Abstract
Mechanical ventilation (MV) can augment sepsis-induced organ injury. Previous studies indicate that human mesenchymal stem cells (hMSCs) have immune-modulatory effect. We hypothesize that human adipose tissue-derived stromal cells (hADSCs) could attenuate MV and sepsis-induced organ injury. Male C57BL/6 mice were randomized to five groups: Sham group; MV group; cecal ligation and puncture (CLP) group; CLP + MV group; and CLP + MV + hADSC group. Anesthetized mice were subjected to cecal ligation and puncture surgery. The mice then received mechanical ventilation (12 ml/kg), with or without the intervention of hADSCs. The survival rate, organ injury of the liver and kidney, total protein and cells in bronchoalveolar lavage fluid (BALF), and histological changes of the lung and liver were examined. The level of IL-6 in BALF was measured by ELISA. Real-time quantitative PCR was used to analyze mRNA of IL-6 and tumor necrosis factor-α (TNF-α). hADSC treatment increased survival rate of septic mice with MV. hADSCs attenuated dysfunction of the liver and kidney and decreased lung inflammation and tissue injury of the liver and lung. IL-6 level in BALF and TNF-α and IL-6 mRNA expression in the tissue of the lung, liver, and kidney were significantly reduced by hADSC treatment. MV with conventional tidal volume aggravates CLP-induced multiple organ injuries. hADSCs inhibited the compound injuries possibly through modulation of immune responses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Sepsis induces extensive systemic inflammation caused by infection, then breaks the blood coagulation system, which eventually leads to organ dysfunction, septic shock, and even death [1]. Every year, about 180 million people suffer from sepsis all around the world, and more than 200 thousand people die from sepsis in the USA [2].
Mechanical ventilation (MV) is an essential respiratory support method for sepsis in the late period. MV can protect organs from hypoxemic injury by increasing oxygen, protecting the lung, and improving the internal environment, but it also can induce lung injury which is called ventilator-induced lung injury (VILI) [3]. At the same time, the lung inflammation caused by MV can result in systemic inflammation. In addition, MV can increase the intrathoracic pressure and reduce the cardiac output to affect the blood flows of other organs. Consequently, MV can induce injuries to other organs apart from the lung [4].
MV can aggravate organ injuries if the organ already has been damaged. Yehya, et al. [5] tested the inflammation, permeability, CT, HE, and function of the lungs in rats which were ventilated after sepsis; then, they found that MV exacerbated the lung injury induced by sepsis. It may be because that MV increases the cytokines secreted by the damaged lung and breaks the alveolar barrier further [6].
MSCs have been a new favor in stem cell therapy. MSCs are a kind of multi-functional stromal cells which can be derived from almost all tissues, such as bone marrow, placenta, umbilical cord blood, fat, cartilage, and other tissues [7, 8]. There is no need to worry that MSCs would form teratomas, provoke immune response, or raise ethical questions compared to embryonic stem cells and hematopoietic stem cells [9]. MSCs had therapeutic effects on sepsis [10, 11] or VILI [12, 13], but there were no reports about the effects of MSCs on the two hit model of sepsis combined with MV.
Our study used cecal ligation and puncture (CLP) surgery in mice to simulate the clinical sepsis. Twelve hours after the CLP surgery, the mice were ventilated with conventional tidal volume (12 ml/kg) for 2 h. We aimed to prove whether MV with conventional tidal volume would aggravate multi-organ injuries induced by sepsis. We also intervened the mice with human adipose tissue-derived stromal cells (hADSCs) to test if it had therapeutic effects on the compound injuries induced by sepsis and MV.
METHODS
Mice
All studies were authorized by the Institutional Animal Care and Use Committee of Tongji University. Eight- to twelve-week-old male C57BL/6 mice (SPF level) were purchased from the Laboratory Animal Research Center of Shanghai. All animals were raised in cages in an air-conditioned room (20 ± 1 °C) with controlled 12-h light/dark cycles and free access to water and chow (Global Diet; Shanghai, China). All process to deal with the animals accorded with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (Bethesda, MD, USA).
Experimental Design
The mice were randomly divided into five groups: (1) Sham group; (2) MV group; (3) CLP group; (4) CLP + MV group; and (5) CLP + MV+ hADSC group. Mice in our study were anesthetized by intraperitoneal injections of fentanyl (75 μg/kg), midazolam (1.5 mg/kg), and pentobarbital (60 mg/kg) [14]. A 2-cm abdominal incision in the midline of mice in the Sham group were made and sutured. In MV group, 12 h after the Sham operation, mice were ventilated for 2 h. Mice in the CLP group were performed with CLP surgery. In the CLP + MV group, 12 h after the CLP surgery, mice were ventilated for 2 h. Mice in the CLP + MV + hADSC group received an intraperitoneal injection of 3 × 106 hADSCs resuspended in 0.2 ml Dulbecco’s phosphate-buffered saline (DPBS) when they were ventilated after CLP surgery. At the same time, the mice in the other groups received an intraperitoneal injection of 0.2 ml DPBS.
Cecal Ligation and Puncture Surgery
Two-centimeter skin incisions were made on the midabdomen to locate the cecum and exteriorize it. The cecal contents were gently pushed toward the distal cecum. The cecum was ligated at 75%:25% position. Then, the cecum was perforated between the ligation and the tip of the cecum by single through-and-through puncture with 20G needle avoiding to rupture the vessels. After removing the needle, a droplet of feces was extruded to ensure the patency of the punctured hole. The cecum was relocated into the abdominal cavity without spreading feces onto the abdominal wall wound. The peritoneum was closed by applying simple interrupted sutures. Pre-warmed normal saline was injected subcutaneously to resuscitate mice (37 1C; 5 ml per 100 g body weight) [15]. All mice received antibiotic and fluid therapy subcutaneously (a combination of imipenem and cilastatin; 14 mg/kg in 1.5 ml of normal saline at 6 h and 7 mg/kg in 1.5 ml of normal saline at 18 h after surgery) [2].
Mechanical Ventilation
Twelve hours after the Sham operation or CLP surgery, the mice were anesthetized, then an orotracheal intubation was performed with a 20G catheter. The mice in the Sham group and CLP group breathed spontaneously, while the mice in the MV group, CLP + MV group, and CLP + MV + hADSC group were connected to the mechanical ventilator for 2 h. The tidal volume was set to 12 ml/kg, and the respiratory rate was set to 140 min−1 [16].
Culture and Identification of hADSCs
hADSCs were bought from Cyagen company (HUXMD-01001) and cultured at 37 °C/5% CO2 in control medium (DMEM, 15% FBS, 1% Penicillin-Streptomycin Solution, 1%NEAA, 1%Glu, EGF 5 ng/ml, FGE 5 ng/ml). Every 3 days, the adherent cells were washed extensively with DPBS and maintained in new control medium. When the adherent cells have reached confluence, cells were trypsinized (0.25% trypsin; Sigma, USA), resuspended in control medium, and replated at 30% concentration [17].
The cells would be used for identification and experimentations when they were expanded to 5th passage. For flow cytometric analyses, cells were incubated in the dark with CD11b (Ebioscience, 11-0113-41), CD34 (Ebioscience, 11-0349-41), CD44 (Ebioscience, 9400-11S), CD45 (Ebioscience, 11-9459-41), CD73 (Ebioscience, 17-0739-41), CD90 (Ebioscience, 17-0909-41), and CD105 (Ebioscience, 17-1057-41) and its isotype antibody at the room temperature. At least 10,000 events were collected, and data was analyzed using FlowJo software.
Survival Rates
Survival rates were observed after surgery every 12 h for 7 days. We began antibiotic (a combination of imipenem and cilastatin injection and fluid resusCitation): 14 mg/kg in 1.5 ml of normal saline at 6 h and 7 mg/kg in 1.5 ml of normal saline every 12 h for 7 days [2]. We killed all the alive mice at the end of the seventh day.
Organ Function
Mice were anesthetized 24 h after model establishment, and blood sample was isolated by eyeball extirpation. Blood sample was protect overnight at 4 °C, then centrifuged (3000g × 10 min, 4 °C). The supernatants were collected and sent to the clinical lab of Shanghai East Hospital for testing the serum SCr, BUN, AST, and ALT.
Histology of the Lung and Liver
The left lung and liver tissue were fixed in 4% paraformaldehyde, then sectioned and stained with hematoxylin and eosin (HE). Analysis of the histology of the lung from four items is as follows: alveolar congestion, hemorrhage, infiltration or aggregation of neutrophils in airspaces or vessel walls, and thickness of alveolar wall membrane formation. Quantitative grade on a scale was from 0 to 4 (0, normal; 1, light; 2, moderate; 3, strong; 4, intense) [18]. Liver injury was analyzed by Suzuki classification from three items: sinusoidal congestion, vacuolization of hepatocyte cytoplasm, and parenchymal necrosis [19].
Analysis of BALF
The trachea was incubated with a 20G catheter and instilled with 1 ml DPBS, retrieved about 80% instilled volume. Retrieved bronchoalveolar lavage fluid (BALF) was centrifuged (3000g × 10 min, 4 °C). The supernatants were used to measure the total protein concentration by using BCA kit (Beyotime, P009), and the redundant supernatants were stored at − 80 °C for ELISA (enzyme-linked immunosorbent assay). The deposits were resuspended in 0.1 ml DPBS to count the total cell number using the hemocytometer.
Quantitative Real-Time Polymerase Chain Reaction
Total RNA was extracted from the right lung, liver, and kidney by using the Trizol reagent (Life Technologies, 15596018). Total RNA was reverse transcribed using a PrimeScript™ RT reagent kit (TaKaRa, RR037A). Quantitative real-time polymerase chain reaction (qPCR) was performed using SYBR Green assay (TaKaRa, RR420A). The primers for amplification were:
-
β-actin forward 5′-GGCTGTATTCCCCTCCATCG-3′;
-
β-actin reverse 5′-CCAGTTGGTAACAATGCCATGT-3′;
-
IL-6 forward 5′-TAGTCCTTCCTACCCCAATTTCC-3′;
-
IL-6 reverse 5′-TTGGTCCTTAGCCACTCCTTC-3′;
-
TNF-a forward 5′-CCCTCACACTCAGATCATCTTCT-3′;
-
TNF-a reverse 5′-GCTACGACGTGGGCTACAG-3′.
Measurement of IL-6 Cytokine in Serum and BALF
Serum and BALF samples were collected from each group and stored at − 80 °C for analysis. IL-6 were measured by ELISA kits (RayBio, ELM-IL6-001C) that have been specifically analyzed for mouse cytokines.
Statistical Analysis
Survival rates were evaluated using the Kaplan-Meier method, and significance was determined by the generalized Wilcoxon method. Data were presented as the mean ± SD and analyzed using either a one-way or two-way analysis of variance (ANOVA); post hoc testing was performed with Bonferroni’s correction of the t test. The individual studies performed throughout this work represent at least five independent studies. Power analyses were performed by using a type I error probability of 0.05, with a power of 0.9, to determine the sample size necessary to reject the null hypothesis. All statistical analyses were carried out using the GraphPad Prism 5 program.
RESULTS
Identification of hADSCs
It has been reported that long-term cultures of MSCs were predisposed to malignant transformation and loss of biological activity [20]. So hADSCs in our study were cultured to the 5th passage, then were used for identification and experimentation. hADSCs harvested were phenotyped by their ability to adhere to plastic and form CFU-Fs, to express specific-cell surface markers including CD44, CD73, CD90, and CD105, but not to express the receptor of CD11b, CD34, and CD45 (Fig. 1), which were accorded with the standard of MSCs put forward by the International Society for Cellular Therapy [21].
hADSCs Improved the Survival Rates of the Mice in the CLP + MV + hADSC Group
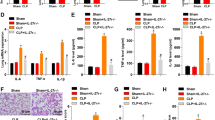
All mice in the Sham group (10/10) and MV group (10/10) survived throughout the 7-day period. The survival rate of the CLP group decreased to 13% (2/15), which was significantly different from that of the Sham group (P = 0.006). Mice in CLP + MV group all died during the 7 days (15/15), but it had no significant difference to the CLP group. hADSCs prominently improved the survival rates of CLP + MV + hADSC group to 33% (5/15) compared to CLP + MV group (P = 0.015) (Fig. 2).
Survival curves of mice in the Sham, MV, CLP, CLP + MV, and CLP + MV + MSC groups during the first 7 days. The survival rates of each group were as follows: Sham 100% (10/10), MV 100% (10/10), CLP 13% (2/15), CLP + MV 0(0/15), and CLP + MV + MSC 33.3% (5/15). hADSCs prominently improved the survival rates of the CLP + MV + MSC group compared to the CLP + MV group (P = 0.015), *P < 0.05.
hADSCs Alleviated the Liver Injury of Mice in the CLP + MV + hADSC Group
The third international consensus definitions for sepsis and septic shock (sepsis-3) defined sepsis as a life-threatening organ dysfunction caused by a dysregulated host response to infection [22]. It was also emphasized that even a modest degree of organ dysfunction when infection was first suspected was associated with an in-hospital mortality in excess of 10% [23]. We examined the liver and kidney function of each group. Liver function, as measured by serum AST, ALT, had no significant difference between the Sham group and MV group. Dramatically increased serum AST, ALT was observed in the CLP group. The serum AST, ALT of the CLP + MV group was not obviously higher than CLP group. hADSCs evidently alleviated the liver function of the CLP + MV + hADSC group (Fig. 3). The pathological changes in the liver coincided with the liver function in each group: sinusoidal congestion and vacuolization of hepatocyte cytoplasm could be seen in the MV group. CLP group had markedly hepatocellular necrosis, congestion, and vacuolization. MV further aggravated the liver injury induced by CLP, even non-normal hepatic lobule could be seen. hADSCs improved the histopathologic changes in CLP + MV + hADSC (Fig. 4). The kidney function of each group had no significant difference.
Liver and kidney function of the Sham, MV, CLP, CLP + MV, and CLP + MV + MSC groups 24 h after Sham or CLP operation. ALT and AST concentrations were tested in the liver. Kidney function was reflected by serum concentration of SCr and BUN. The number of mice in all measurements was five. Error bars represent means ± SD; *P < 0.05; **P < 0.01; ***P < 0.001.
hADSCs Attenuated the Lung Injury of Mice in the CLP + MV + hADSC Group
No abnormal histological alterations of the lung samples were observed in the Sham group. In the MV group, the alveolar wall was slightly thick. The alveolar congestion and hemorrhage could be observed in the local lung tissues. In the CLP group, thickness of alveolar wall and alveolar congestion were apparent. MV exactly aggravated the thickness of alveolar wall and congestion induced by CLP in CLP + MV group. hADSCs attenuated the histological injury of the lung in the CLP + MV + hADSC group. Consistent with these findings, the histological scoring of MV group was not significantly different from the Sham group (3.4 ± 0.5099 vs 2.2 ± 0.3742, P > 0.05). The histological scoring of the CLP group was evidently higher than the Sham group (7.5 ± 0.3073 vs 2.2 ± 0.3742, P < 0.0001). The CLP + MV group scored higher compared with the CLP group (8.8 ± 0.3887 vs 7.5 ± 0.3073, P < 0.05). hADSCs decreased the histological scoring of the CLP + MV + hADSC group compared to the CLP + MV group (7.1 ± 0.2333 vs 8.8 ± 0.3887, P < 0.0001).(Fig. 5).
The total protein and cells of BALF were measured to observe the alveolar permeability. The total protein (0.4805 ± 0.04374 vs 0.4001 ± 0.02841, P > 0.05) and cells (0.5203 ± 0.05654 vs 0.375 ± 0.05, P > 0.05) of BALF in the MV group had no significant difference to the Sham group. The total protein (0.6425 ± 0.0239 vs 0.4001 ± 0.02841, P < 0.001) and cells (1.013 ± 0.1065 vs 0.375 ± 0.05, P < 0.001) in the CLP group obviously increased compared to the Sham group. MV further raised the total protein of BALF in the CLP + MV group compared to the CLP group (0.8031 ± 0.03961 vs 0.6425 ± 0.0239, P < 0.05). hADSCs dramatically decreased the total protein (0.5741 ± 0.03775 vs 0.8031 ± 0.03961, P < 0.001) and cells (0.8637 ± 0.07639 vs 1.337 ± 0.1019, P < 0.05) in the CLP + MV + hADSC group compared with the CLP + MV group (Fig. 6).
hADSCs Decreased the Inflammation of Mice in the CLP + MV + hADSC Group
MSCs could alter the immune response to infection; therefore, we test TNF-α and IL-6 proinflammatory cytokines that had a central role in organ injury. The mRNA level of IL-6 and TNF-α in the lung, liver, and kidney and protein level in serum and BALF of IL-6 of the MV group had no significant difference to the Sham group. CLP dramatically increased the mRNA level of IL-6 and TNF-α in the lung, liver, and kidney and the protein level of IL-6 in serum and BALF compared to the Sham group. MV accentuated the local and systemic inflammation increased by CLP. hADSCs obviously decreased the high inflammatory level in the CLP + MV + hADSC group (Fig. 7).
The inflammation of the lung, liver, kidney, serum, and BALF in the Sham, MV, CLP, CLP + MV, and CLP + MV + MSC groups 24 h after Sham or CLP operation. The RNA level of IL-6 and TNF-α in the lung, liver, and kidney was assayed by qPCR. The protein level of IL-6 in serum and BALF was measured by ELISA. The number of mice in all measurements was five. Error bars represent means ± SD; *P < 0.05; **P < 0.01; ***P < 0.001.
DISCUSSION
CLP in rodents has turned into the most extensive model for experimental sepsis and is perceived as the gold standard in sepsis research at present. The animals started to appear clinical signs of sepsis at around 12 h after CLP [15]. Yehya et al. [5] found that the lung was injured 12 h following CLP in rats; mechanical ventilation deteriorated the injury. We use the CLP model to simulate sepsis; 12 h later, the mice were ventilated. Our results showed CLP decreased the survival rates in mice, exacerbated the live function, destructed the pathological changes of the lung, and increased the alveolar permeability and inflammation of the lung, liver, and kidney.
Though low tidal volume was advocated as a protective ventilation strategy, it has been pointed out further injury generated by lung protective ventilation was equal to the injury induced by the tidal volume of 12 ml/kg ventilation in ARDS (acute respiratory disease syndrome) animals [24]. Fuller, et al. [25] revealed that the delivered tidal volume of patients with severe sepsis and septic shock receiving MV in the emergency department (June 2005 to May 2010 n = 251) was from 5.2 to 14.6 ml/kg. Lung protective ventilation was only used in 68 patients (27.1%). Xuan et al. [26] found low tidal volume (6 ml/kg) MV caused some heart rate fluctuation and insufficient oxygen index in early phase of sepsis, while conventional tidal volume (12 ml/kg), MV provided better oxygenation and hemodynamic stability. A large number of animal model has proved the conventional tidal volume (12 ml/kg) of MV could result in lung injury in mice. Papaiahgari et al. [16] studied that the protein concentration and neutrophil counts of BALF obtained from the mice ventilated for 2 h at a tidal volume of 12 ml/kg had no difference to the mice in spontaneous respiration, but it had difference when the time of mechanical ventilation was prolonged to 4 h. In our study, 12 h after the CLP surgery, the mice were ventilated for 2 h at the tidal volume of 12 ml/kg. The results of our study showed that conventional tidal volume MV for 2 h did not make significant injury to the lung, liver, and kidney, but it could aggravate the pathological changes of the lung and increase the alveolar permeability and inflammation which were induced by sepsis. It was reported that sepsis could result in destruction to oxidation and antioxidation system and immune system, which could be deteriorated by MV, and there was no effective immune response to the second hit [27,28,29]. In our study, MV aggravated the organ injuries induced by sepsis.
MSCs were initially derived from the bone marrow, so it used to employ the bone marrow-derived MSCs (BMSCs) for experimentation [30, 31]. But now it has been found that MSCs also could be derived from the adipose tissue which was much easier to be acquired and had less injuries [32]. It was reported that ADSCs were more competent in immunoregulation and more suitable for treating inflammatory diseases compared to BMSCs [33, 34]. hADSCs were used in our study to intervene in the compound injury.
It has been reported that MSCs could reduce bacteria in BALF peritoneal irrigation fluid and peripheral blood of infectious lung injury model [35,36,37,38,39]. Kol et al. [40] studied the interaction between gastrointestinal microbes and ADSCs in vitro then found neither microbe induced MSC to death degeneration or diminished proliferation; microbial association did not induce potentially harmful phenotype shift; MSC-microbial interaction significantly increased transcription of key immunomodulatory genes. Yip et al. [33, 41] has proved intraperitoneal injection of ADSCs had effects on sepsis in rats. In our study, hADSCs were intraperitoneally injected in order to amplify the effect of ADSCs in the peritoneal cavity.
MSCs not only can play direct role in antimicrobial through paracrine of antibacterial peptide, but also can indirectly affect the phagocytosis of immunocytes. Hall et al. [39] found that MSCs cleared the bacteria by adjusting the neutrophils; Chao et al. [1] discovered that MSCs increased the number and proportion of regulatory T cells and strengthened the immunosuppressive action of regulatory T cells to decrease the IL-6 and TNF-α in serum; Németh et al. [2] found that MSCs promoted the macrophages to transform from M1 to M2 to secrete IL-10[2]. It could be seen that MSCs could adjust neutrophils, regulatory T cells, macrophages, and other immune cells to regulate the immune system to improve the bacterial clearance and survival rates. The results of our study showed that hADSCs improved the survival rates, liver function, and alveolar permeability, decreased the pathological changes of the lung and inflammation caused by the compound injuries induced by sepsis and MV.
There are still limitations in our study: Firstly, the mice were ventilated and transplanted hADSCs 12 h after CLP, because 12 h after CLP, the animals started to show clinical signs of sepsis and the lung was injured [5, 15]. There should be more time points to find the best therapeutic time. Secondly, we should use more types of injection to compare with intraperitoneal injection to find the best ways to treat the multi-organ injuries. Thirdly, more inflammatory mediators apart from IL-6 and TNF-α that need to be tested to assure the organ injury; the mechanisms of the therapeutic effects of hADSCs on the compound injuries induced by sepsis and MV was not explained in our study; it will be studied in our future study.
Our study proved that hADSCs had therapeutic effects on the compound injuries induced by sepsis and MV in mice. When we solve the questions of safety, optimum time, dosage, and transplanted way, we believe MSC will be an effective treatment for the clinical compound injuries induced by sepsis and MV.
CONCLUSION
Mechanical ventilation with conventional tidal volume did not make significant injuries to the lung, liver, and kidney, but it could aggravate the multi-organ injuries induced by sepsis. hADSCs had therapeutic effects on the compound injuries induced by sepsis combined with the mechanical ventilation in mice.
References
Chao, Y.H., H.P. Wu, K.H. Wu, Y.G. Tsai, C.T. Peng, K.C. Lin, W.R. Chao, M.S. Lee, and Y.C. Fu. 2014. An increase in cd3+cd4+cd25+ regulatory t cells after administration of umbilical cord-derived mesenchymal stem cells during sepsis. PLoS One 9: e110338.
Krisztián Németh, A Leelahavanichkul, Peter S T Yuen, Balázs Mayer, Alissa Parmelee1, Kent Doi, Pamela G Robey, Kantima Leelahavanichkul, Beverly H Koller, Jared M Brown, Xuzhen Hu, Ivett Jelinek, Robert A Star, and Éva Mezey. Bone marrow stromal cells attenuate sepsis via prostaglandin e2—Dependent reprogramming of host macrophages to increase their interleukin-10 production. Nature Medicine 2009;15.
Pinhu, L., T. Whitehead, T. Evans, and M. Griffiths. 2003. Ventilator-associated lung injury. Lancet (London, England) 361: 332–340.
Kuiper, J.W., A.B. Groeneveld, A.S. Slutsky, and F.B. Plotz. 2005. Mechanical ventilation and acute renal failure. Critical Care Medicine 33: 1408–1415.
Yehya, N., Y. Xin, Y. Oquendo, M. Cereda, R.R. Rizi, and S.S. Margulies. 2015. Cecal ligation and puncture accelerates development of ventilator-induced lung injury. American Journal of Physiology. Lung Cellular and Molecular Physiology 308: L443–L451.
Villar, J., N. Cabrera, M. Casula, C. Flores, F. Valladares, M. Muros, L. Blanch, A.S. Slutsky, and R.M. Kacmarek. 2010. Mechanical ventilation modulates toll-like receptor signaling pathway in a sepsis-induced lung injury model. Intensive Care Medicine 36: 1049–1057.
Matthay, M.A., B.T. Thompson, E.J. Read, D.H. McKenna Jr., K.D. Liu, C.S. Calfee, and J.W. Lee. 2010. Therapeutic potential of mesenchymal stem cells for severe acute lung injury. Chest 138: 965–972.
Dimarino, A.M., A.I. Caplan, and T.L. Bonfield. 2013. Mesenchymal stem cells in tissue repair. Frontiers in Immunology 4: 201.
Ma, S., N. Xie, W. Li, B. Yuan, Y. Shi, and Y. Wang. 2014. Immunobiology of mesenchymal stem cells. Cell Death and Differentiation 21: 216–225.
Rocheteau, P., L. Chatre, D. Briand, M. Mebarki, G. Jouvion, J. Bardon, C. Crochemore, P. Serrani, P.P. Lecci, M. Latil, B. Matot, P.G. Carlier, N. Latronico, C. Huchet, A. Lafoux, T. Sharshar, M. Ricchetti, and F. Chretien. 2015. Sepsis induces long-term metabolic and mitochondrial muscle stem cell dysfunction amenable by mesenchymal stem cell therapy. Nature Communications 6: 10145.
Anderson, P., L. Souza-Moreira, M. Morell, M. Caro, F. O'Valle, E. Gonzalez-Rey, and M. Delgado. 2013. Adipose-derived mesenchymal stromal cells induce immunomodulatory macrophages which protect from experimental colitis and sepsis. Gut 62: 1131–1141.
Hayes, M., C. Masterson, J. Devaney, F. Barry, S. Elliman, T. O'Brien, D. O'Toole, G.F. Curley, and J.G. Laffey. 2015. Therapeutic efficacy of human mesenchymal stromal cells in the repair of established ventilator-induced lung injury in the rat. Anesthesiology 122: 363–373.
Curley, G.F., M. Hayes, B. Ansari, G. Shaw, A. Ryan, F. Barry, T. O'Brien, D. O'Toole, and J.G. Laffey. 2012. Mesenchymal stem cells enhance recovery and repair following ventilator-induced lung injury in the rat. Thorax 67: 496–501.
Muller-Redetzky, H.C., D. Will, K. Hellwig, W. Kummer, T. Tschernig, U. Pfeil, R. Paddenberg, M.D. Menger, O. Kershaw, A.D. Gruber, N. Weissmann, S. Hippenstiel, N. Suttorp, and M. Witzenrath. 2014. Mechanical ventilation drives pneumococcal pneumonia into lung injury and sepsis in mice: Protection by adrenomedullin. Critical Care (London, England) 18: R73.
Rittirsch, Daniel, M.S. Huber-Lang, M.A. Flierl, and Peter A. Ward. 2009. Immunodesign of experimental sepsis by cecal ligation and puncture. Nature Protocols 4.
Papaiahgari, S., A. Yerrapureddy, S.R. Reddy, N.M. Reddy, O.J. Dodd, M.T. Crow, D.N. Grigoryev, K. Barnes, R.M. Tuder, M. Yamamoto, T.W. Kensler, S. Biswal, W. Mitzner, P.M. Hassoun, and S.P. Reddy. 2007. Genetic and pharmacologic evidence links oxidative stress to ventilator-induced lung injury in mice. American Journal of Respiratory and Critical Care Medicine 176: 1222–1235.
Schaffler, A., and C. Buchler. 2007. Concise review: Adipose tissue-derived stromal cells--Basic and clinical implications for novel cell-based therapies. Stem Cells (Dayton, Ohio) 25: 818–827.
Ding, X., X. Wang, X. Zhao, S. Jin, Y. Tong, H. Ren, Z. Chen, and Q. Li. 2015. Rgd peptides protects against acute lung injury in septic mice through wisp1-integrin beta6 pathway inhibition. Shock (Augusta, Ga.) 43: 352–360.
Suzuki, S., S. Nakamura, T. Koizumi, S. Sakaguchi, S. Baba, H. Muro, et al. 1991. The beneficial effect of a prostaglandin I2 analog on ischemic rat liver. Transplantation 52: 979–983.
Gupta, N., A. Krasnodembskaya, M. Kapetanaki, M. Mouded, X. Tan, V. Serikov, and M.A. Matthay. 2012. Mesenchymal stem cells enhance survival and bacterial clearance in murine Escherichia coli pneumonia. Thorax 67: 533–539.
Dominici, M., K. Le Blanc, I. Mueller, I. Slaper-Cortenbach, F. Marini, D. Krause, R. Deans, A. Keating, D. Prockop, and E. Horwitz. 2006. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy 8: 315–317.
Singer, M., C.S. Deutschman, C.W. Seymour, M. Shankar-Hari, D. Annane, M. Bauer, R. Bellomo, G.R. Bernard, J.D. Chiche, C.M. Coopersmith, R.S. Hotchkiss, M.M. Levy, J.C. Marshall, G.S. Martin, S.M. Opal, G.D. Rubenfeld, T. van der Poll, J.L. Vincent, and D.C. Angus. 2016. The third international consensus definitions for sepsis and septic shock (sepsis-3). Journal of the American Medical Association 315: 801–810.
Seymour, C.W.L.V., T.J. Iwashyna, F.M. Brunkhorst, T.D. Rea, A. Scherag, G. Rubenfeld, J.M. Kahn, M. Shankar-Hari, M. Singer, C.S. Deutschman, G.J. Escobar, and D.C. Angus. 2016. Assessment of clinical criteria for sepsis: For the third international consensus definitions for sepsis and septic shock (sepsis-3). Journal of the American Medical Association 15: 762–774.
Muller, H.C., K. Hellwig, S. Rosseau, T. Tschernig, A. Schmiedl, B. Gutbier, B. Schmeck, S. Hippenstiel, H. Peters, L. Morawietz, N. Suttorp, and M. Witzenrath. 2010. Simvastatin attenuates ventilator-induced lung injury in mice. Critical Care (London, England) 14: R143.
Fuller, B.M., N.M. Mohr, M. Dettmer, S. Kennedy, K. Cullison, R. Bavolek, N. Rathert, and C. McCammon. 2013. Mechanical ventilation and acute lung injury in emergency department patients with severe sepsis and septic shock: An observational study. Academic Emergency Medicine 20: 659–669.
Xuan, W., Q. Zhou, S. Yao, Q. Deng, T. Wang, and Q. Wu. 2015. Mechanical ventilation induces an inflammatory response in preinjured lungs in late phase of sepsis. Oxidative Medicine and Cellular Longevity 2015: 364020.
Tasaka, S., F. Amaya, S. Hashimoto, and A. Ishizaka. 2008. Roles of oxidants and redox signaling in the pathogenesis of acute respiratory distress syndrome. Antioxidants & Redox Signaling 10: 739–753.
Chapman, K.E., S.E. Sinclair, D. Zhuang, A. Hassid, L.P. Desai, and C.M. Waters. 2005. Cyclic mechanical strain increases reactive oxygen species production in pulmonary epithelial cells. American Journal of Physiology—Lung Cellular and Molecular Physiology 289: 834–841.
Deng, J.C., G. Cheng, M.W. Newstead, X. Zeng, K. Kobayashi, R.A. Flavell, and T.J. Standiford. 2006. Sepsis-induced suppression of lung innate immunity is mediated by IRAK-M. The Journal of Clinical Investigation 116: 2532–2542.
Pati, S., M.H. Gerber, T.D. Menge, K.A. Wataha, Y. Zhao, J.A. Baumgartner, J. Zhao, P.A. Letourneau, M.P. Huby, L.A. Baer, J.R. Salsbury, R.A. Kozar, C.E. Wade, P.A. Walker, P.K. Dash, C.S. Cox Jr., M.F. Doursout, and J.B. Holcomb. 2011. Bone marrow derived mesenchymal stem cells inhibit inflammation and preserve vascular endothelial integrity in the lungs after hemorrhagic shock. PLoS One 6: e25171.
Goodwin, M., V. Sueblinvong, P. Eisenhauer, N.P. Ziats, L. LeClair, M.E. Poynter, C. Steele, M. Rincon, and D.J. Weiss. 2011. Bone marrow-derived mesenchymal stromal cells inhibit th2-mediated allergic airways inflammation in mice. Stem Cells (Dayton, Ohio) 29: 1137–1148.
Ivanova-Todorova, E., I. Bochev, M. Mourdjeva, R. Dimitrov, D. Bukarev, S. Kyurkchiev, P. Tivchev, I. Altunkova, and D.S. Kyurkchiev. 2009. Adipose tissue-derived mesenchymal stem cells are more potent suppressors of dendritic cells differentiation compared to bone marrow-derived mesenchymal stem cells. Immunology Letters 126: 37–42.
Chang, C.L., S. Leu, H.C. Sung, Y.Y. Zhen, C.L. Cho, A. Chen, T.H. Tsai, S.Y. Chung, H.T. Chai, C.K. Sun, C.H. Yen, and H.K. Yip. 2012. Impact of apoptotic adipose-derived mesenchymal stem cells on attenuating organ damage and reducing mortality in rat sepsis syndrome induced by cecal puncture and ligation. Journal of Translational Medicine 10: 244.
Zhang, S., S.D. Danchuk, R.W. Bonvillain, B. Xu, B.A. Scruggs, A.L. Strong, J.A. Semon, J.M. Gimble, A.M. Betancourt, D.E. Sullivan, and B.A. Bunnell. 2014. Interleukin 6 mediates the therapeutic effects of adipose-derived stromal/stem cells in lipopolysaccharide-induced acute lung injury. Stem Cells (Dayton, Ohio) 32: 1616–1628.
Mei, S.H., J.J. Haitsma, C.C. Dos Santos, Y. Deng, P.F. Lai, A.S. Slutsky, W.C. Liles, and D.J. Stewart. 2010. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. American Journal of Respiratory and Critical Care Medicine 182: 1047–1057.
Asmussen, S., H. Ito, D.L. Traber, J.W. Lee, R.A. Cox, H.K. Hawkins, D.F. McAuley, D.H. McKenna, L.D. Traber, H. Zhuo, J. Wilson, D.N. Herndon, D.S. Prough, K.D. Liu, M.A. Matthay, and P. Enkhbaatar. 2014. Human mesenchymal stem cells reduce the severity of acute lung injury in a sheep model of bacterial pneumonia. Thorax 69: 819–825.
Lee, J.W., X. Fang, N. Gupta, V. Serikov, and M.A. Matthay. 2009. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proceedings of the National Academy of Sciences of the United States of America 106: 16357–16362.
Lee, J.W., A. Krasnodembskaya, D.H. McKenna, Y. Song, J. Abbott, and M.A. Matthay. 2013. Therapeutic effects of human mesenchymal stem cells in ex vivo human lungs injured with live bacteria. American Journal of Respiratory and Critical Care Medicine 187: 751–760.
Hall, S.R., K. Tsoyi, B. Ith, R.F. Padera Jr., J.A. Lederer, Z. Wang, X. Liu, and M.A. Perrella. 2013. Mesenchymal stromal cells improve survival during sepsis in the absence of heme oxygenase-1: The importance of neutrophils. Stem Cells (Dayton, Ohio) 31: 397–407.
Kol, A., S. Foutouhi, N.J. Walker, N.T. Kong, B.C. Weimer, and D.L. Borjesson. 2014. Gastrointestinal microbes interact with canine adipose-derived mesenchymal stem cells in vitro and enhance immunomodulatory functions. Stem Cells and Development 23: 1831–1843.
Chen, H.H., K.C. Lin, C.G. Wallace, Y.T. Chen, C.C. Yang, S. Leu, Y.C. Chen, C.K. Sun, T.H. Tsai, Y.L. Chen, S.Y. Chung, C.L. Chang, and H.K. Yip. 2014. Additional benefit of combined therapy with melatonin and apoptotic adipose-derived mesenchymal stem cell against sepsis-induced kidney injury. Journal of Pineal Research 57: 16–32.
Acknowledgements
Thanks for Yiteng Liao, Hao Li, and Hongming Zhu’s help during the experiment.
Author information
Authors and Affiliations
Contributions
Shuya Mei mainly completed this experiment and wrote this manuscript. Shuang Wang, Shuqing Jin, Xiang Zhao, Zhenzhen Shao, Renlingzi Zhang, and Xiangsheng Yu helped to finish this experiment. Yao Tong and Shibiao Chen have seen the original data. Zhixia Chen and Quan Li modified the final manuscript. All authors approved the final manuscript. Zhixia Chen and Quan Li were responsible for finalizing this manuscript.
Corresponding authors
Ethics declarations
Conflicts of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Mei, S., Wang, S., Jin, S. et al. Human Adipose Tissue-Derived Stromal Cells Attenuate the Multiple Organ Injuries Induced by Sepsis and Mechanical Ventilation in Mice. Inflammation 42, 485–495 (2019). https://doi.org/10.1007/s10753-018-0905-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-018-0905-5