Abstract
To identify the effects of the neurokinin-1 receptor (NK1R) antagonist aprepitant in treating pelvic pain, micturition symptoms, and bladder inflammation in mice with experimental autoimmune cystitis (EAC) similar to bladder pain syndrome/interstitial cystitis (BPS/IC). Female C57BL/6 mice were divided into the following three groups: normal control, EAC, and EAC plus aprepitant. EAC was induced in mice by duplicate immunization with bladder homogenate. In the EAC model group, EAC mice were given PBS by gavage once a day during the fourth week. In the EAC plus aprepitant group, aprepitant was administered instead of PBS in the same way. After 4 weeks, pelvic pain threshold and urination habits of mice were analyzed, as well as the bladder weight to body weight ratio, and histologic assessment of the expression of IL-1β, TNF-α, intercellular adhesion molecule 1 (ICAM-1), and NK1R in bladder tissue. EAC mice mimicked the phenotype and pathophysiologic lesions of BPS/IC well. Compared to PBS-treated EAC mice, the mice treated with aprepitant exhibited higher pain threshold values, less number of total urine spots or small urine spots, lower bladder weight to body weight ratio, and reduced bladder inflammation with less mast cell infiltration and decreased expressions of IL-1β, TNF-α, and ICAM-1 in bladder tissue. There was no difference in NK1R expression in bladders treated with or without aprepitant. The NK1R antagonist aprepitant relieved pelvic pain, urinary symptoms, and bladder inflammation in EAC mice. This indicated that NK1R may be a novel therapeutic target in BPS/IC treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Bladder pain syndrome/interstitial cystitis (BPS/IC) mainly occurs in women with chronic pelvic pain related to bladder filling, and it is accompanied by urinary frequency and urgency without infection [2, 4]. BPS/IC causes intense physical and mental suffering and may result in restricted social and daily life. However, after Alexander Skene first investigated IC in 1887, numerous studies on its etiology and pathophysiology have been performed, but they have revealed more problems than answers [7]. There is a lack of understanding regarding the pathogenesis and the various therapies of BPS/IC in clinical practice and traditional treatment has proved ineffective in alleviating the syndrome [14, 17, 21]. Thus, it is necessary to identify new effective and therapeutic strategies for BPS/IC.
Neurokinin-1 receptor (NK1R) has a high affinity for substance P (SP) which is an important inflammatory and pain stimulating factor [1, 18]. SP-NK1R is the major tachykinin signal involved in bladder function regulation [5]. A specific NK1R antagonist, aprepitant, was approved by the US Food and Drug Administration in 2003 for the treatment of chemotherapy-induced nausea and vomiting therapy [16]. However, this NK1R antagonist may have other indications besides the treatment of chemotherapy-induced nausea and vomiting. The results of previous clinical studies showed that blocking NK1R could relieve urinary urgency and frequency in patients with overactive bladder who share similar urination symptoms to those with BPS/IC [8, 10]. However, it is not yet known if this NK1R inhibitor could also be used in the treatment of BPS/IC as well. In the present study, we investigated whether the NK1R antagonist aprepitant may be a novel therapeutic approach in BPS/IC using our new experimental autoimmune cystitis (EAC) mouse model.
METHODS
Ethics Statement
All animal experiments were approved by the Animal Care and Use Committee of Ruijin Hospital affiliated to Shanghai Jiao Tong University School of Medicine. Procedures were performed in accordance with the Animal Management Rule of the Ministry of Health, People’s Republic of China (documentation no. 55, 2001). All vaccinations were performed under isoflurane anesthesia, and the mice were sacrificed by an overdose of sodium pentobarbital.
Animals
Female C57BL/6 mice (aged 6 to 8 weeks) were purchased from the Shanghai Laboratory Animal Center and raised in the Animal Center of Ruijin Hospital. The mice were randomly assigned to each experimental group and were housed under standard laboratory conditions (temperature 24 ± 1 °C, humidity 60–70%, 12-h light-dark cycle) with free access to rodent pellets and water.
EAC Model
Experimental autoimmune cystitis in C57BL/6 mice was carried out, which was successfully introduced in our previous study [11]. Briefly, bladders were removed from C57BL/6 mice after euthanasia and then homogenized in phosphate-buffered saline (PBS). The homogenate was centrifuged (12,000g, 4 °C, 15 min) and the supernatant was obtained. A micro-spectrophotometer (Nano-100, Allsheng Ltd., China) was used to detect the protein concentration in the supernatant which was diluted to 1 mg/ml with PBS. An emulsion of vaccines was prepared with a certain volume of the above-mentioned supernatant (or PBS only as the control) and an equal volume of complete Freund’s adjuvant (CFA; Sigma-Aldrich, St. Louis, MO, USA) or incomplete Freund’s adjuvant (IFA; Sigma-Aldrich). The mice were injected subcutaneously with 400-μl emulsion on the back at first and at 2-week intervals to achieve immunization. Aprepitant (Merck & Co, Inc., Whitehouse Station, NJ, USA) was chosen to block NK1R [16]. Three experimental groups were included as follows: (1) control group, PBS-immunized mice were given PBS by gavage once daily during the fourth week; (2) EAC group, bladder homogenate-immunized mice were given PBS by gavage once daily during the fourth week; and (3) aprepitant group, bladder homogenate-immunized mice were given aprepitant (1.2 mg/kg) by gavage once daily during the fourth week. At the end of the fourth week, the phenotype, pathology, and inflammatory cytokines present in the bladder were assessed in the three groups. The procedures carried out in these three groups are shown in Fig. 1.
Pain Threshold Detection
Pain threshold was measured using an electronic von Frey anesthesiometer (IITC, Inc., Life Science Instruments, Woodland Hills, CA, USA) [6, 11, 19]. The mice were placed in transparent cages (10 × 10 × 16 cm high) with a wire grid floor. Following 30-min environmental adaptation, an increasing vertical force was applied to the central area of the pelvic region or hind paw using the tip of the electronic von Frey anesthesiometer. The intensity of the force applied was recorded automatically by the instrument when it induced avoidance behaviors in mice. Three behaviors were considered as positive responses: (1) instant scratching and/or licking of the stimulated area, (2) sharp retraction of the stimulated body part (abdomen or hind paw), or (3) jumping. The pain threshold was measured at 09:00, 18:00, and 21:00, and the pain threshold value for each mouse was the average of three measurements. The measurements were taken by a researcher who was blind to the treatments in the three groups.
Voiding Behavior Assessments
Spontaneous void spot assays were used to assess the voiding behavior as described previously [26, 27]. At the end of the fourth week, the mice were gently placed in three standard cages according to the different treatments for 1 h with solid food only. A filter paper (Grade 540, Whatman, Wohua Ltd., China) was placed on the bottom of each cage to collect urine. The filter paper was photographed under ultraviolet light and all void spots were observed. The Fiji version of ImageJ software (http://fiji.sc/wiki/index.php/Fiji) was used to analyze numbers and areas of void spots on each filter paper. The total number of void spots represented urine frequency. The void spot with an area ≤ 0.2 cm2 was defined as a small urine spot [26] and the number of small void spots represented low urine output per micturition. The void spots with an area < 6.6 mm2 were excluded as they may have been due to claw or tooth marks [27].
Histopathologic Evaluation
At the end of the fourth week, bladders were excised and processed as described previously [11]. Specimens were fixed in 10% phosphate-buffered formalin, embedded in paraffin, cut into 5 μm thickness sections, and stained with hematoxylin/eosin (H&E) or toluidine blue. A light microscope (Eclipse E600, Nikon, Japan) was used to observe the inflammatory changes and mast cell status of bladders. A four-grade scale was used to evaluate the inflammatory changes in bladders as described previously [22]. Bladder with no lesions was defined as grade 1, simple edema as grade 2, serious edema accompanied by epithelial thinning, cleavage or the initial stage of leukocyte infiltration as grade 3, and bladder with increased extent and scope of all the above manifestations plus petechial hemorrhage as grade 4. Mast cells stained by toluidine blue were counted in two different fields of view in the area with greatest infiltration. The number of mast cells was calculated as the average of the two fields examined.
Real-Time Quantitative Reverse Transcription-Polymerase Chain Reaction
Bladder total RNA was extracted with TRIzol reagent (Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s protocol. The wavelength absorption ratio (260/280 nm) of all specimens was from 1.8 to 2.0. The purity of total RNA was measured and 1000 ng of total RNA was reverse transcribed into cDNA using the FastQuant RT Kit (Tiangen, Beijing, China). Quantitative PCR was performed in duplicate with SYBR Premix EX Taq™ (TaKaRa, Dalian, China) by the QuantStudio Dx Real-time PCR Instrument (Life Technologies). The sequence of each primer is listed in Table 1. The results of target gene expression were first normalized against GAPDH and then compared to the control.
Statistical Analysis
ANOVA with post hoc Tukey’s test for multiple comparisons was used to evaluate pain threshold values, numbers of void spots, bladder weight to body weight ratios, mast cell counts, and RT-PCR results. The Mann-Whitney U test was used to analyze bladder inflammation grade. Mast cell count data were expressed as the mean with the range in parentheses. All calculations were performed using IBM SPSS Statistics 23. Graphs were generated using GraphPad Prism 6. p < 0.05 was considered statistically significant.
RESULTS
NK1R Antagonist Relieved Pelvic Pain in EAC Mice
Suprapubic-pelvic pain is the most prominent symptom of BPS/IC. The pain threshold changes in the three groups were determined and compared. The lower the threshold, the more noticeable the pain was. The results showed that mice in the EAC group were extremely sensitive to pressure applied to the suprapubic area compared to the control group (p < 0.001; Fig. 2a). Mice treated with aprepitant showed some sensitivity to pressure applied to the suprapubic area compared to the control group (p < 0.001; Fig. 2a), but lower than that in the EAC group (p < 0.001; Fig. 2a). Following administration of aprepitant, mice were less sensitive to pelvic stimulation compared to EAC mice. In order to exclude individual sensitivity, pain threshold values of the hind paws were also determined. There were no significant differences between the three groups in hind paw pain threshold values (Fig. 2b). These findings indicated that the application of NK1R antagonist relieved pelvic pain in EAC mice.
Behavioral changes in the three groups. a Pain threshold changes in the pelvic area. Compared with the control, EAC mice showed extensive sensitivity to pressure on the suprapubic area, and the sensitivity was down-regulated after aprepitant administration. b Pain threshold changes in the hind paw. There was no significant difference in the hind paw pain threshold among the three groups. c Changes in total urine spot number. Compared with the control group, EAC mice showed a higher number of total urine spots, and this number was reduced after aprepitant administration. d Changes in small urine spot number. EAC mice showed a higher number of small urine spots which was also reduced by aprepitant. *** represents p < 0.001, ** represents p < 0.01, * represents p < 0.05 (analyzed using Tukey’s multiple comparisons test, n = 10 per group).
NK1R Antagonist Improved Voiding Behavior in EAC Mice
Changes in voiding behavior were evaluated by the pad test. Numbers of total urine spots and small urine spots in the EAC model group increased compared to those in the control group (all p < 0.001; Fig. 2c, d). The number of total urine spots in the aprepitant group decreased compared to that in the EAC model group (p = 0.011; Fig. 2c), which indicated that urine frequency was improved by aprepitant. The number of small urine spots in the aprepitant group was also decreased compared to the EAC group (p = 0.001; Fig. 2d), indicating that urine urgency was also improved by aprepitant. There was no significant difference in the number of small urine spots between the aprepitant group and the control group (p = 0.502, Fig. 2d). These results suggested that aprepitant improved urinary symptoms in EAC mice.
NK1R Antagonist Reduced Bladder Inflammation in EAC Mice
The weight of each mouse and removed bladder were obtained to calculate the bladder weight to body weight ratio. This ratio reflected general edema in the bladder by eliminating the effects of individual differences. The bladder weight to body weight ratios in EAC mice were much higher than those in control mice (p < 0.001, Fig. 3). Following treatment with aprepitant for 1 week, the bladder weight to body weight ratio significantly declined compared to PBS-treated EAC mice (p = 0.003, Fig. 3). There was no significant difference in the bladder weight to body weight ratio between the control group and aprepitant group (Fig. 3).
Changes in bladder weight (mg) to body weight (g) ratio in the three groups. Compared with the control group, EAC mice had a higher bladder weight to body weight ratio, and this ratio was decreased after aprepitant administration. *** represents p < 0.001, ** represents p < 0.01 (analyzed using Tukey’s multiple comparisons test, n = 10 per group).
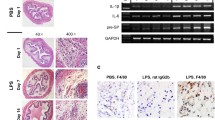
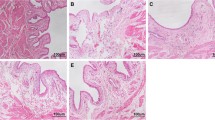
The histopathologic results are presented in Fig. 4. Bladder histologic analysis in the control group showed no inflammatory lesions and almost no mast cell infiltration. In the EAC group, bladders showed obvious mucosal edema, perivascular leukocyte infiltration, and petechial hemorrhage. Following treatment with aprepitant, bladder edema and leukocyte infiltration were reduced, and mast cell infiltration disappeared. A comparison of bladder inflammation grade and mast cell counts in the three groups is shown in Table 2. These results indicated that aprepitant improved bladder inflammation in EAC mice.
Inflammation of bladder tissue in the three groups. H&E staining (100×) was used for histologic analysis and toluidine blue staining (200×) was used for mast cell analysis. EAC mice showed obvious mucosal edema, perivascular leukocyte infiltration, and petechial hemorrhage. Following aprepitant administration, bladder edema decreased with less leukocyte infiltration, and mast cell infiltration disappeared.
Changes in Inflammatory Cytokines and ICAM-1 in Bladder Tissue
Changes in bladder tissue IL-1β and TNF-α levels were determined. Compared with the control group, the expression of IL-1β and TNF-α was significantly elevated in the EAC group (all p < 0.001, Fig. 5). Aprepitant lowered the expression of IL-1β and TNF-α compared to EAC mice (p = 0.001 and p = 0.025, respectively; Fig. 5). However, there were significant differences in IL-1β and TNF-α levels between the aprepitant group and control group (p = 0.004 and p = 0.007, respectively, Fig. 5). We also determined the expression of ICAM-1 in the bladder, which is closely related to BPS/IC [9, 12, 22]. The expression of ICAM-1 in the EAC group was significantly higher than that in the control group (p < 0.001, Fig. 5). Treatment with aprepitant decreased the expression of ICAM-1 compared to EAC mice (p = 0.025; Fig. 5). NK1R expression in the bladder in the three groups was also measured. NK1R expression was elevated following immunization with bladder homogenate (EAC group and aprepitant group, all p < 0.001, Fig. 5). There was no statistically significant difference in NK1R expression between the EAC group and aprepitant group, indicating that aprepitant down-regulated bladder inflammatory cytokines with no effect on NK1R.
Gene expression of inflammation-related factors in mouse bladder, including IL-1β, TNF-α, ICAM-1, and NK1R. Compared with the control group, the expression of IL-1β, TNF-α, and ICAM-1 in EAC mice was elevated and was down-regulated following aprepitant administration. Aprepitant had no effect on NK1R expression. *** represents p < 0.001, ** represents p < 0.01, * represents p < 0.05 (analyzed using Tukey’s multiple comparison test, n = 4 per group).
DISCUSSION
This study investigated the effect of an NK1R antagonist using our new experimental autoimmune cystitis mouse model, which showed remarkably lower pelvic pain threshold, urinary frequency, and obvious bladder inflammation. This model mimicked the phenotype and pathophysiologic lesions of BPS/IC as described previously [11]. Compared to PBS-treated EAC mice, NK1R antagonist administration resulted in higher pain threshold values, fewer total urine spots and small urine spots, lower bladder weight to body weight ratios, and decreased bladder inflammation on histopathology, as well as decreased expression of inflammatory cytokines and ICAM-1 in bladder tissue. Thus, the NK1R antagonist aprepitant may relieve pain, urinary symptoms, and inflammation in bladder lesions such as BPS/IC.
The precise etiology of BPS/IC is unknown, and long-term, effective treatments for BPS/IC have not yet been established [15]. Current therapies for BPS/IC can be divided into two categories: intravesical treatments and oral medications. Intravesical treatments require repetitive catheterization, and intravesical drug dilution occurs as urine is continuously produced and voiding behavior results in short drug duration in the bladder. Tight junctions and umbrella cells in the bladder result in urothelial impermeability which hinders drug delivery [28]. Therefore, intravesical treatments are often considered second-line therapies. Oral medications are more acceptable to patients due to their convenience and long-term tolerance. However, to date, there is no effective and well-tolerated drug for BPS/IC. An NK1R antagonist may be a promising treatment option according to our findings. Aprepitant is a specific NK1R antagonist which has been approved by the US FDA for the prevention of chemotherapy-induced nausea and vomiting [16]. The safety and tolerance of aprepitant in humans have been confirmed. Thus, it would be beneficial to patients if the effects of NK1R antagonist on BPS/IC could be confirmed in the near future.
The potential mechanism of NK1R antagonist in treating lesions such as BPS/IC is unknown, although previous studies have provided some evidence. Intravesical instillation of hyaluronic acid can relieve symptoms in BPS/IC patients [23] by down-regulating the expression of ICAM-1 in the bladder [9, 12, 22]. In fact, NK1R activation can up-regulate ICAM-1 expression [13]. Aprepitant can also relieve urinary urgency and frequency in patients with overactive bladder [8]. Moreover, mast cells play a major role in BPS/IC [20, 25]. The NK1R has been demonstrated to be of functional relevance and leads to an increase in the sensitivity of mast cell [3, 24]. Our mouse study showed that the NK1R antagonist aprepitant improved urinary symptoms, increased the pain threshold, and inhibited mast cell infiltration and relevant cytokines in autoimmune cystitis, suggesting that further research to investigate the value and mechanism of NK1R in BS/IC is warranted.
There are some limitations in this study. We did not measure the underlying signaling pathway of aprepitant in bladder tissue, which is essential to understand the mechanism of NK1R inhibition. When the effect of NK1R antagonist is confirmed, clinical trials of aprepitant in BPS/IC patients should be considered.
CONCLUSIONS
This research showed that the NK1R antagonist aprepitant relieved pain, urinary symptoms, and bladder inflammation in a mouse model of EAC, which resembles BPS/IC. Our results indicated that the NK1R may be a novel therapeutic target in BPS/IC and the NK1R antagonist, aprepitant, may be a promising drug in the treatment of BPS/IC.
Abbreviations
- NK1R:
-
neurokinin 1 receptor
- BPS:
-
bladder pain syndrome
- IC:
-
interstitial cystitis
- EAC:
-
experimental autoimmune cystitis
- CFA:
-
complete Freund’s adjuvant
- IFA:
-
incomplete Freund’s adjuvant
- PBS:
-
phosphate-buffered saline
- IL-1β:
-
interleukin 1β
- TNF-α:
-
tumor necrosis factor α
- ICAM-1:
-
intercellular adhesion molecule 1
- SP:
-
substance P
References
Abbadie, C., J.L. Brown, P.W. Mantyh, and A.I. Basbaum. 1996. Spinal cord substance P receptor immunoreactivity increases in both inflammatory and nerve injury models of persistent pain. Neuroscience 70 (1): 201–209.
Abrams, P., L. Cardozo, M. Fall, D. Griffiths, P. Rosier, U. Ulmsten, et al. 2002. The standardisation of terminology of lower urinary tract function: Report from the standardisation sub-committee of the international continence society. Neurourology and Urodynamics 21 (2): 167–178.
Ansel, J.C., J.R. Brown, D.G. Payan, and M.A. Brown. 1993. Substance P selectively activates TNF-alpha gene expression in murine mast cells. Journal of Immunology 150 (10): 4478–4485.
Bogart, L.M., S.H. Berry, and J.Q. Clemens. 2007. Symptoms of interstitial cystitis, painful bladder syndrome and similar diseases in women: A systematic review. The Journal of Urology 177 (2): 450–456. https://doi.org/10.1016/j.juro.2006.09.032.
Candenas, L., A. Lecci, F.M. Pinto, E. Patak, C.A. Maggi, and J.N. Pennefather. 2005. Tachykinins and tachykinin receptors: Effects in the genitourinary tract. Life Sciences 76 (8): 835–862. https://doi.org/10.1016/j.lfs.2004.10.004.
Chou, L.W., J. Wang, P.L. Chang, and Y.L. Hsieh. 2011. Hyaluronan modulates accumulation of hypoxia-inducible factor-1 alpha, inducible nitric oxide synthase, and matrix metalloproteinase-3 in the synovium of rat adjuvant-induced arthritis model. Arthritis Research & Therapy 13 (3): R90. https://doi.org/10.1186/ar3365.
Colaco, M., and R. Evans. 2015. Current guidelines in the management of interstitial cystitis. Translational Andrology and Urology 4 (6): 677–683. https://doi.org/10.3978/j.issn.2223-4683.2015.11.03.
Frenkl, T.L., H. Zhu, T. Reiss, O. Seltzer, E. Rosenberg, and S. Green. 2010. A multicenter, double-blind, randomized, placebo controlled trial of a neurokinin-1 receptor antagonist for overactive bladder. The Journal of Urology 184 (2): 616–622. https://doi.org/10.1016/j.juro.2010.03.147.
Green, M., A. Filippou, G. Sant, and T.C. Theoharides. 2004. Expression of intercellular adhesion molecules in the bladder of patients with interstitial cystitis. Urology 63 (4): 688–693. https://doi.org/10.1016/j.urology.2003.11.022.
Green, S.A., A. Alon, J. Ianus, K.S. McNaughton, C.A. Tozzi, and T.F. Reiss. 2006. Efficacy and safety of a neurokinin-1 receptor antagonist in postmenopausal women with overactive bladder with urge urinary incontinence. The Journal of Urology 176 (6 Pt 1): 2535–2540; discussion 2540. https://doi.org/10.1016/j.juro.2006.08.018.
Jin, X.W., B.K. Liu, X. Zhang, Z.H. Zhao, and Y. Shao. 2017. Establishment of a novel autoimmune experimental model of bladder pain syndrome/interstitial cystitis in C57BL/6 mice. Inflammation 40 (3): 861–870. https://doi.org/10.1007/s10753-017-0531-7.
Leppilahti, M., P. Hellstrom, and T.L. Tammela. 2002. Effect of diagnostic hydrodistension and four intravesical hyaluronic acid instillations on bladder ICAM-1 intensity and association of ICAM-1 intensity with clinical response in patients with interstitial cystitis. Urology 60 (1): 46–51.
Li, P.C., W.C. Chen, L.C. Chang, and S.C. Lin. 2008. Substance P acts via the neurokinin receptor 1 to elicit bronchoconstriction, oxidative stress, and upregulated ICAM-1 expression after oil smoke exposure. American Journal of Physiology. Lung Cellular and Molecular Physiology 294 (5): L912–L920. https://doi.org/10.1152/ajplung.00443.2007.
Moutzouris, D.A., and M.E. Falagas. 2009. Interstitial cystitis: An unsolved enigma. Clinical Journal of the American Society of Nephrology 4 (11): 1844–1857. https://doi.org/10.2215/CJN.02000309.
Ogawa, T., O. Ishizuka, T. Ueda, P. Tyagi, M.B. Chancellor, and N. Yoshimura. 2015. Current and emerging drugs for interstitial cystitis/bladder pain syndrome (IC/BPS). Expert Opinion on Emerging Drugs 20 (4): 555–570. https://doi.org/10.1517/14728214.2015.1105216.
Patel, L., and C. Lindley. 2003. Aprepitant--a novel NK1-receptor antagonist. Expert Opinion on Pharmacotherapy 4 (12): 2279–2296. https://doi.org/10.1517/14656566.4.12.2279.
Pazin, C., A.M. de Souza Mitidieri, A.P. Silva, M.B. Gurian, O.B. Poli-Neto, and E.S.J.C. Rosa. 2016. Treatment of bladder pain syndrome and interstitial cystitis: A systematic review. International Urogynecology Journal 27 (5): 697–708. https://doi.org/10.1007/s00192-015-2815-5.
Pennefather, J.N., A. Lecci, M.L. Candenas, E. Patak, F.M. Pinto, and C.A. Maggi. 2004. Tachykinins and tachykinin receptors: A growing family. Life Sciences 74 (12): 1445–1463.
Pinto, L.G., T.M. Cunha, S.M. Vieira, H.P. Lemos, W.A. Verri Jr., F.Q. Cunha, and S.H. Ferreira. 2010. IL-17 mediates articular hypernociception in antigen-induced arthritis in mice. Pain 148 (2): 247–256. https://doi.org/10.1016/j.pain.2009.11.006.
Regauer, S. 2016. Mast cell activation syndrome in pain syndromes bladder pain syndrome/interstitial cystitis and vulvodynia. Translational Andrology and Urology 5 (3): 396–397. https://doi.org/10.21037/tau.2016.03.12.
Seth, A., and J.M. Teichman. 2008. What's new in the diagnosis and management of painful bladder syndrome/interstitial cystitis? Current Urology Reports 9 (5): 349–357.
Shao, Y., G.L. Lu, Z.J. Shen, and H.C. He. 2013. Reduction of intercellular adhesion molecule 1 may play a role in anti-inflammatory effect of hyaluronic acid in a rat model of severe non-bacterial cystitis. World Journal of Urology 31 (3): 535–540. https://doi.org/10.1007/s00345-012-0839-8.
Shao, Y., Z.J. Shen, W.B. Rui, and W.L. Zhou. 2010. Intravesical instillation of hyaluronic acid prolonged the effect of bladder hydrodistention in patients with severe interstitial cystitis. Urology 75 (3): 547–550. https://doi.org/10.1016/j.urology.2009.09.078.
van der Kleij, H.P., D. Ma, F.A. Redegeld, A.D. Kraneveld, F.P. Nijkamp, and J. Bienenstock. 2003. Functional expression of neurokinin 1 receptors on mast cells induced by IL-4 and stem cell factor. Journal of Immunology 171 (4): 2074–2079.
Wang, X., W. Liu, M. O'Donnell, S. Lutgendorf, C. Bradley, A. Schrepf, and Y. Luo. 2016. Evidence for the role of mast cells in cystitis-associated lower urinary tract dysfunction: A multidisciplinary approach to the study of chronic pelvic pain research network animal model study. PLoS One 11 (12): e0168772. https://doi.org/10.1371/journal.pone.0168772.
Wang, Z.Y., P. Wang, and D.E. Bjorling. 2014. Treatment with a cannabinoid receptor 2 agonist decreases severity of established cystitis. The Journal of Urology 191 (4): 1153–1158. https://doi.org/10.1016/j.juro.2013.10.102.
Yu, W., C. Ackert-Bicknell, J.D. Larigakis, B. MacIver, W.D. Steers, G.A. Churchill, et al. 2014. Spontaneous voiding by mice reveals strain-specific lower urinary tract function to be a quantitative genetic trait. American Journal of Physiology. Renal Physiology 306 (11): F1296–F1307. https://doi.org/10.1152/ajprenal.00074.2014.
Zacche, M.M., S. Srikrishna, and L. Cardozo. 2015. Novel targeted bladder drug-delivery systems: a review. Research and Reports in Urology 7: 169–178. https://doi.org/10.2147/RRU.S56168.
Funding
This research was supported by the Research Program of Shanghai Municipal Commission of Health and Family Planning (No. 201540146) and the General Programs of the National Natural Science Foundation of China (No. 81270846).
Author information
Authors and Affiliations
Contributions
BKL: data collection, analysis, and manuscript writing. JXW: protocol development, data collection, and manuscript writing. HZL and XZ: data collection. ZHZ: pathologic evaluation. YS: research design, data analysis, and manuscript editing.
Corresponding author
Ethics declarations
Ethics Statement
All animal experiments were approved by the Animal Care and Use Committee of Ruijin Hospital affiliated to Shanghai Jiao Tong University School of Medicine. Procedures were performed in accordance with the Animal Management Rule of the Ministry of Health, People’s Republic of China (documentation no. 55, 2001).
Competing Interests
No competing interests in this research.
Rights and permissions
About this article
Cite this article
Liu, BK., Jin, XW., Lu, HZ. et al. The Effects of Neurokinin-1 Receptor Antagonist in an Experimental Autoimmune Cystitis Model Resembling Bladder Pain Syndrome/Interstitial Cystitis. Inflammation 42, 246–254 (2019). https://doi.org/10.1007/s10753-018-0888-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-018-0888-2