Abstract
Bacterial lipopolysaccharide (LPS) induces inflammatory response via toll-like receptor 4 (TLR4). However, this response must be strictly regulated because unbalanced overproduction of pro-inflammatory cytokines can lead to tissue damage and even be fatal. Herein, we explore whether Mer receptor tyrosine kinase (MerTK) regulates Escherichia coli (E. coli) LPS-induced inflammation and mediates phagocytosis of E. coli by macrophages. The results showed that LPS activated TLR4 signaling pathway and induced MerTK pathway in RAW264.7 macrophages, including suppressor of cytokine signaling1 (SOCS1). Preincubation with MerTK-specific blocking antibody (MerTK-Ab) markedly suppressed LPS-induced expression of phosphorylated MerTK, while further promoted LPS-induced production of TNF-α, IL-6, and IL-1β as well as phosphorylation of IκB-α and p65. Likewise, MerTK-Ab prevented LPS-induced SOCS1 expression. Furthermore, LPS-induced production of pro-inflammatory cytokines and activation of NF-κB were increased by transfection with SOCS1 siRNA. Additionally, we demonstrated that MerTK was dispensable in phagocytosis of E. coli by RAW264.7 or peritoneal macrophages. Collectively, these results indicate that MerTK downregulates LPS-induced inflammation through SOCS1 protein without affecting phagocytosis of E. coli in macrophages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Inflammation is the first defense response of the immune system against infection, which involves phagocytes such as macrophages [1]. Macrophages are the main effector cells of the immune system and play an essential role in host defense against infectious microorganisms [2]. Toll-like receptors (TLRs) are “pattern recognition receptors” that are predominantly expressed in immune cells, including macrophages and dendritic cells (DCs) [3]. TLRs initiate the innate immune responses by recognizing bacterial components such as lipopolysaccharide (LPS) and lipoteichoic acid (LTA) [4]. As a component of the cell membrane of Gram-negative bacteria, LPS is a potent microbial trigger of the inflammatory response [5]. Bacterial LPS elicits a variety of effects on the immune system, and the major target cells of LPS are macrophages and monocytes [6].
Among all TLRs, TLR4 recognizes bacterial LPS and then triggers a strong inflammatory response in host macrophages via MyD88-dependent and MyD88-independent signaling pathways [5, 7]. MyD88 exerts an essential role in TLR signaling pathways except for TLR3 [8, 9]. After the activation of TLR4, mitogen-activated protein kinase (MAPK) pathways, interferon response factor (IRF), and NF-κB are activated, subsequently resulting in the production of pro-inflammatory cytokines [10]. Then, macrophages are recruited by pro-inflammatory cytokines, which eventually phagocytize and eliminate the invading bacteria [11]. This rapid inflammatory response and subsequent macrophage phagocytosis are essential for the elimination of bacterial infection.
The TLR4-mediated immune response is critical in controlling bacterial infection, but it must be tightly regulated because unbalanced overproduction of pro-inflammatory cytokines can lead to tissue damage and even be fatal [5, 12]. Hence, multiple negative regulatory mechanisms to downregulate TLR signaling have been clarified [8]. Mer receptor tyrosine kinase (MerTK), a subfamily of the Tyro3/Axl/Mer (TAM) receptors, is predominantly expressed in macrophages and DCs [13]. It has been reported that MerTK in DCs negatively regulates bacterial LPS-induced inflammatory response via suppressor of cytokine signaling (SOCS) proteins [10]. Additionally, macrophages isolated from MerTK knockdown (MerTKKD) mice have been shown to be hypersensitive to bacterial LPS [14]. However, the underlying mechanisms by which MerTK downregulates the bacterial LPS-induced inflammation in macrophages need to be further clarified.
It has been clearly demonstrated that MerTK is involved in phagocytosis of apoptotic cells [15, 16], whereas its involvement in phagocytosis of Escherichia coli (E. coli) remains controversial. Phagocytosis of E. coli by macrophages derived from MerTKKD and wild-type mice is reported to be similar [11]. However, macrophages from TAMKD mice phagocytize E. coli more effectively than those from wild-type mice [17].
Therefore, we aimed to further elucidate the mechanisms by which MerTK downregulated E. coli LPS-induced inflammation and to further investigate the role of MerTK in the phagocytosis of E. coli in macrophages.
MATERIALS AND METHODS
Reagents and Antibodies
LPS derived from E. coli 055: B5 was acquired from Sigma-Aldrich (St. Louis, MO, USA). E. coli (ATCC25922) was purchased from Shanghai Fuxiang Biotech, Co, LTD, China.
The antibodies against phosphorylated (P)-MerTK (PMKT-140AP) and MerTK were from Fab Gennix (Frisco, TX, USA). The antibodies against P-IκB-α (Ser32) and SOCS1 were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The antibodies against MyD88, P-p38/p38, P-ERK/ERK, P-JNK/JNK, P-NF-κBp65 (Ser536)/NF-κBp65, GAPDH, and β-tubulin were from Cell Signaling Technology (Beverly, MA, USA). The HRP-conjugated secondary antibodies were from KangChen Biotech Company (Shanghai, China).
Animals
C57BL/6 mice were purchased from Shanghai Laboratory Animal Center (Shanghai, China), and mice were housed and bred under specific pathogen-free conditions. MerTKKD mice were established as previously described [14]. Briefly, the tyrosine kinase domain of MerTK was replaced with a neomycin resistance gene, and C57BL/6 MerTKKD mice were established. C57BL/6 mice were used as wild-type controls. All experimental protocols in the current study were approved by the Animal Ethical Committee of Anhui Medical University.
Cell Culture and Stimulation
Murine macrophage cell line RAW264.7 (Cell Bank of the Chinese Academy of Sciences, Shanghai, China) was grown in Dulbecco’s modified Eagle’s medium (DMEM; Sigma-Aldrich) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gibco, Grand Island, NY, USA), 100 U/ml penicillin G, and 100 μg/ml streptomycin in a humidified 37 °C, 5% CO2 incubator.
Primary peritoneal macrophages were obtained as previously described [18]. Briefly, 6-week-old wild-type and MerTKKD mice were injected with 1 ml of 1.5% (w/v) starch solution. After 5 days, macrophages were obtained by peritoneal lavage, washed twice with 5 ml of phosphate-buffered saline (PBS). Then, 1 × 106/ml cells were added to each well of 6-well plates. After 3 h, the non-adherent cells were washed away, and adherent cells were incubated in 3 ml of DMEM. Before LPS or E. coli stimulation, the cell culture medium was replaced with antibiotic- and serum-free DMEM.
MerTK-Specific Blocking Antibody
Polyclonal goat anti-mouse MerTK antibody (AF591) and control goat IgG were purchased from R&D Systems (Minneapolis, MN, USA). The MerTK-specific blocking antibody (MerTK-Ab), which specifically prevents MerTK activation by selectively blocking MerTK binding to its ligand Gas6 [19,20,21,22,23], was dissolved in PBS at 20-μg/ml working concentration.
RNA Interference of SOCS1
Small interfering RNA (siRNA) oligonucleotides targeting SOCS1 were obtained from Shanghai GenePharma Corporation. The siRNA sequences used in the present study were SOCS1 siRNA (sense: 5′-CAGCCAGUUUAGGUAAUAATT-3′ and antisense: 5′-UUAUUACCUAAACUGGCUGTT-3′) and negative control siRNA (sense: 5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense: 5′-ACGUGACACGUUCGGAGAATT-3′). RAW264.7 macrophages were transfected with siRNA oligonucleotides using Lipofectamine 2000 (Invitrogen, Carlsbad, USA). Before further experiments, the transfected cells were incubated for 48 h.
Western Blot
RAW264.7 macrophages were seeded into 12-well plates at 5.0 × 105/well. The treated and control macrophages were lysed in lysis buffer containing 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% TritonX-100, 0.25% sodium deoxycholate, 0.1% SDS, and 1 mM EDTA and supplemented with phosphatase inhibitor PhosSTOP (Roche, Indianapolis, IN, USA) and protease inhibitor cocktail (Roche). Proteins were separated by 8–12% SDS-PAGE and then transferred onto PVDF membranes. The membranes were blocked at room temperature for 60 min and then incubated overnight at 4 °C with appropriate primary antibodies. On the next day, after incubation with HRP-conjugated secondary antibodies for 60 min, all proteins were detected by enhanced chemiluminescence (ECL) reagent.
Enzyme-Linked Immunosorbent Assay
RAW264.7 macrophages were incubated with 1 μg/ml LPS for 24 h after pretreatment with MerTK-Ab or transfection with SOCS1 siRNA. The culture supernatants were harvested, and concentrations of TNF-α, IL-6, and IL-1β were measured by respective ELISA kits (Cusabio Biotech, Co, LTD, China).
Phagocytosis Assays
Macrophage phagocytosis of bacteria was examined using E. coli (ATCC25922) transfected with green fluorescent protein (GFP) plasmid. Briefly, RAW264.7 and peritoneal macrophages were respectively seeded onto glass coverslips placed in 12-well plates (2.5 × 105/well) for 12 h. RAW264.7 macrophages were pretreated with 20 μg/ml MerTK-Ab or IgG for 60 min. RAW264.7 and peritoneal macrophages were then incubated with a MOI of ten GFP-E. coli for 60 min. After incubation for 60 min, non-associating GFP-E. coli was washed away with 3 × PBS. The macrophages were fixed with 4% paraformaldehyde and stained with DAPI to visualize the nuclei in blue, then observed under a Zeiss LMS710 confocal laser microscope. Percent phagocytosis was expressed as a percentage of phagocytes with associating (binding and uptake) at least one GFP-E. coli in the total number of phagocytes. Phagocytic index was expressed as the mean number of associating GFP-E. coli per phagocytizing cell. At least 100 macrophages were counted.
Macrophage phagocytosis of apoptotic cells was examined using apoptotic thymocytes. The phagocytosis assay was performed as previously described [15]. Briefly, thymocytes isolated from 4- to 6-week-old wild-type mice were washed and incubated in media containing 2 μM dexamethasone. After the incubation for 5 h, cells were fluorescein-labeled with Cell Tracker Red (Molecular Probes). Cells were washed with PBS and incubated in media for 30 min prior to experiments. RAW264.7 macrophages were transfected with GFP plasmid and allowed to recover for at least 48 h before co-culturing with apoptotic thymocytes. RAW264.7 macrophages were pretreated with 20 μg/ml MerTK-Ab or IgG for 60 min and then co-cultured with apoptotic thymocytes for 2 h at a ratio of 1:10. After incubation for 2 h, non-associating apoptotic thymocytes were washed away with 3 × PBS. The percent phagocytosis and phagocytic index were tested by two-color FACScan flow cytometer.
Statistical Analysis
Data are expressed as mean ± SEM from three independent experiments. One-way ANOVA with a post hoc Bonferroni’s test was used for multiple comparisons. Independent sample t test was applied for comparisons of two sample means. A p value of < 0.05 was considered statistically significant.
RESULTS
LPS Activates TLR4 and MerTK Signaling Pathways in RAW264.7 Macrophages
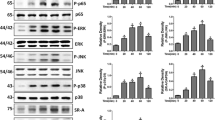
To analyze whether TLR4 and MerTK pathways could be triggered by LPS stimulation, we incubated RAW264.7 macrophages with LPS for the indicated time, then examined the expression levels of proteins related to these signaling pathways. As shown in Fig. 1a, LPS resulted in activation of MyD88, MAPKs (JNK, ERK, and p38), and NF-κB (IκB-α and p65), which peaked at 60 min after LPS treatment (Fig. 1b). Similarly, LPS also activated MerTK signaling pathway, including SOCS1, with the same kinetics (Fig. 1a, b).
Multiple signaling molecules were activated in LPS-stimulated RAW264.7 macrophages. a RAW264.7 macrophages were stimulated with 1 μg/ml LPS for the indicated time. Whole cell lysates were subjected to western blot analysis of MyD88, P-p38, P-ERK, P-JNK, P-IκB-α (Ser32), P-p65 (Ser536), P-MerTK, and SOCS1. b The graph represents quantitative analysis of the band intensity. The results are expressed as the mean ± SEM from three independent experiments. *p < 0.05 compared with the control group.
MerTK-Ab Promotes LPS-Induced Inflammation
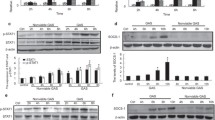
TNF-α, IL-6, and IL-1β are well-known pro-inflammatory cytokines in the induction of inflammation in macrophages. To investigate the role of MerTK in LPS-induced inflammation, we pretreated LPS-stimulated RAW264.7 macrophages with MerTK-Ab or IgG and measured the levels of these pro-inflammatory cytokines. We found that MerTK-Ab, but not control IgG, significantly attenuated the level of p-MerTK after LPS stimulation (Fig. 2a). As expected, LPS significantly increased the levels of TNF-α, IL-6, and IL-1β (Fig. 2b). Preincubation with MerTK-Ab further promoted LPS-induced pro-inflammatory cytokine production (Fig. 2b).
The inhibitory effect of MerTK on LPS-induced pro-inflammatory cytokine production. a RAW264.7 macrophages were stimulated with 1 μg/ml LPS for 60 min after preincubation with 20 μg/ml MerTK-Ab or IgG for 60 min. Whole cell lysates were subjected to western blot analysis of P-MerTK. b RAW264.7 macrophages were stimulated with 1 μg/ml LPS for 24 h after preincubation with 20 μg/ml MerTK-Ab or IgG for 60 min. The levels of TNF-α, IL-6, and IL-1β in culture supernatants were measured by ELISA. The graph represents a quantitative analysis of the band intensity and b the levels of these pro-inflammatory cytokines. The results are expressed as the mean ± SEM from three independent experiments. *p < 0.05 compared with the control group; #p < 0.05 compared with the LPS + MerTK-Ab group versus the LPS-only group or the LPS + IgG group.
MerTK-Ab Selectively Enhances LPS-Induced IκB-α and p65 Phosphorylation
Next, we analyzed the effect of MerTK on TLR4 signaling molecules, including MyD88, MAPKs, IκB-α, and p65, in the LPS-induced inflammation. We pretreated LPS-stimulated RAW264.7 macrophages with or without MerTK-Ab and found that MerTK-Ab further promoted LPS-induced phosphorylation of IκB-α and p65, whereas LPS-induced MyD88 expression and MAPK phosphorylation were unaffected (Fig. 3).
LPS-induced IκB-α and p65 phosphorylation was inhibited by MerTK in RAW264.7 macrophages. RAW264.7 macrophages were stimulated with 1 μg/ml LPS for 60 min after preincubation with 20 μg/ml MerTK-Ab or IgG for 60 min. Whole cell lysates were subjected to western blot analysis of MyD88, P-IκB-α (Ser32), P-p65 (Ser536), P-ERK, P-JNK, and P-p38. The graph represents quantitative analysis of the band intensity. The results are expressed as the mean ± SEM from three independent experiments. *p < 0.05 compared with the control group; #p < 0.05 compared with the LPS + MerTK-Ab group versus the LPS-only group or the LPS + IgG group.
SOCS1 Is Involved in MerTK-Dependent Inhibition of LPS-Induced Inflammation
Recent studies have shown that SOCS1 is a downstream effector of MerTK signaling pathway [10, 24], and SOCS1 is reported to be one of the negative intracellular regulators of TLR-mediated inflammation [8]. These findings prompted us to examine whether SOCS1 contributed to the MerTK-dependent inhibition of LPS-induced inflammation. Our present study demonstrated that pretreatment with MerTK-Ab significantly blocked LPS-induced SOCS1 expression (Fig. 4a), while the control IgG had no impact on the expression of SOCS1 in RAW264.7 macrophages (Fig. 4a).
MerTK inhibition of LPS-induced inflammation was mediated through SOCS1 in RAW264.7 macrophages. a RAW264.7 macrophages were stimulated with 1 μg/ml LPS for 60 min after preincubation with 20 μg/ml MerTK-Ab or IgG for 60 min. Whole cell lysates were subjected to western blot analysis of SOCS1. b, c RAW264.7 macrophages were transfected with SOCS1 siRNA or control siRNA and then stimulated with 1 μg/ml LPS for 60 min. Whole cell lysates were subjected to western blot analysis of SOCS1, P-IκB-α (Ser32), and P-p65 (Ser536). d RAW264.7 macrophages were transfected with SOCS1 siRNA or control siRNA and then stimulated with 1 μg/ml LPS for 24 h. The levels of TNF-α, IL-6, and IL-1β in culture supernatants were measured by ELISA. The graph represents a–c quantitative analysis of the band intensity and d the levels of these pro-inflammatory cytokines. The results are expressed as the mean ± SEM from three independent experiments. *p < 0.05 compared with the control group; a#p < 0.05 compared with the LPS + MerTK-Ab group versus the LPS-only group or the LPS + IgG group; b–d#p < 0.05 compared with the LPS + SOCS1 siRNA group versus the LPS + control siRNA group.
To confirm the involvement of SOCS1 in the inflammatory response of LPS-stimulated macrophages, we incubated RAW264.7 macrophages with LPS after transfection with SOCS1 siRNA or control siRNA and tested whether SOCS1 siRNA could affect the levels of TNF-α, IL-6, and IL-1β as well as the expression of p-IκB-α and p-p65. As shown in Fig. 4b, transfection with SOCS1 siRNA, but not with control siRNA, markedly inhibited LPS-induced SOCS1 expression, and the knockdown efficiency was also approximately 70%. Furthermore, LPS-induced IκB-α and p65 phosphorylation (Fig. 4c) as well as TNF-α, IL-6, and IL-1β production (Fig. 4d) was markedly increased in SOCS1 siRNA-transfected RAW264.7 macrophages.
MerTK Is Dispensable in Phagocytosis of E. coli by Macrophages
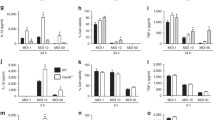
To explore whether the role of MerTK in the phagocytosis of E. coli by macrophages, we incubated RAW264.7 macrophages with GFP-E. coli in the presence of MerTK-Ab or IgG preincubation. By phagocytosis assays, we observed that there was no statistically significant difference in percent phagocytosis between MerTK-Ab group and IgG group (Fig. 5a). We further performed a phagocytic index. In line with the percent phagocytosis results, there was still no difference in phagocytic index between MerTK-Ab group and IgG group (Fig. 5b). Meanwhile, we confirmed our findings using primary peritoneal macrophages. Peritoneal macrophages from wild-type and MerTKKD mice were incubated with GFP-E. coli for 60 min, respectively. As shown in Fig. 5c, d, we also found that there was no difference in percent phagocytosis or phagocytic index between macrophages from MerTKKD and wild-type mice.
MerTK had no effect on phagocytosis of E. coli by macrophages. a, b RAW264.7 macrophages were stimulated with a MOI of ten GFP-E. coli for 60 min with pretreatment with 20 μg/ml MerTK-Ab or IgG for 60 min. c, d Peritoneal macrophages from wild-type (WT) and MerTKKD mice were respectively stimulated with a MOI of ten GFP-E. coli for 60 min. e, f RAW264.7 macrophages were stimulated with apoptotic thymocytes at a ratio of 1:10 for 2 h with pretreatment with 20 μg/ml MerTK-Ab or IgG for 60 min. Phagocytosis assays were performed as indicated in “MATERIALS AND METHODS.” The graph represents a, c, e percent phagocytosis or b, d, f phagocytic index. The results are expressed as the mean ± SEM from three independent experiments. *p < 0.05 compared with the control IgG group.
In addition, to further demonstrate that MerTK was essential for phagocytosis of apoptotic cells, we incubated RAW264.7 macrophages with apoptotic thymocytes in the presence of MerTK-Ab or IgG preincubation. Expectedly, we found that the percent phagocytosis and phagocytic index in MerTK-Ab group were significantly lower than those in IgG group (Fig. 5e, f).
DISCUSSION
This study demonstrates that MerTK downregulates LPS-induced inflammation through SOCS1 protein, but does not affect phagocytosis of E. coli in macrophages.
Treatment of LPS results in the activation of TLR4 signaling and concomitantly activates PI3K/Akt anti-inflammatory pathway in human monocytic cells [25]. Recent studies have shown that LPS induces the activation of TLR4 and MerTK signaling pathways with the same kinetics [24]. Similarly, our studies show that activation of MerTK signaling is most significant at 60 min after stimulation of LPS. Additionally, MerTK activation was reported to inhibit inflammatory response in vivo in LPS-induced acute lung injury [24]. In agreement with this, our results suggest that MerTK activation downregulates LPS-induced inflammation and indicate that the negative regulation of MerTK follows TLR-mediated immune responses in macrophages.
NF-κB is a pivotal transcription factor in TLR-mediated immune responses, and its activation is necessary for the production of pro-inflammatory cytokines [9, 26]. As for the mechanism of NF-κB activation, IκB is phosphorylated and degraded, followed by NF-κB heterodimer translocating into the nucleus, and finally, NF-κB p65 subunit is phosphorylated [8, 26]. Interestingly, our studies show that MerTK only inhibits LPS-induced IκB-α and p65 phosphorylation in RAW264.7 macrophages, but has no significant effect on MAPK phosphorylation or MyD88 expression. These findings were consistent with our previous studies that LTA-induced MerTK activation selectively suppresses NF-κB activation in macrophages [27]. Altogether, these observations support the idea that blocking the activation of NF-κB is necessary for MerTK negative regulation on TLR signaling in macrophages.
Although the molecular mechanisms by which MerTK downregulates LPS-induced inflammation in macrophages have not been fully understood, our current studies indicate that SOCS1 protein may participate in this process. We found that LPS induced the activation of SOCS1and MerTK with the same kinetics. Meanwhile, MerTK-Ab suppressed LPS-induced SOCS1 expression. These findings indicate that SOCS1 activation is MerTK-dependent during LPS-induced inflammatory response in macrophages. Numerous studies have showed that SOCS1 is a potent regulatory protein which inhibits TLR-mediated inflammatory responses in macrophages and DCs [6, 10, 28,29,30]. Similarly, our studies also show that SOCS1 siRNA enhances the levels of TNF-α, IL-6, and IL-1β as well as expression of p-IκB-α and p-p65. These results indicate that activation of MerTK pathway downregulates LPS-induced inflammation through SOCS1 protein in macrophages.
Since MerTK downregulates E. coli LPS-induced inflammation in RAW264.7 macrophages, we hypothesized that RAW264.7 macrophages might be able to phagocytize and eliminate E. coli more efficiently with preincubation of MerTK-Ab. Most notably, however, the involvement of MerTK in phagocytosis of E. coli in previous studies has provided conflicting results [11, 17]. In the present study, we found that MerTK-Ab did not affect the phagocytosis of E. coli by RAW264.7 macrophages. To further confirm the necessity of MerTK in phagocytosis of E. coli by macrophages, we also assessed phagocytosis of E. coli using primary peritoneal macrophages. Likewise, we observed that there was no statistically significant difference in the phagocytosis of E. coli between MerTKKD and wild-type macrophages, consistent with a previous study [11]. In contrast, macrophages from MerTKKD mice were markedly deficient in phagocytosis of apoptotic cells similar to previous studies [15, 16]. Therefore, although MerTK play an important role in the phagocytosis of apoptotic cells, it is dispensable in phagocytosis of E. coli by macrophages. This may be because the cell membrane of bacteria does not express phosphatidylserine, which binds to MerTK ligands Gas6. However, Gas6 binds to phosphatidylserine expressed on the membrane of apoptotic cells to form the Gas6-phosphatidylserine complex, which MerTK binds to apoptotic cells [31,32,33].
In conclusion, our current studies show that LPS stimulation induces the activation of MerTK signaling pathway in macrophages. MerTK activation downregulates LPS-induced inflammation through SOCS1 protein, but it does not affect phagocytosis of E. coli in macrophages. Therefore, our studies deepen the understanding of TLR signaling regulation and provide new targets to control TLR-mediated autoimmune and inflammatory diseases.
References
Galli, S.J., M. Grimbaldeston, and M. Tsai. 2008. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nature Reviews. Immunology 8: 478–486.
Ma, W., Y. Dumont, F. Vercauteren, and R. Quirion. 2010. Lipopolysaccharide induces calcitonin gene-related peptide in the RAW264.7 macrophage cell line. Immunology 130: 399–409.
Medzhitov, R. 2001. Toll-like receptors and innate immunity. Nature Reviews. Immunology 1: 135–145.
Takeda, K., and S. Akira. 2005. Toll-like receptors in innate immunity. International Immunology 17: 1–14.
Seeley, J.J., and S. Ghosh. 2017. Molecular mechanisms of innate memory and tolerance to LPS. Journal of Leukocyte Biology 101: 107–119.
Kinjyo, I., T. Hanada, K. Inagaki-Ohara, H. Mori, D. Aki, M. Ohishi, H. Yoshida, M. Kubo, and A. Yoshimura. 2002. SOCS1/JAB is a negative regulator of LPS-induced macrophage activation. Immunity 17: 583–591.
Zielen, S., J. Trischler, and R. Schubert. 2015. Lipopolysaccharide challenge: immunological effects and safety in humans. Expert Review of Clinical Immunology 11: 409–418.
Liew, F.Y., D. Xu, E.K. Brint, and L.A.J. O'Neill. 2005. Negative regulation of toll-like receptor-mediated immune responses. Nature Reviews. Immunology 5: 446–458.
Takeuchi, O., and S. Akira. 2010. Pattern recognition receptors and inflammation. Cell 140: 805–820.
Rothlin, C.V., S. Ghosh, E.I. Zuniga, M.B.A. Oldstone, and G. Lemke. 2007. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell 131: 1124–1136.
Williams, J.C., R.R. Craven, H.S. Earp, et al. 2009. TAM receptors are dispensable in the phagocytosis and killing of bacteria. Cellular Immunology 259: 128–134.
Tan, Y., and J.C. Kagan. 2014. A cross-disciplinary perspective on the innate immune responses to bacterial lipopolysaccharide. Molecular Cell 54: 212–223.
Behrens, E.M., P. Gadue, S.Y. Gong, et al. 2003. The mer receptor tyrosine kinase: expression and function suggest a role in innate immunity. European Journal of Immunology 33: 2160–2167.
Camenisch, T.D., B.H. Koller, H.S. Earp, et al. 1999. A novel receptor tyrosine kinase, Mer, inhibits TNF-alpha production and lipopolysaccharide-induced endotoxic shock. Journal of Immunology 162: 3498–3503.
Scott, R.S., E.J. McMahon, S.M. Pop, E.A. Reap, R. Caricchio, P.L. Cohen, H.S. Earp, and G.K. Matsushima. 2001. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature 411: 207–211.
Seitz, H.M., T.D. Camenisch, G. Lemke, H.S. Earp, and G.K. Matsushima. 2007. Macrophages and dendritic cells use different Axl/Mertk/Tyro3 receptors in clearance of apoptotic cells. Journal of Immunology 178: 5635–5642.
Lu, Q., and G. Lemke. 2001. Homeostatic regulation of the immune system by receptor tyrosine kinases of the Tyro 3 family. Science 293: 306–311.
Wu, H.M., J. Wang, B. Zhang, et al. 2016. CpG-ODN promotes phagocytosis and autophagy through JNK/P38 signal pathway in Staphylococcus aureus-stimulated macrophage. Life Sciences 161: 51–59.
Sen, P., M.A. Wallet, Z. Yi, Y. Huang, M. Henderson, C.E. Mathews, H.S. Earp, G. Matsushima, A.S. Baldwin, and R.M. Tisch. 2007. Apoptotic cells induce Mer tyrosine kinase-dependent blockade of NF-kappaB activation in dendritic cells. Blood 109: 653–660.
Alciato, F., P.P. Sainaghi, D. Sola, L. Castello, and G.C. Avanzi. 2010. TNF-alpha, IL-6, and IL-1 expression is inhibited by GAS6 in monocytes/macrophages. Journal of Leukocyte Biology 87: 869–875.
Anwar, A., A.K. Keating, D. Joung, S. Sather, G.K. Kim, K.K. Sawczyn, L. Brandão, P.M. Henson, and D.K. Graham. 2009. Mer tyrosine kinase (MerTK) promotes macrophage survival following exposure to oxidative stress. Journal of Leukocyte Biology 86: 73–79.
Park, H.J., J.Y. Baen, Y.J. Lee, Y.H. Choi, and J.L. Kang. 2012. The TAM-family receptor Mer mediates production of HGF through the RhoA-dependent pathway in response to apoptotic cells. Molecular Biology of the Cell 23: 3254–3265.
Wallet, M.A., P. Sen, R.R. Flores, Y. Wang, Z. Yi, Y. Huang, C.E. Mathews, H.S. Earp, G. Matsushima, B. Wang, and R. Tisch. 2008. MerTK is required for apoptotic cell-induced T cell tolerance. The Journal of Experimental Medicine 205: 219–232.
Lee, Y.J., J.Y. Han, J. Byun, H.J. Park, E.M. Park, Y.H. Chong, M.S. Cho, and J.L. Kang. 2012. Inhibiting Mer receptor tyrosine kinase suppresses STAT1, SOCS1/3, and NF-kappaB activation and enhances inflammatory responses in lipopolysaccharide-induced acute lung injury. Journal of Leukocyte Biology 91: 921–932.
Guha, M., and N. Mackman. 2002. The phosphatidylinositol 3-kinase-Akt pathway limits lipopolysaccharide activation of signaling pathways and expression of inflammatory mediators in human monocytic cells. The Journal of Biological Chemistry 277: 32124–32132.
Graham, B., and S.B. Gibson. 2005. The two faces of NFkappaB in cell survival responses. Cell Cycle 4: 1342–1345.
Zhang, B., H. Wu, L. Fang, P. Ding, K. Xu, Q. Yang, and R. Liu. 2017. MerTK does not mediate phagocytosis of Staphylococcus aureus but attenuates inflammation induced by staphylococcal lipoteichoic acid through blocking NF-κB activation. Inflammation 40: 1543–1552.
Dalpke, A.H., S. Opper, S. Zimmermann, and K. Heeg. 2001. Suppressors of cytokine signaling (SOCS)-1 and SOCS-3 are induced by CpG-DNA and modulate cytokine responses in APCs. Journal of Immunology 166: 7082–7089.
Mansell, A., R. Smith, S.L. Doyle, P. Gray, J.E. Fenner, P.J. Crack, S.E. Nicholson, D.J. Hilton, L.A.J. O'Neill, and P.J. Hertzog. 2006. Suppressor of cytokine signaling 1 negatively regulates toll-like receptor signaling by mediating Mal degradation. Nature Immunology 7: 148–155.
Zheng, J., P. Yang, Y. Tang, et al. 2015. Respiratory syncytial virus nonstructural proteins upregulate SOCS1 and SOCS3 in the different manner from endogenous IFN signaling. Journal of Immunology Research 2015: 738547.
Wu, Y., N. Tibrewal, and R.B. Birge. 2006. Phosphatidylserine recognition by phagocytes: a view to a kill. Trends in Cell Biology 16: 189–197.
Lemke, G., and C.V. Rothlin. 2008. Immunobiology of the TAM receptors. Nature Reviews. Immunology 8: 327–336.
Mark, M.R., J. Chen, R.G. Hammonds, M. Sadick, and P.J. Godowsk. 1996. Characterization of Gas6, a member of the superfamily of G domain-containing proteins, as a ligand for Rse and Axl. The Journal of Biological Chemistry 271: 9785–9789.
Acknowledgments
We thank Dr. HuiMei Wu and Lei Fang for their technical assistance in performing the experiments.
Funding
This study was supported by the National Key Clinical Specialist Construction Programs of China (respiratory medicine), National Education Ministry of China (No. 20113420110006), and Key Lab of Geriatric Molecular Medicine of Anhui Province (1206c0805028).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All experimental protocols in the current study were approved by the Animal Ethical Committee of Anhui Medical University.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhang, B., Lu, H., Jiang, A. et al. MerTK Downregulates Lipopolysaccharide-Induced Inflammation Through SOCS1 Protein but Does Not Affect Phagocytosis of Escherichia coli in Macrophages. Inflammation 42, 113–123 (2019). https://doi.org/10.1007/s10753-018-0877-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-018-0877-5