Abstract
Sirtuin 2 (SIRT2), a member of the mammalian sirtuin family, plays an important role in the pathogenesis of various neurological diseases. However, whether SIRT2 is involved in the regulation of neuropathic pain remains unclear. In this study, we aimed to investigate the potential role of SIRT2 in regulating neuropathic pain in a rat model induced by chronic constriction injury (CCI). We found that SIRT2 was downregulated in the dorsal root ganglion (DRG) in CCI rats. Intrathecal injection of a recombinant adenovirus expressing SIRT2 markedly alleviated mechanical allodynia and thermal hyperalgesia in CCI rats. This also inhibited the expression of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and IL-6 in the DRG of CCI rats. Moreover, our results showed that overexpression of SIRT2 inhibited the acetylation of the nuclear factor-kappa B (NF-κB) p65 protein in the DRG of CCI rats. Additionally, treatment with a SIRT2 specific inhibitor significantly aggravated neuropathic pain and attenuated the inhibitory effect of SIRT2 overexpression on neuropathic pain development. Taken together, these results suggest that overexpression of SIRT2 alleviates neuropathic pain associated with inhibition of NF-κB signaling and neuroinflammation. Therefore, SIRT2 may serve as a potential therapeutic target for treatment of neuropathic pain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Neuropathic pain is a chronic disorder of the nervous system characterized by hyperalgesia in response to noxious stimuli or allodynia in response to non-painful stimuli [1, 2]. Neuropathic pain is currently a notable public health problem and greatly impacts on individual quality of life, affecting a broader population worldwide [3, 4]. However, the therapeutic effects of current pharmacological agents for neuropathic pain remain unsatisfied [5]. Therefore, a better understanding of the molecular mechanisms contributing to neuropathic pain development may help the development of novel therapeutics towards pain management.
Neuroinflammation is a hallmark of neuropathic pain [6]. Astrocytes and microglia in the spinal cord dorsal horn react to nerve injury, leading to persistent neuroinflammation [7]. The release of proinflammatory cytokines, including tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6, contributes to the development of neuropathic pain development [8]. Various transcription factors, such as nuclear factor-kappa B (NF-κB), are involved in regulating neuroinflammation during neuropathic pain development [9], and inhibition of NF-κB significantly attenuates neuropathic pain. Therefore, suppression of neuroinflammation may be beneficial in preventing the development of neuropathic pain.
Sirtuin 2 (SIRT2) is a member of the sirtuin family, which is a group of nicotinamide adenine dinucleotide-dependent deacetylases [10]. SIRT2 is involved in regulating various biological functions, including proliferation, apoptosis, senescence, metabolism, and inflammation [11, 12]. A growing body of evidence has reported that SIRT2 regulates various physiological and pathological processes, including tumorigenesis [13], sepsis [14], and neurodegenerative disorders [15]. SIRT2 has been reported to regulate various signaling pathways through deacetylation of forkhead box O1 (FoxO1), forkhead box 3a (FOXO3a), and p53 [16,17,18]. In addition, studies have shown that SIRT2 inhibits inflammation through deacetylation of the NF-κB p65 protein and inhibits NF-κB-dependent proinflammatory cytokine expression [19, 20].
An increasing number of studies have reported that SIRT2 is involved in the regulation of neurological diseases and neuroinflammation [21, 22]. However, whether SIRT2 is involved in regulating neuropathic pain remains unknown. In this study, we aimed to investigate the role of SIRT2 in regulating neuroinflammation and neuropathic pain development using a chronic constriction injury (CCI)-induced neuropathic pain model. We found that SIRT2 was downregulated in the dorsal root ganglion (DRG) in CCI rats. Intrathecal injection of recombinant adenovirus expressing SIRT2 (Ad-SIRT2) markedly alleviated mechanical allodynia and thermal hyperalgesia in CCI rats, and inhibited the expression of TNF-α, IL-1β, and IL-6 in the DRG of CCI rats. Moreover, we found that overexpression of SIRT2 inhibited the acetylation of the NF-κB p65 protein in the DRG of CCI rats. Additionally, treatment with the SIRT2 specific inhibitor, AK-7, significantly aggravated neuropathic pain and attenuated the inhibitory effect of SIRT2 overexpression on neuropathic pain development. Taken together, these results suggest that overexpression of SIRT2 alleviates neuropathic pain associated with inhibition of NF-κB signaling-mediated neuroinflammation. Therefore, SIRT2 may serve as a potential therapeutic target for preventing neuropathic pain.
MATERIALS AND METHODS
Animals
Adult male Sprague-Dawley rats weighing 180–200 g were purchased from the Experimental Animal Center of Xi’an Jiaotong University. The rats were raised in separated cages at 22 ± 1 °C and 50–60% humidity under a 12/12-h light/dark cycle with free access to water and food. The animal experiments were performed according to the guidelines of the International Association for the Study of Pain and the National Institute of Health Guide for the Care and Use of Laboratory Animals. This study was reviewed and approved by the Institutional Animal Care and Use Committee of Shaanxi Provincial People’s Hospital.
Establishment of Neuropathic Pain Model Induced by Chronic Constriction Injury
The rat model of neuropathic pain was induced by bilateral chronic constriction injury (CCI) according to a previously described method [23]. Briefly, rats were anesthetized with an intraperitoneal injection of sodium pentobarbital (40 mg/kg). The sciatic nerves on both sides were exposed and isolated from surrounding tissues. Then, the sciatic nerves were loosely ligated with a 4-0 catgut thread at four sites with an interval of 1 mm. In sham surgery, the sciatic nerves were exposed but not ligated. Rats with sham surgery were used as control.
Implantation of Intrathecal Catheter
Intrathecal catheter implantation was conducted according to a method described previously [24]. Briefly, rats were anesthetized, and the polyethylene catheter was inserted in the cisterna magna through an incision advanced 7.0 cm caudally to the lumbar enlargement. Proper location of the intrathecal implantation was confirmed by bilateral hind limb paralysis with injection of 2% lidocaine (Sigma, St. Louis, MO, USA). Afterwards, the catheter was fixed and the incision was sealed.
Administration of Recombinant Adenoviruses
Recombinant adenoviruses-SIRT2 (Ad-SIRT2) were purchased from Applied Biological Materials Inc. (Richmond, Canada). The recombinant adenoviruses were amplified using 293T cells and then purified using double CsCl purification. For gene transfer, 1 × 108 pfu recombinant adenoviruses were injected with a microinjection syringe linked with the intrathecal catheter. For biological experiments, the L4–L5 lumbar spinal cords were removed and the DRG from L4–L5 lumbar spinal cords were dissected for detection.
Real-Time Quantitative Polymerase Chain Reaction Analysis
Total RNAs were isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), and cDNA was synthesized using PrimeScript™ RT Master Mix (Takara, Dalian, China), according to the manufacturer’s instructions. PCR amplification was performed using Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA) and specific primers on an ABI 7500 real-time PCR system (Applied Biosystems). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the internal control for normalizing gene expression. The data were analyzed using the 2−ΔΔCt method.
Western Blot Analysis
Proteins from DRGs were extracted using a Tissue Protein Extraction Kit (Weiao Biotech, Shanghai, China). Protein concentrations were determined using a bicinchoninic acid (BCA) kit (Beyotime Biotechnology, Haimen, China). Equal amounts of proteins (40 μg) were separated using 10% sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto a polyvinylidene fluoride membrane (Millipore, Billerica, MA, USA). The membrane was rinsed in blocking buffer for 1 h at 37 °C and then incubated with primary antibodies, including anti-SIRT2 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-NF-κB p65 (Santa Cruz Biotechnology), anti-NF-κB p65 (acetyl K310) (Abcam, Cambridge, UK), and anti-GAPDH (Santa Cruz Biotechnology) at 4 °C overnight. Afterwards, the membrane was incubated with horseradish peroxidase-conjugated secondary antibody (1:2000; Beyotime Biotechnology). The staining was visualized using enhanced chemiluminescence reagents (Pierce, Rockford, IL, USA). The intensity of protein bands was quantified by Image-Pro Plus 6.0 software.
Enzyme-Linked Immunosorbent Assay
Levels of IL-1β, IL-6, and TNF-α were determined by using commercially available ELISA kits (R&D Systems, Minneapolis, MN, USA) following the manufacturer’s recommended protocol.
Evaluation of Thermal Hyperalgesia and Mechanical Allodynia
Thermal hyperalgesia was determined by measuring the paw withdrawal latencies (PWLs) in response to radiant heat stimulation. Briefly, the rats were placed in a plastic chamber upon a radiant heat source. The time duration between the start and paw withdrawal in response to the heat source was automatically recorded by a digital timer. A 30-s cutoff time was used to avoid tissue damage. Mechanical allodynia was determined by measuring the paw withdrawal threshold (PWT) in response to the stimulation of Von Frey hair (IITC, Woodland Hills, CA, USA). Rats were placed in a plastic cage with a plexiglass floor and then subjected to an ascending series of Von Frey hairs. The withdrawal reflex of at least three of the five applications was defined as a positive response.
Data Analysis
Data are presented as means ± standard deviation. Statistical analyses were performed using one-way analysis of variance followed by a Bonferroni post hoc test, with SPSS 18.0 software (SPSS Inc., Chicago, IL, USA). Differences with p < 0.05 were considered statistically significant.
RESULTS
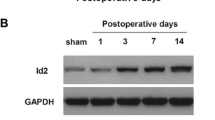
SIRT2 Is Downregulated in the DRG of CCI Rats
To investigate the potential relevance of SIRT2 in neuropathic pain, we firstly examined the expression status of SIRT2 in CCI rats. RT-qPCR analysis showed that the mRNA expression of SIRT2 was significantly downregulated in the DRG of CCI rats compared with the sham group in a time-dependent manner (Fig. 1a). Moreover, western blot analysis demonstrated that the protein expression of SIRT2 was also decreased in CCI rats (Fig. 1b). These results indicate that the decreased expression of SIRT2 may contribute to the development of neuropathic pain.
Decreased expression of SIRT2 in the DRG of CCI rats. a The relative mRNA expression of SIRT2 was detected by RT-qPCR. b The relative protein expression of SIRT2 was detected by western blot. The L4–L5 lumbar spinal cords were removed at postoperative days 1, 3, 7, and 14 after CCI, and the DRG was dissected for detection. N = 3, *p < 0.05 vs. sham group.
Overexpression of SIRT2 Attenuates Mechanical Allodynia and Thermal Hyperalgesia in CCI Rats
To investigate the biological function of SIRT2 in regulating neuropathic pain, we overexpressed SIRT2 in CCI rats by intrathecal injection of recombinant adenovirus expressing SIRT2 (Ad-SIRT2). RT-qPCR and western blot analysis showed that infection of Ad-SIRT2 significantly upregulated the mRNA and protein expression of SIRT2 in the DRG of CCI rats (Fig. 2a, b). We then examined the effect of SIRT2 overexpression on neuropathic pain development by assessment of thermal hyperalgesia and mechanical allodynia. The results showed that overexpression of SIRT2 significantly alleviated thermal hyperalgesia (Fig. 3a) and mechanical allodynia (Fig. 3b) of CCI rats, implying that overexpression of SIRT2 inhibited neuropathic pain development.
Intrathecal injection of recombinant ad-SIRT2 upregulates SIRT2 expression in CCI rats. The rats were infected with recombinant ad-SIRT2 by intrathecal injection 1 day before CCI surgery. The L4–L5 lumbar spinal cords were removed at postoperative day 7, and DRG was dissected for detection. The mRNA (a) and protein (b) expression in the DRG were detected by RT-qPCR and western blot, respectively. N = 3, *p < 0.05.
Overexpression of SIRT2 alleviates mechanical allodynia and thermal hyperalgesia in CCI rats. a Thermal hyperalgesia was determined by measuring the paw withdrawal latencies (PWLs) in response to radiant heat stimulation. b Mechanical allodynia was determined by measuring the paw withdrawal latencies (PWT) in response to von Frey hair stimulation. N = 6, *p < 0.05 vs. sham; & p < 0.05 vs. CCI + Ad-control.
Overexpression of SIRT2 Inhibits Neuroinflammation in CCI Rats
To further investigate the biological function of SIRT2 in regulating neuropathic pain, we examined the effect of SIRT2 overexpression on neuroinflammation. RT-qPCR analysis showed that the mRNA expression levels of TNF-α, IL-1β, and IL-6 (Fig. 4a–c) were significantly downregulated by SIRT2 overexpression in the DRG of CCI rats. Moreover, ELISA detection also showed that the protein expression of TNF-α, IL-1β, and IL-6 (Fig. 4d–f) were markedly decreased by SIRT2 overexpression in the DRG of CCI rats. Overall, these data indicate that overexpression of SIRT2 inhibits neuroinflammation in CCI rats.
Overexpression of SIRT2 suppresses neuroinflammation in CCI rats. Relative mRNA expression levels of TNF-α (a), IL-1β (b), and IL-6 (c) in the L4–L5 DRG at postoperative day 7 were detected by RT-qPCR. Protein expression levels of TNF-α (d), IL-1β (e), and IL-6 (f) were assessed by ELISA method. N = 3, *p < 0.05.
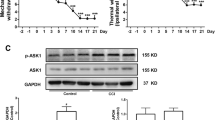
Overexpression of SIRT2 Decreases the Acetylation of NF-κB p65
To investigate the molecular mechanism of SIRT2 in regulating neuropathic pain, we detected the effect of SIRT2 on NF-κB, which is a key transcription factor controlling the expression of pro-inflammatory cytokines during the development of neuropathic pain development. We found that overexpression of SIRT2 significantly suppressed the acetylation of NF-κB p65 protein in the DRG of CCI rats (Fig. 5a–c). The results suggest that SIRT2 may inhibit NF-κB signaling by suppressing the acetylation of NF-κB p65.
Overexpression of SIRT2 decreases the acetylation of NF-κB p65. The DRG dissected from the L4–L5 lumbar spinal cords at postoperative day 7 was subjected to detection. a Western blot analysis of total NF-κB p65 and acetylated NF-κB p65 (ac-NF-κB p65) protein expression. b Quantitative analysis of total NF-κB p65 protein expression. c Quantitative analysis of acetylated NF-κB p65 protein expression. N = 3, *p < 0.05; # p > 0.05.
Inhibition of SIRT2 Aggravates Neuropathic Pain in CCI Rats
To confirm whether SIRT2 is involved in the regulation of neuropathic pain, we further detected the effect of SIRT2 inhibition on neuropathic pain. We treated CCI rats with AK-7, a selective inhibitor of SIRT2, and then detected the effect of AK-7 on neuropathic pain development. The results showed that inhibition of SIRT2 significantly aggravates neuropathic pain in CCI rats (Fig. 6a, b). Furthermore, inhibition of SIRT2 attenuated the inhibitory effect of SIRT2 overexpression on neuropathic pain (Fig. 6a, b). Moreover, we detected the effect of SIRT2 inhibition on neuroinflammation and NF-κB signaling. The results showed that inhibition of SIRT2 enhanced the expression of TNF-α, IL-1β, and IL-6 (Fig. 7a–c) and promoted the acetylation of NF-κB p65 (Fig. 7d–f). In addition, inhibition of SIRT2 abrogated the inhibitory effect of SIRT2 overexpression on the expression of TNF-α, IL-1β, and IL-6 (Fig. 7a–c) and the acetylation of NF-κB p65 (Fig. 7d–f). Overall, these results confirmed that SIRT2 inhibits neuropathic pain development in CCI rats.
Inhibition of SIRT2 enhances neuroinflammation. Protein expression levels of TNF-α (a), IL-1β (b), and IL-6 (c) were assessed by ELISA method. d Western blot analysis of total NF-κB p65 and acetylated NF-κB p65 (ac-NF-κB p65) protein expression. e Quantitative analysis of total NF-κB p65 protein expression. f Quantitative analysis of acetylated NF-κB p65 protein expression. N = 3, *p < 0.05; # p > 0.05.
DISCUSSION
In the present study, our results showed that SIRT2 was downregulated in the DRG of CCI rats. Overexpression of SIRT2 inhibited mechanical allodynia and thermal hyperalgesia in CCI rats. Moreover, overexpression of SIRT2 reduced the acetylation of NF-κB p65 protein and inhibited NF-κB signaling-mediated neuroinflammation. These findings suggest an important role of SIRT2 in the pathogenesis of neuropathic pain.
In recent years, an increasing number of studies have reported that SIRT2 is involved in various neurological diseases. Inhibition of SIRT2 inhibits alpha-synuclein toxicity in a cellular model of Parkinson’s disease [25]. Wang et al. reported that SIRT2 inhibition diminished striatal dopamine depletion and improved anti-oxidation ability and behavior abnormality in a rat model of Parkinson’s disease [26]. Loss of SIRT2 recovers microtubule stabilization and facilitates the elimination of toxic Aβ oligomers in Alzheimer’s disease [27]. SIRT2 inhibition reduces sterol levels and diminishes mutant huntingtin toxicity in Huntington’s disease [28]. SIRT2 inhibition induces antidepressant-like action, and overexpression of SIRT2 inhibits depressive behaviors [29, 30]. Xie et al. reported that downregulation of SIRT2 protects the mouse brain against ischemic stroke [31]. Krey et al. reported that knockdown of SIRT2 preserved neurological function after experimental stroke in mice [32]. In contrast, Yuan et al. reported that SIRT2 inhibition exacerbated blood-brain barrier disruption and traumatic brain injury in a mouse model [21]. However, whether SIRT2 is involved in regulating neuropathic pain remains unclear. In this study, we showed that SIRT2 was decreased in CCI rats, and overexpression of SIRT2 significantly alleviated neuropathic pain. Moreover, we found that SIRT2 inhibition aggravated neuropathic pain in CCI rats. Our findings imply an important role of SIRT2 in regulating neuropathic pain.
Studies have shown that SIRT2 plays an important role in regulating inflammatory diseases. SIRT2 has been reported to regulate the inflammation processes of sepsis, colitis, and arthritis associated with suppression of NF-κB signaling [14, 33,34,35]. It is reported that SIRT2 regulates NF-κB signaling and gene expression of proinflammatory mediators through deacetylation of the NF-κB p65 protein [19]. The hyperacetylation of the NF-κB p65 protein can increase the transcriptional activity of NF-κB and promote the expression of proinflammatory cytokines [36]. Overexpression of SIRT2 inhibits the inflammatory responses in collagen-induced arthritis by increasing the deacetylation of the NF-κB p65 protein [35]. Deletion of SIRT2 promotes inflammatory responses by increasing NF-κB acetylation in experimental colitis [34]. Overexpression of SIRT2 reduces the expression of proinflammatory cytokines and the activation of NF-κB signaling in murine macrophages [37]. Pais et al. reported that silencing of SIRT2 promoted the acetylation level of NF-κB p65 and enhanced the expression of inflammatory genes and reactive oxygen species in microglia with treatment of lipopolysaccharide [20]. Inhibition of SIRT2 exacerbates neuroinflammation in experimental traumatic brain injury of a mouse model by increasing NF-κB p65 acetylation and activation [21]. Consistent with these findings, our study showed that overexpression of SIRT2 significantly downregulated the acetylation level of the NF-κB p65 protein in CCI rats and reduced the expression of TNF-α, IL-1β, and IL-6 in the DRG of CCI rats. Moreover, inhibition of SIRT2 by a selective inhibitor, AK-7, enhanced the acetylation level of the NF-κB p65 protein and promoted neuroinflammation in CCI rats. The protective role of SIRT2 overexpression in neuropathic pain was also significantly abrogated by the SIRT2 inhibitor. These findings suggest an anti-inflammatory role of SIRT2. However, the pro-inflammatory role of SIRT2 has also been reported. SIRT2 has been reported to promote the activation of macrophages and microglia induced by lipopolysaccharide [38, 39]. Wang et al. reported that SIRT2 inhibition suppressed lipopolysaccharide-induced neuroinflammation and brain injury in mice [40]. These reports support a proinflammatory role of SIRT2. Therefore, the precise role of SIRT2 in regulating inflammation requires further investigation. Nevertheless, our results suggest an anti-inflammatory role of SIRT2 in regulating neuropathic pain.
Previous studies have shown that SIRT2 is expressed in microglia [40], dopaminergic neurons [41], and hippocampal neurons of central nervous system [42]. Our study showed that SIRT2 was also expressed in DRG of peripheral nervous system. Consistently, a recent study reports that SIRT2 expression is significantly decreased in the DRG of peripheral nervous system in rats with spinal nerve ligation [43]. Multiple studies also show that SIRT2 is expressed in spinal cord [44,45,46]. These findings suggest that SIRT2 is expressed in the peripheral nervous system and intrathecal injection method for SIRT2 overexpression or inhibition may be a promising therapeutic strategy for treatment of related diseases.
Taken together, our study reveals an important role of SIRT2 in regulating neuroinflammation and neuropathic pain development in CCI rats. We showed that overexpression of SIRT2 alleviated neuropathic pain through deacetylation of the NF-κB p65 protein and inhibition of NF-κB-mediated neuroinflammation. Recombinant adenovirus-mediated gene transfer to nociceptive neurons is a promising approach to determine mechanisms of pain in animal models and is a potential therapeutic strategy for the treatment of neuropathic pain. Our study suggests that recombinant adenovirus-mediated gene transfer of SIRT2 may have a potential application for preventing neuropathic pain.
Abbreviations
- SIRT2:
-
Sirtuin 2
- CCI:
-
Chronic constriction injury
- DRG:
-
Dorsal root ganglion
- TNF-α:
-
Tumor-necrosis factor-α
- IL-1β:
-
Interleukin
- NF-κB:
-
Nuclear factor-kappa B
- FoxO1:
-
Forkhead box O1
- FOXO3a:
-
Forkhead box 3a
- Ad-SIRT2:
-
Adenoviruses-SIRT2
- RT-qPCR:
-
Real-time quantitative polymerase chain reaction
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- BCA:
-
Bicinchoninic acid
- SDS-PAGE:
-
Sodium dodecylsulfate-polyacrylamide gel electrophoresis
- ELISA:
-
Enzyme-linked immunosorbent assay
- PWL:
-
Paw withdrawal latencies
- PWT:
-
Paw withdrawal threshold
References
Baron, R. 2000. Peripheral neuropathic pain: from mechanisms to symptoms. The Clinical Journal of Pain 16: S12–S20. https://doi.org/10.1097/00002508-200006001-00004.
Sorge, R.E., T. Trang, R. Dorfman, S.B. Smith, S. Beggs, J. Ritchie, J.S. Austin, D.V. Zaykin, H. Vander Meulen, M. Costigan, et al. 2012. Genetically determined P2X7 receptor pore formation regulates variability in chronic pain sensitivity. Nature Medicine 18: 595–599. https://doi.org/10.1038/nm.2710.
Breivik, H., B. Collett, V. Ventafridda, R. Cohen, and D. Gallacher. 2006. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. European Journal of Pain 10: 287–333. https://doi.org/10.1016/j.ejpain.2005.06.009.
Bouhassira, D., M. Lanteri-Minet, N. Attal, B. Laurent, and C. Touboul. 2008. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain 136: 380–387. https://doi.org/10.1016/j.pain.2007.08.013.
O'Connor, A.B., and R.H. Dworkin. 2009. Treatment of neuropathic pain: an overview of recent guidelines. The American Journal of Medicine 122: S22–S32. https://doi.org/10.1016/j.amjmed.2009.04.007.
Myers, R.R., W.M. Campana, and V.I. Shubayev. 2006. The role of neuroinflammation in neuropathic pain: mechanisms and therapeutic targets. Drug Discovery Today 11: 8–20. https://doi.org/10.1016/S1359-6446(05)03637-8.
Scholz, J., and C.J. Woolf. 2007. The neuropathic pain triad: neurons, immune cells and glia. Nature Neuroscience 10: 1361–1368. https://doi.org/10.1038/nn1992.
Moalem, G., and D.J. Tracey. 2006. Immune and inflammatory mechanisms in neuropathic pain. Brain Research Reviews 51: 240–264. https://doi.org/10.1016/j.brainresrev.2005.11.004.
Sun, T., W.G. Song, Z.J. Fu, Z.H. Liu, Y.M. Liu, and S.L. Yao. 2006. Alleviation of neuropathic pain by intrathecal injection of antisense oligonucleotides to p65 subunit of NF-kappaB. British Journal of Anaesthesia 97: 553–558. https://doi.org/10.1093/bja/ael209.
Houtkooper, R.H., E. Pirinen, and J. Auwerx. 2012. Sirtuins as regulators of metabolism and healthspan. Nature Reviews Molecular Cell Biology 13: 225–238. https://doi.org/10.1038/nrm3293.
Inoue, T., M. Hiratsuka, M. Osaki, and M. Oshimura. 2007. The molecular biology of mammalian SIRT proteins: SIRT2 in cell cycle regulation. Cell Cycle 6: 1011–1018.
Harting, K., and B. Knoll. 2010. SIRT2-mediated protein deacetylation: an emerging key regulator in brain physiology and pathology. European Journal of Cell Biology 89: 262–269. https://doi.org/10.1016/j.ejcb.2009.11.006.
Cha, Y.I., and H.S. Kim. 2013. Emerging role of sirtuins on tumorigenesis: possible link between aging and cancer. BMB Reports 46: 429–438. https://doi.org/10.5483/BMBRep.2013.46.9.180.
Buechler, N., X. Wang, B.K. Yoza, C.E. McCall, and V. Vachharajani. 2017. Sirtuin 2 regulates microvascular inflammation during sepsis. Journal of Immunology Research 2017: 2648946. https://doi.org/10.1155/2017/2648946.
Starke, R.M., R.J. Komotar, and E.S. Connolly. 2013. The role of SIRT2 in programmed necrosis: implications for stroke and neurodegenerative disorders. Neurosurgery 72: N20–N22. https://doi.org/10.1227/01.neu.0000430740.01610.74.
Wang, Y., Y. Mu, X. Zhou, H. Ji, X. Gao, W.W. Cai, Q. Guan, and T. Xu. 2017. SIRT2-mediated FOXO3a deacetylation drives its nuclear translocation triggering FasL-induced cell apoptosis during renal ischemia reperfusion. Apoptosis 22: 519–530. https://doi.org/10.1007/s10495-016-1341-3.
Zhao, Y., J. Yang, W. Liao, X. Liu, H. Zhang, S. Wang, D. Wang, J. Feng, L. Yu, and W.G. Zhu. 2010. Cytosolic FoxO1 is essential for the induction of autophagy and tumour suppressor activity. Nature Cell Biology 12: 665–675. https://doi.org/10.1038/ncb2069.
Hoffmann, G., F. Breitenbucher, M. Schuler, and A.E. Ehrenhofer-Murray. 2014. A novel sirtuin 2 (SIRT2) inhibitor with p53-dependent pro-apoptotic activity in non-small cell lung cancer. Journal of Biological Chemistry 289: 5208–5216. https://doi.org/10.1074/jbc.M113.487736.
Rothgiesser, K.M., S. Erener, S. Waibel, B. Luscher, and M.O. Hottiger. 2010. SIRT2 regulates NF-kappaB dependent gene expression through deacetylation of p65 Lys310. Journal of Cell Science 123: 4251–4258. https://doi.org/10.1242/jcs.073783.
Pais, T.F., E.M. Szego, O. Marques, L. Miller-Fleming, P. Antas, P. Guerreiro, R.M. de Oliveira, B. Kasapoglu, and T.F. Outeiro. 2013. The NAD-dependent deacetylase sirtuin 2 is a suppressor of microglial activation and brain inflammation. The EMBO Journal 32: 2603–2616. https://doi.org/10.1038/emboj.2013.200.
Yuan, F., Z.M. Xu, L.Y. Lu, H. Nie, J. Ding, W.H. Ying, and H.L. Tian. 2016. SIRT2 inhibition exacerbates neuroinflammation and blood-brain barrier disruption in experimental traumatic brain injury by enhancing NF-kappaB p65 acetylation and activation. Journal of Neurochemistry 136: 581–593. https://doi.org/10.1111/jnc.13423.
Yu, J., Y. Wu, and P. Yang. 2016. High glucose-induced oxidative stress represses sirtuin deacetylase expression and increases histone acetylation leading to neural tube defects. Journal of Neurochemistry 137: 371–383. https://doi.org/10.1111/jnc.13587.
Bennett, G.J., and Y.K. Xie. 1988. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 33: 87–107. https://doi.org/10.1016/0304-3959(88)90209-6.
Yaksh, T.L., and T.A. Rudy. 1976. Chronic catheterization of the spinal subarachnoid space. Physiology & Behavior 17: 1031–1036. https://doi.org/10.1016/0031-9384(76)90029-9.
Outeiro, T.F., E. Kontopoulos, S.M. Altmann, I. Kufareva, K.E. Strathearn, A.M. Amore, C.B. Volk, M.M. Maxwell, J.C. Rochet, P.J. McLean, et al. 2007. Sirtuin 2 inhibitors rescue alpha-synuclein-mediated toxicity in models of Parkinson's disease. Science 317: 516–519. https://doi.org/10.1126/science.1143780.
Wang, X., Q. Guan, M. Wang, L. Yang, J. Bai, Z. Yan, Y. Zhang, and Z. Liu. 2015. Aging-related rotenone-induced neurochemical and behavioral deficits: role of SIRT2 and redox imbalance, and neuroprotection by AK-7. Drug Design Development and Therapy 9: 2553–2563.
Silva, D.F., A.R. Esteves, C.R. Oliveira, and S.M. Cardoso. 2017. Mitochondrial metabolism power SIRT2-dependent deficient traffic causing Alzheimer's-disease related pathology. Molecular Neurobiology 54: 4021–4040. https://doi.org/10.1007/s12035-016-9951-x.
Luthi-Carter, R., D.M. Taylor, J. Pallos, E. Lambert, A. Amore, A. Parker, H. Moffitt, D.L. Smith, H. Runne, O. Gokce, et al. 2010. SIRT2 inhibition achieves neuroprotection by decreasing sterol biosynthesis. Proceedings of the National Academy of Sciences of the United States of America 107: 7927–7932. https://doi.org/10.1073/pnas.1002924107.
Erburu, M., I. Munoz-Cobo, T. Diaz-Perdigon, P. Mellini, T. Suzuki, E. Puerta, and R.M. Tordera. 2017. SIRT2 inhibition modulate glutamate and serotonin systems in the prefrontal cortex and induces antidepressant-like action. Neuropharmacology 117: 195–208. https://doi.org/10.1016/j.neuropharm.2017.01.033.
Liu, R., W. Dang, Y. Du, Q. Zhou, K. Jiao, and Z. Liu. 2015. SIRT2 is involved in the modulation of depressive behaviors. Scientific Reports 5: 8415.
Xie, X.Q., P. Zhang, B. Tian, and X.Q. Chen. 2016. Downregulation of NAD-dependent deacetylase SIRT2 protects mouse brain against ischemic stroke. Molecular Neurobiology. https://doi.org/10.1093/biomet/asw035.
Krey, L., F. Luhder, K. Kusch, B. Czech-Zechmeister, B. Konnecke, T. Fleming Outeiro, and G. Trendelenburg. 2015. Knockout of silent information regulator 2 (SIRT2) preserves neurological function after experimental stroke in mice. Journal of Cerebral Blood Flow and Metabolism 35: 2080–2088. https://doi.org/10.1038/jcbfm.2015.178.
Wang, X., N.L. Buechler, A. Martin, J. Wells, B. Yoza, C.E. McCall, and V. Vachharajani. 2016. Sirtuin-2 regulates sepsis inflammation in ob/ob mice. PLoS One 11: e0160431. https://doi.org/10.1371/journal.pone.0169417.
Lo, Sasso G., K.J. Menzies, A. Mottis, A. Piersigilli, A. Perino, H. Yamamoto, K. Schoonjans, and J. Auwerx. 2014. SIRT2 deficiency modulates macrophage polarization and susceptibility to experimental colitis. PLoS One 9: e103573. https://doi.org/10.1371/journal.pone.0114617.
Lin, J., B. Sun, C. Jiang, H. Hong, and Y. Zheng. 2013. Sirt2 suppresses inflammatory responses in collagen-induced arthritis. Biochemical and Biophysical Research Communications 441: 897–903. https://doi.org/10.1016/j.bbrc.2013.10.153.
Chen, L., W. Fischle, E. Verdin, and W.C. Greene. 2001. Duration of nuclear NF-kappaB action regulated by reversible acetylation. Science 293: 1653–1657. https://doi.org/10.1126/science.1062374.
Kim, M.J., D.W. Kim, J.H. Park, S.J. Kim, C.H. Lee, J.I. Yong, E.J. Ryu, S.B. Cho, H.J. Yeo, J. Hyeon, et al. 2013. PEP-1-SIRT2 inhibits inflammatory response and oxidative stress-induced cell death via expression of antioxidant enzymes in murine macrophages. Free Radical Biology and Medicine 63: 432–445. https://doi.org/10.1016/j.freeradbiomed.2013.06.005.
Lee, A.S., Y.J. Jung, D. Kim, T. Nguyen-Thanh, K.P. Kang, S. Lee, S.K. Park, and W. Kim. 2014. SIRT2 ameliorates lipopolysaccharide-induced inflammation in macrophages. Biochemical and Biophysical Research Communications 450: 1363–1369. https://doi.org/10.1016/j.bbrc.2014.06.135.
Chen, H., D. Wu, X. Ding, and W. Ying. 2015. SIRT2 is required for lipopolysaccharide-induced activation of BV2 microglia. Neuroreport 26: 88–93. https://doi.org/10.1097/WNR.0000000000000305.
Wang, B., Y. Zhang, W. Cao, X. Wei, J. Chen, and W. Ying. 2016. SIRT2 plays significant roles in lipopolysaccharides-induced neuroinflammation and brain injury in mice. Neurochemical Research 41: 2490–2500. https://doi.org/10.1007/s11064-016-1981-2.
Szego, E.M., E. Gerhardt, and T.F. Outeiro. 2017. Sirtuin 2 enhances dopaminergic differentiation via the AKT/GSK-3beta/beta-catenin pathway. Neurobiology of Aging 56: 7–16. https://doi.org/10.1016/j.neurobiolaging.2017.04.001.
Park, J.H., C.K. Kim, S.B. Lee, K.H. Lee, S.W. Cho, and J.Y. Ahn. 2016. Akt attenuates apoptotic death through phosphorylation of H2A under hydrogen peroxide-induced oxidative stress in PC12 cells and hippocampal neurons. Scientific Reports 6: 21857.
Fu, Z., and J. Shi. 2017. Differential expression of tubulin acetylase and deacetylase between the damaged dentral and peripheral branch of dorsal root ganglion neurons. Medical Science Monitor 23: 3673–3678. 10.12659/MSM.902829.
Valle, C., I. Salvatori, V. Gerbino, S. Rossi, L. Palamiuc, F. Rene, and M.T. Carri. 2014. Tissue-specific deregulation of selected HDACs characterizes ALS progression in mouse models: pharmacological characterization of SIRT1 and SIRT2 pathways. Cell Death & Disease 5: e1296. https://doi.org/10.1038/cddis.2014.247.
Maxwell, M.M., E.M. Tomkinson, J. Nobles, J.W. Wizeman, A.M. Amore, L. Quinti, V. Chopra, S.M. Hersch, and A.G. Kazantsev. 2011. The Sirtuin 2 microtubule deacetylase is an abundant neuronal protein that accumulates in the aging CNS. Human Molecular Genetics 20: 3986–3996. https://doi.org/10.1093/hmg/ddr326.
Ma, B., J. Shi, L. Jia, W. Yuan, J. Wu, Z. Fu, Y. Wang, N. Liu, and Z. Guan. 2013. Over-expression of PUMA correlates with the apoptosis of spinal cord cells in rat neuropathic intermittent claudication model. PLoS One 8: e56580. https://doi.org/10.1371/journal.pone.0084903.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was reviewed and approved by the Institutional Animal Care and Use Committee of Shaanxi Provincial People’s Hospital. The animal experiments were performed according to the guidelines of the International Association for the Study of Pain and the National Institute of Health Guide for the Care and Use of Laboratory Animals.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhang, Y., Chi, D. Overexpression of SIRT2 Alleviates Neuropathic Pain and Neuroinflammation Through Deacetylation of Transcription Factor Nuclear Factor-Kappa B. Inflammation 41, 569–578 (2018). https://doi.org/10.1007/s10753-017-0713-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-017-0713-3