTTTTGGTCATGAAG-3′ and reverse 5′-AAAGGTGTTCAGTGGCTTCTTT-3′, TNF-α forward 5′-CCAGACCCTCACACTCACAA-3′ and reverse 5′-TTGAGATCCATGCCGTTG-3′, IL-6 forward 5′-CCTCTCTGCAAGAGACTTCCAT-3′ and reverse 5′-AGTCTCCTCTCCGGACTTGT-3′, CD40 forward 5′-AATCTAGATGCCGCCTGGTCTCACCTCG-3′ and reverse 5′-AAAAGCTTGCCAACTGCCTGTTTGCCCACG-3′, CD40L forward 5′-GACGTCAGCATGATAGAAACATACAGCCAACCT-3′ and reverse 5′-GCCGAATTCTCAGAGTTTGAGTAAGCCAAAAGA-3′, GAPDH forward 5′-GGTGAAGGTCGGAGTCAACG-3′ and reverse 5′-TGGGTGGAATCATATTGGAACA-3′.
Western Blot Analysis
Total protein from the MAECs was extracted by RIPA Lysis Buffer containing PMSF, according to the manufacturer’s instructions (Beyotime, Shanghai, China). Protein concentration was measured using the Bradford method. Equal amounts of protein were electrophoresed by 15% SDS-PAGE and transferred to polyvinyl difluoride membranes. The membranes were blocked in 5% non-fat milk in Tris-buffered saline, followed by incubation with primary antibodies, including anti-CTRP3 (1:1000; Abcam, Cambridge, UK), anti-PI3K (1:1000, Sigma), anti-p-PI3K (1:1000, Sigma), anti-Akt (1:1000, Sigma), anti-p-Akt (1:1000, Sigma), anti-eNOS (1:200, Santa Cruz Biotechnology Inc., CA, USA), anti-p-eNOS (1:500, Santa Cruz Biotechnology Inc.), and β-actin (1:2000, Santa Cruz Biotechnology Inc.) at 4 °C overnight. Secondary antibodies were labeled with HRP (1:10000, Bio-Rad Laboratories), and the signals were detected using an ECL kit (Pierce Biotech, Rockford, IL, USA). Subsequently, the images were analyzed by Image J 1.43 software. Each measurement was normalized to GAPDH.
ELISA Assay
The concentrations of CRP, TNF-a, IL-6, CD40, CD40L, Ang II, ICAM-1, VCAM-1, ET-1, and NO in MAECs were measured using the corresponding ELISA kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. Plates were read using an ELISA reader (Bio-Tek, Winooski, VT, USA) at a wavelength of 450 nm.
MTT Assay
Cell viability was investigated by measuring the metabolism of MTT. The MTT solution (5 mg/mL) was added to the culture medium, and the pretreated arterial endothelial cells were sustained for 4 h at 37 °C, after which the MTT solution was removed. The addition of 20% SDS and 50% dimethylformamide were used to dissolve the formazan. Absorptions at 490 nm were measured. Cell viability was determined as the percentage of living cells.
Lactated Hydrogenase Activity Assay
Cell injury was quantified by measuring LDH release at 6 h, using a colorimetric LDH cytotoxicity detection kit (Clontech, Mountain View, CA, USA) according to the manufacturer’s manuals. LDH activity was determined via a colorimetric assay using an absorbance wavelength of 492 nm and a reference wavelength of 655 nm in a spectrophotometer (Bio-Rad Laboratories). Background absorbance was subtracted, and the percentage of LDH release was calculated based on the LDH standard curve.
Flow Cytometry Analysis
Cell apoptosis was detected using the flow cytometry method and an Annexin V-Propidium Iodide (PI) Apoptosis Detection Kit (Abcam). Briefly, the cells were collected, washed with PBS, and suspended in 500 mL of binding buffer. The cells were incubated with Annexin V at room temperature for 10 min and then stained with propidium iodide. The percentage of apoptotic cells was analyzed using flow cytometry.
Statistical Analysis
The data were expressed as the mean ± standard deviation (SD). Levels of significance between or among groups were analyzed by two-tailed t test or one-way ANOVA, respectively. All statistical parameters were calculated using SPSS version 19.0 software. P values < .05 were considered statistically significant.
RESULTS
CTRP3 Expression Is Downregulated in Atherosclerotic Lesions
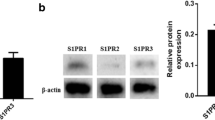
To investigate the role of CTRP3 during atherosclerosis, we detected the mRNA and protein levels of CTRP3 in the atherosclerotic lesions of ApoE−/− mice. We confirmed that the mRNA level of CTRP3 was remarkably downregulated in the atherogenic diet-induced atherosclerosis group, compared to the control (Fig. 1a). Similarly, the protein level of CTRP3 also was distinctly downregulated in mice with atherosclerosis (Fig. 1b). These findings suggest that the abnormally low level of CTRP3 is highly relative in atherosclerosis, implying an important role of CTRP3 in atherosclerotic lesions.
Expression of CTRP3 in atherosclerotic lesions of atherogenic ApoE−/− mice. ApoE−/− mice were fed an atherogenic diet for 12 weeks to construct the mouse model of atherosclerosis. Aortas of the two different groups were collected. a The mRNA level of CTRP3 in atherosclerotic lesions was assessed by RT-PCR assay. b The protein level of CTRP3 in atherosclerotic lesions was assessed by Western blot analysis. *P < 0.05 vs. control group.
CTRP3 Overexpression Relieved Inflammatory Response in Ox-LDL–Induced MAECs
MAECs damage can lower the effectiveness of the endothelium, which serves as a barrier against plasma components. MAECs dysfunction is closely associated with atherosclerosis etiology. To elucidate the effect of CTRP3 in atherosclerosis, we assessed the role of CTRP3 in MAECs inflammation, as mediated by ox-LDL. We co-transfected pcDNA-CTRP3 and siCTRP3 into MAECs to verify the change in CTRP3 expression and its effect on the inflammatory response triggered by ox-LDL. After 48 h, CTRP3 was distinctly upregulated with pcDNA-CTRP3 treatment and downregulated with siCTRP3 treatment (Fig. 2a, b).
Effect of CTRP3 on the inflammatory response in ox-LDL–induced MAECs. After transfection with pcDNA-CTRP3 (10 μg) or siCTRP3 (5 μg), MAECs were stimulated with ox-LDL (100 μg/mL) for 12 h. a The transfection efficiency of pcDNA-CTRP3 and siCTRP3 was detected by RT-PCR assay. b The protein level of CTRP3 was detected by Western blot. c–e The mRNA levels of CRP, TNF-α, IL-6, CD40, and CD40L were detected by RT-PCR assay. f–h CRP, TNF-α, IL-6, CD40, and CD40L levels were detected by ELISA assay. *P < 0.05 vs. control group; #P < 0.05 vs. ox-LDL group.
Then, we detected the expression of inflammatory markers CRP, TNF-α, and IL-6 and found that all were distinctly upregulated in ox-LDL–induced MAECs (Fig. 2c–d, f–g), which were remarkably decreased by pcDNA-CTRP3 transfection. On the contrary, siCTRP3 obviously enhanced ox-LDL–induced CRP, TNF-α, and IL-6 production. CD40 and CD40L also were upregulated in ox-LDL–induced MAECs, compared with normal controls (Fig. 2e, h). Moreover, expression of CD40 and CD40L was reduced after CTRP3 overexpression. Meanwhile, CTRP3 downregulation further enhanced ox-LDH-increased transcripts of CD40 and CD40L, as well as their production. These findings indicate that CTRP3 negatively regulated the inflammatory response in MAECs exposed to ox-LDL.
Overexpression of CTRP3 Ameliorated Ox-LDL–Induced Endothelial Dysfunction
We further evaluated the effect of CTRP3 on ox-LDL–induced endothelial dysfunction and observed that ox-LDL treatment led to a significant decrease in cell viability, whereas LDH release was strongly elevated (Fig. 3a, b). Enhancing CTRP3 expression reversed the decreased cell activity and increased LDH release in ox-LDL–induced MAECs. CTRP3 silencing promoted ox-LDL–induced cell viability decrease and LDH leakage increase (Fig. 3a, b). In marked contrast to the untreated group, the ox-LDL–induced MAECs contained more apoptotic cells (Fig. 3c, d). We also found that pcDNA-CTRP3 treatment diminished apoptosis in MAECs exposed to ox-LDL. However, the rate of apoptosis cells was enhanced after siCTRP3 transfection. Further study revealed that CTRP3 overexpression remarkably attenuated the increase of Ang II in ox-LDL–induced MAECs, but this increase was observed after siCTRP treatment (Fig. 3e). Moreover, the elevated level of circulating ET-1 was found to be expressed in atherosclerotic patients [25] and increased in atherosclerotic lesions. Overexpression of CTRP3 effectively reduced ET-1, increased NO, and reversed the unfavorable imbalance between ET-1 and NO in ox-LDL–induced MAECs (Fig. 3f, g). CTRP3 inhibition increased ET-1 in ox-LDL–treated cells but reduced NO. Additionally, both ICAM-1 and VCAM-1 were reduced by pcDNA-CTRP3 transfection (Fig. 3h). Meanwhile, significantly higher levels of ICAM-1 and VCAM-1 were found in siCTRP3-transfected MAECs than ox-LDL group. These results suggest that overexpression of CTRP3 may repress the endothelial dysfunction induced by ox-LDL, thus preventing atherosclerosis progression.
Effect of CTRP3 on endothelial dysfunction in ox-LDL–induced MAECs. After transfection with pcDNA-CTRP3 (10 μg) or siCTRP3 (5 μg), MAECs were stimulated with ox-LDL (100 μg/mL) for 12 h. a Cell activity was measured by MTT assay. b The change of LDH release was determined by a colorimetric LDH cytotoxicity detection kit. c Quantitative analysis of cell apoptosis. d The cell apoptosis was detected by flow cytometry analysis in each group. e–h Ang II, ET-1, NO, ICAM-1, and VCAM-1 levels were detected by ELISA assay.*P < 0.05 vs. control group; #P < 0.05 vs. ox-LDL group.
CTRP3 Reversed the Inactivation of the PI3K/Akt/eNOS Pathway Triggered by Ox-LDL
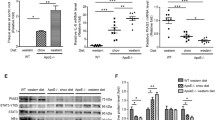
To further determine how CTRP3 is involved in atherosclerosis, we aimed to clarify the underlying mechanism regarding the protective effects of CTRP3 on the inflammatory response and on endothelial dysfunction. According to Fig. 4a and b, protein levels of p-PI3K, p-Akt, and p-eNOS were significantly decreased in MAECs after ox-LDL treatment. However, enhanced CTRP3 reversed the decrease in p-PI3K, p-Akt, and p-eNOS in the ox-LDL group, suggesting that CTRP3 can activate the PI3K/Akt/eNOS pathway in atherosclerosis. Unsurprisingly, the expression of p-PI3K, p-Akt, and p-eNOS was shown a further decrease in ox-LDL–induced MAECs after downregulating CTRP3. These data indicate that CTRP3 assisted in PI3K/Akt/eNOS pathway activation in ox-LDL–induced MAECs.
CTRP3 antagonized ox-LDL–induced inflammation and endothelial dysfunction in MAECs by activating the PI3K/Akt/eNOS pathway. After treatment with LY294002 or L-NAME, MAECs were transfected with pcDNA-CTRP3 or siCTRP3 plasmids and exposed to ox-LDL. a Representative blots of p-PI3K, p-Akt, and p-eNOS were determined by Western blot. b Quantitative analysis of p-PI3K, p-Akt, and p-eNOS expression. *P < 0.05 vs. control group; #P < 0.05 vs. ox-LDL group. c Cell activity was measured by MTT assay. d The change in LDH release was determined by a colorimetric LDH cytotoxicity detection kit. e Quantitative analysis of cell apoptosis. f–j CRP, TNF-α, IL-6, Ang II, ET-1, and NO levels were detected by ELISA assay. #P < 0.05 vs. ox-LDL group; &P < 0.05 vs. pcDNA-CTRP3 group.
CTRP3 Inhibited Ox-LDL–Induced Inflammation and Endothelial Dysfunction, Probably via PI3K/Akt/eNOS Pathway Activation
To test whether PI3K/Akt/eNOS pathway activation exerts a vital role in CTRP3-inhibited inflammation and dysfunction in ox-LDL–induced MAECs, we measured the effects of PI3K/Akt/eNOS pathway antagonists on inflammation and endothelial dysfunction. As selective blockers of the PI3K/Akt/eNOS pathway, LY294002 and L-NAME abolish decreases in p-PI3K, p-Akt, and p-eNOS expression during atherosclerosis. We observed that the protective effect of CTRP3 on cell activity, LDH leakage, and apoptosis were reduced when the PI3K/Akt/eNOS pathway was inhibited by LY294002 or L-NAME (Fig. 4c–e). Further mechanism analysis showed that, after pretreatment with LY294002 or L-NAME alone, the inhibitory effects of CTRP3 on ox-LDL–triggered CRP, TNF-α, and IL-6 production decreased, accompanied by increases in Ang II and ET-1 and a decrease in NO production (Fig. 4f–j). Thus, the inhibitory effects of CTRP3 overexpression on ox-LDL–induced inflammation and endothelial dysfunction appear to be closely associated with activation of the PI3K/Akt/eNOS pathway.
DISCUSSION
Many factors contribute to severe cardiovascular disease, including inflammation, thrombosis, platelet activation, and endothelial dysfunction [26, 27]. The rupture of atherosclerotic plaques and secondary thrombosis are the major causes of most acute vascular events, such as stroke and acute myocardial infarction [2]. Inflammation and endothelial disorders facilitate plaque rupture [28]. Therefore, preventing inflammation and inhibiting endothelial dysfunction are critical for slowing atherosclerosis. It is therefore necessary to seek a new strategy for treating inflammation and endothelial dysfunction caused by atherosclerotic lesion.
CTRP3, a paralog of adiponectin, is expressed in adipose tissue and can restrain the release of chemokines and cytokines in adipocytes and monocytes [29]. CTRP3 may promote the proliferation and migration of endothelial cells [30]. However, no study has investigated the association between CTRP3 and inflammation or endothelial dysfunction in atherosclerosis. Our study preliminary verified that the expression of CTRP3 is significantly decreased in mice with atherosclerosis, suggesting that CTRP3 upregulation may be required for remission.
Ox-LDL is a major factor in endothelial disorders during atherosclerosis [31]. Ox-LDL damages endothelial cells and enhances endothelial cell adhesion and adhesion molecules [32]. ICAM-1 and VCAM-1 are important endothelial adhesion molecules that are overexpressed at atherosclerosis-prone sites on the endothelium in ApoE-deficient mice [33]. Ox-LDL can increase the release of the ICAM-1 and VCAM-1 from damaged endothelial cells, which promotes the attachment of inflammatory factors to dysfunctional endothelial cells [34]. Our study showed that ox-LDL caused a decrease in cell viability, an increase in LDH leakage, and an increase in ICAM-1 and VCAM-1 expression and that CTRP3 reversed these events.
Vascular homeostasis is maintained by the release of vasoactive substances. NO promotes angiogenesis in endothelial cells, and vasodilation opposes the effects of endothelium-derived vasoconstrictors such as ET-1 [35]. Determination of the Ang II level is a basic method for assessing endothelial function [36]. Increased Ang II has been implicated in atherogenesis, and Ang II can promote atherosclerotic lesions [37]. We confirmed that CTRP3 can suppress Ang II in MEACs exposed to ox-LDL. We found that CTRP3 counteracted ox-LDL, decreased production of ET-1, and increased production of NO. Apoptosis of endothelial cells induced by ox-LDL could also promote endothelial dysfunction, which is a necessary condition for atherosclerosis [38]. CTRP3 replenishment attenuates cardiomyocyte apoptosis in the ischemic mouse heart [8]. It also protects against high-glucose–induced apoptosis in rats with diabetic cardiomyopathy [39]. Our results indicate that CTRP3 distinctly reversed MAEC apoptosis induced by ox-LDL, indicating that CTRP3 may be available for endothelial repair during atherosclerosis by suppressing endothelial cell apoptosis.
Inflammation plays a key part in atherosclerosis, including plaque rupture [40]. Numerous studies have proposed that ox-LDL contributes to atherosclerotic inflammation [41]. Ox-LDL stimulation allows LDL particles to penetrate the endothelial barrier, and it allows phenotypic changes in cells to be captured in endothelial cells [42]. Ox-LDL triggers an increase in the expression of proinflammatory cytokines and in chemokine expression in vascular endothelial cells [43]. Most studies have shown that circulating inflammatory markers, such as CRP, TNF-α, and IL-6, are elevated in atherosclerosis plaque and commonly accompany atherosclerosis [44]. Our results demonstrate that CTRP3 downregulated CRP, TNF-α, and IL-6 in ox-LDL–treated MEACs. CD40-CD40L interactions have been shown to promote atherosclerotic plaques and to aggravate established lesions [45]. Our data indicate that CTRP3 reduced expression of CD40 and CD40L in ox-LDL–induced MAECs. Taken together, these results hint that CTRP9 protected against endothelial dysfunction and inhibited the inflammatory response to ox-LDL.
The PI3K/Akt signaling pathway is essential for regulating various cellular and molecular functions [18]. It is reported that eNOS is involved in regulating vascular function and can produce NO [46]. In the endothelial cells, PI3K activates Akt, thereby directly phosphorylating eNOS [47]. The PI3K/Akt/eNOS pathway plays a crucial role in ox-LDL–induced endothelial cells [48] and atherosclerosis [17]. Endothelial dysfunction is relative to the suppression of the PI3K/AKT/eNOS pathway [49], which has an important role in improving endothelial function and alleviating atherosclerosis [50]. Herein, we showed that CTRP3 ameliorates the ox-LDL–induced inhibition of PI3K/Akt/eNOS phosphorylation. Pretreatment with the depressor LY294002 or L-NAME remarkably abrogated the effect of CTRP3 on ox-LDL–induced inflammation and endothelial dysfunction. These findings suggest that the protective effect of CTRP3 on ox-LDL–induced inflammation and endothelial dysfunction may be achieved partly by regulating the PI3K/Akt/eNOS pathway during atherosclerosis.
In summary, these results confirm that overexpressed CTRP3 may provide a novel approach for preventing inflammation and arterial endothelial dysfunction induced by ox-LDL. They also show that the inhibitory effect of CTRP3 in atherosclerosis is due at least in part to its ability to activate the PI3K/Akt/eNOS pathway and thus CTRP3 may be a novel target for preventing atherosclerosis and plaque instability.
References
Guo, Y., X.C. Liu, Y.J. Wang, Q. Li, Q. Yang, X.G. Weng, Y. Chen, W.Y. Cai, X.X. Kan, and X. Chen. 2016. Effects of Shenlian extract on experimental atherosclerosis in ApoE-deficient mice based on ultrasound biomicroscopy. Bmc Complementary & Alternative Medicine 16: 469.
Badimon, L., and G. Vilahur. 2014. Thrombosis formation on atherosclerotic lesions and plaque rupture. Journal of Internal Medicine 276: 618–632.
Ragino, Y.I., A.M. Chernjavski, Y.V. Polonskaya, A.M. Volkov, E.V. Kashtanova, A.V. Tikhonov, and S.Y. Tcimbal. 2012. Oxidation and endothelial dysfunction biomarkers of atherosclerotic plaque instability. Studies of the vascular wall and blood. Bulletin of Experimental Biology & Medicine 153: 331–335.
Chong, D.P.H., and B.S. Bachenheimer. 2000. Current, new and future treatments in Dyslipidaemia and atherosclerosis. Drugs 60: 55–93.
Yoo, H.J., S.Y. Hwang, H.C. Hong, H.Y. Choi, S.J. Yang, S.C. Dong, S.H. Baik, M. Blüher, B.S. Youn, and K.M. Choi. 2013. Implication of progranulin and C1q/TNF-related protein-3 (CTRP3) on inflammation and atherosclerosis in subjects with or without metabolic syndrome. PLoS One 8: e55744.
Kim, J.Y., J.Y. Min, J.M. Baek, S.J. Ahn, H.Y. Jun, K.H. Yoon, M.K. Choi, M.S. Lee, and J. Oh. 2015. CTRP3 acts as a negative regulator of osteoclastogenesis through AMPK-c-Fos-NFATc1 signaling in vitro and RANKL-induced calvarial bone destruction in vivo. Bone 79: 242–251.
Sun, H., X. Zhu, Y. Zhou, W. Cai, and L. Qiu. 2017. C1q/TNF-related Protein-9 ameliorates ox-LDL-induced endothelial dysfunction via PGC-1α/AMPK-mediated antioxidant enzyme induction. International Journal of Molecular Sciences 18: 1097.
Yi, W., Y. Sun, Y. Yuan, W.B. Lau, Q. Zheng, X. Wang, Y. Wang, X. Shang, E. Gao, W.J. Koch, and X.L. Ma. 2012. C1q/tumor necrosis factor-related protein-3, a newly identified adipokine, is a novel antiapoptotic, proangiogenic, and cardioprotective molecule in the ischemic mouse heart. Circulation 125: 3159–3169.
Hofmann, C., N. Chen, F. Obermeier, G. Paul, C. Büchler, A. Kopp, W. Falk, and A. Schäffler. 2011. C1q/TNF-related Protein-3 (CTRP-3) is secreted by visceral adipose tissue and exerts antiinflammatory and antifibrotic effects in primary human colonic fibroblasts. Inflammatory Bowel Diseases 17: 2462–2471.
Schmid, A., A. Kopp, F. Hanses, T. Karrasch, and A. Schäffler. 2014. C1q/TNF-related protein-3 (CTRP-3) attenuates lipopolysaccharide (LPS)-induced systemic inflammation and adipose tissue Erk-1/-2 phosphorylation in mice in vivo. Biochemical & Biophysical Research Communications 452: 8–13.
Wang, Y., L. Tu, Y. Li, D. Chen, and S. Wang. 2016. Notoginsenoside R1 protects against neonatal cerebral hypoxic-ischemic injury through estrogen receptor-dependent activation of endoplasmic reticulum stress pathways. The Journal of Pharmacology and Experimental Therapeutics 357: 591–605.
Pende, A., A. Denegri. 2012. An anti-inflammatory approach in the therapeutic choices for the prevention of atherosclerotic events: InTech, 158–164.
Chang, Y., Q. Wu, T. Tian, L. Li, X. Guo, Z. Feng, J. Zhou, L. Zhang, S. Zhou, and G. Feng. 2015. The influence of SRPK1 on glioma apoptosis, metastasis, and angiogenesis through the PI3K/Akt signaling pathway under normoxia. Tumour Biology the Journal of the International Society for Oncodevelopmental Biology & Medicine 36: 6083–6093.
Ou, H.C., W.J. Lee, S.D. Lee, C.Y. Huang, T.H. Chiu, K.L. Tsai, W.C. Hsu, and W.H. Sheu. 2010. Ellagic acid protects endothelial cells from oxidized low-density lipoprotein-induced apoptosis by modulating the PI3K/Akt/eNOS pathway. Toxicology & Applied Pharmacology 248: 134–143.
Wygrecka, M., D. Zakrzewicz, B. Taborski, M. Didiasova, G. Kwapiszewska, K.T. Preissner, and P. Markart. 2012. TGF-Î21 induces tissue factor expression in human lung fibroblasts in a PI3K/JNK/Akt-dependent and AP-1-dependent manner. American Journal of Respiratory Cell & Molecular Biology 47: 614–627.
Xing, S.S., X.Y. Yang, T. Zheng, W.J. Li, D. Wu, J.Y. Chi, F. Bian, X.L. Bai, G.J. Wu, and Y.Z. Zhang. 2015. Salidroside improves endothelial function and alleviates atherosclerosis by activating a mitochondria-related AMPK/PI3K/Akt/eNOS pathway. 中国药理学通报 72: 141.
Li, T., D. Li, H. Xu, H. Zhang, D. Tang, and H. Cao. 2016. Wen-Xin decoction ameliorates vascular endothelium dysfunction via the PI3K/AKT/eNOS pathway in experimental atherosclerosis in rats. BMC Complementary and Alternative Medicine 16: 27.
Dong, R., W. Chen, W. Feng, C. Xia, D. Hu, Y. Zhang, Y. Yang, D.W. Wang, X. Xu, and L. Tu. 2015. Exogenous bradykinin inhibits tissue factor induction and deep vein thrombosis via activating the eNOS/phosphoinositide 3-kinase/Akt signaling pathway. Cellular Physiology and Biochemistry: International Journal of Experimental Cellular Physiology, Biochemistry, and Pharmacology 37: 1592–1606.
Deng, G., Y. Long, Y.R. Yu, and M.R. Li. 2010. Adiponectin directly improves endothelial dysfunction in obese rats through the AMPK-eNOS pathway. International Journal of Obesity 34: 165–171.
Feng, X., Y. Zhang, R. Xu, X. Xie, L. Tao, H. Gao, Y. Gao, Z. He, and H. Wang. 2010. Lipopolysaccharide up-regulates the expression of Fcalpha/mu receptor and promotes the binding of oxidized low-density lipoprotein and its IgM antibody complex to activated human macrophages. Atherosclerosis 208: 396–405.
Matsuura, E., F. Atzeni, P. Sarzi-Puttini, M. Turiel, L.R. Lopez, and M.T. Nurmohamed. 2014. Is atherosclerosis an autoimmune disease? BMC Medicine 12: 47.
Mitra, S., A. Deshmukh, R. Sachdeva, J. Lu, and J.L. Mehta. 2011. Oxidized low-density lipoprotein and atherosclerosis implications in antioxidant therapy. The American Journal of the Medical Sciences 342: 135–142.
Huang, Y., G. Wan, and J. Tao. 2017. C1q/TNF-related protein-3 exerts the chondroprotective effects in IL-1beta-treated SW1353 cells by regulating the FGFR1 signaling. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 85: 41–46.
Otani, M., S. Furukawa, S. Wakisaka, and T. Maeda. 2015. A novel adipokine C1q/TNF-related protein 3 is expressed in developing skeletal muscle and controls myoblast proliferation and differentiation. Molecular and Cellular Biochemistry 409: 271–282.
Zhou, B.Y., Y.L. Guo, N.Q. Wu, C.G. Zhu, Y. Gao, P. Qing, X.L. Li, Y. Wang, Q. Dong, G. Liu, R.X. Xu, C.J. Cui, J. Sun, and J.J. Li. 2017. Plasma big endothelin-1 levels at admission and future cardiovascular outcomes: A cohort study in patients with stable coronary artery disease. International Journal of Cardiology 230: 76–79.
Jr, W.T. 2007. Coronary atherosclerosis, low-density lipoproteins and markers of thrombosis, inflammation and endothelial dysfunction. International Journal of Angiology 16: 12.
Hua, S., C. Song, C.L. Geczy, S.B. Freedman, and P.K. Witting. 2009. A role for acute-phase serum amyloid a and high-density lipoprotein in oxidative stress, endothelial dysfunction and atherosclerosis. Redox Report Communications in Free Radical Research 14: 187–196.
Tedgui, A. 2011. The role of inflammation in atherothrombosis: Implications for clinical practice. Vascular Medicine 10: 45–53.
Kopp, A., M. Bala, C. Buechler, W. Falk, P. Gross, M. Neumeier, J. Schölmerich, and A. Schäffler. 2010. C1q/TNF-related protein-3 represents a novel and endogenous lipopolysaccharide antagonist of the adipose tissue. Endocrinology 151: 5267–5278.
Akiyama, H., S. Furukawa, S. Wakisaka, and T. Maeda. 2007. CTRP3/cartducin promotes proliferation and migration of endothelial cells. Molecular and Cellular Biochemistry 304: 243–248.
Mitra, S., T. Goyal, and J.L. Mehta. 2011. Oxidized LDL, LOX-1 and atherosclerosis. Cardiovascular Drugs and Therapy 25: 419–429.
Qiu, M.K., S.C. Wang, Y. Tang, C. Pan, Y. Wang, S.Q. Wang, Z.W. Quan, and J.M. Ou. 2017. Tim-3 inhibits low-density lipoprotein-induced atherogenic responses in human umbilical vein endothelial cells. Oncotarget 8: 61001–61010.
Nakashima, Y., E.W. Raines, A.S. Plump, J.L. Breslow, and R. Ross. 1998. Upregulation of VCAM-1 and ICAM-1 at atherosclerosis-prone sites on the endothelium in the ApoE-deficient mouse. Arteriosclerosis, Thrombosis, and Vascular Biology 18: 842–851.
Zhao, W., C. Wu, and X. Chen. 2016. Cryptotanshinone inhibits oxidized LDL-induced adhesion molecule expression via ROS dependent NF-kappaB pathways. Cell Adhesion & Migration 10: 248–258.
Kojda, G., and D. Harrison. 1999. Interactions between NO and reactive oxygen species: Pathophysiological importance in atherosclerosis, hypertension, diabetes and heart failure. Cardiovascular Research 43: 562–571.
MA, B., D. I, and L. JH. 2015. Salt, angiotensin II, superoxide, and Endothelial Function. Comprehensive Physiology 6: 215.
Daugherty, A., M.W. Manning, and L.A. Cassis. 2000. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. The Journal of Clinical Investigation 105: 1605–1612.
Suo, J., L. Zhao, J. Wang, Z. Zhu, H. Zhang, and R. Gao. 2015. Influenza virus aggravates the ox-LDL-induced apoptosis of human endothelial cells via promoting p53 signaling. Journal of Medical Virology 87: 1113–1123.
Ma, Z.G., Y.P. Yuan, S.C. Xu, W.Y. Wei, C.R. Xu, X. Zhang, Q.Q. Wu, H.H. Liao, J. Ni, and Q.Z. Tang. 2017. CTRP3 attenuates cardiac dysfunction, inflammation, oxidative stress and cell death in diabetic cardiomyopathy in rats. Diabetologia 60: 1126–1137.
Pant, S., A. Deshmukh, G.S. Gurumurthy, N.V. Pothineni, T.E. Watts, F. Romeo, and J.L. Mehta. 2014. Inflammation and atherosclerosis--revisited. Journal of Cardiovascular Pharmacology and Therapeutics 19: 170–178.
Farmer, J.A., and G. Torre-Amione. 2002. Atherosclerosis and inflammation. Current Atherosclerosis Reports 4: 92–98.
Steinberg, D. 1997. Lewis A. Conner Memorial Lecture. Oxidative modification of LDL and atherogenesis. Circulation 95: 1062–1071.
Trpkovic, A., I. Resanovic, J. Stanimirovic, D. Radak, S.A. Mousa, D. Cenic-Milosevic, D. Jevremovic, and E.R. Isenovic. 2015. Oxidized low-density lipoprotein as a biomarker of cardiovascular diseases. Critical Reviews in Clinical Laboratory Sciences 52: 70–85.
Wang, S.S., S.W. Hu, Q.H. Zhang, A.X. Xia, Z.X. Jiang, and X.M. Chen. 2015. Mesenchymal stem cells stabilize atherosclerotic vulnerable plaque by anti-inflammatory properties. PLoS One 10: e0136026.
Schönbeck, U., G.K. Sukhova, K. Shimizu, F. Mach, and P. Libby. 2000. Inhibition of CD40 signaling limits evolution of established atherosclerosis in mice. Proceedings of the National Academy of Sciences of the United States of America 97: 7458–7463.
Forstermann, U., and H. Li. 2011. Therapeutic effect of enhancing endothelial nitric oxide synthase (eNOS) expression and preventing eNOS uncoupling. British Journal of Pharmacology 164: 213–223.
Ning, W.H., and K. Zhao. 2013. Propionyl-L-carnitine induces eNOS activation and nitric oxide synthesis in endothelial cells via PI3 and Akt kinases. Vascular Pharmacology 59: 76–82.
Yao, Y., Y. Wang, Y. Zhang, and C. Liu. 2017. Klotho ameliorates oxidized low density lipoprotein (ox-LDL)-induced oxidative stress via regulating LOX-1 and PI3K/Akt/eNOS pathways. Lipids in Health and Disease 16: 77.
Garcia-Prieto, C.F., F. Hernandez-Nuno, D.D. Rio, G. Ruiz-Hurtado, I. Aranguez, M. Ruiz-Gayo, B. Somoza, and M.S. Fernandez-Alfonso. 2015. High-fat diet induces endothelial dysfunction through a down-regulation of the endothelial AMPK-PI3K-Akt-eNOS pathway. Molecular Nutrition & Food Research 59: 520–532.
Xing, S.S., X.Y. Yang, T. Zheng, W.J. Li, D. Wu, J.Y. Chi, F. Bian, X.L. Bai, G.J. Wu, Y.Z. Zhang, C.T. Zhang, Y.H. Zhang, Y.S. Li, and S. Jin. 2015. Salidroside improves endothelial function and alleviates atherosclerosis by activating a mitochondria-related AMPK/PI3K/Akt/eNOS pathway. Vascular Pharmacology 72: 141–152.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, L., Qin, L., Liu, X. et al. CTRP3 Alleviates Ox-LDL–Induced Inflammatory Response and Endothelial Dysfunction in Mouse Aortic Endothelial Cells by Activating the PI3K/Akt/eNOS Pathway. Inflammation 42, 1350–1359 (2019). https://doi.org/10.1007/s10753-019-00996-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-019-00996-1