Abstract
The purpose of this study was to investigate the protective effects of Saikosaponin a (SSa), a triterpene saponin derived from Radix bupleuri, on lipopolysaccharide (LPS)-induced acute lung injury (ALI) using a murine model. The mice were given SSa 1 h after intranasal instillation of LPS. Then, lung histopathological examination, the wet/dry (W/D) ratio, myeloperoxidase (MPO), and inflammatory cytokines in bronchoalveolar lavage fluid (BALF) were detected in this study. The results showed that SSa reduced lung pathological injury induced by LPS. Furthermore, LPS-induced lung W/D ratio, MPO activity, and inflammatory cytokines TNF-α and IL-1β in BALF were significantly inhibited by SSa. In addition, SSa suppressed LPS-induced NF-κB activation and NLRP3 inflammasome expression. In conclusion, we found that SSa played a critical anti-inflammatory effect through inhibition of NF-κB and NLRP3 signaling pathways and protected against LPS-induced ALI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Acute lung injury (ALI), a major cause of acute respiratory failure in critically ill patients, has been reported to have high morbidity and mortality in patients [1]. It is characterized by injury of the alveolar epithelium and endothelium, lung edema, and infiltration of neutrophils [2, 3]. Inflammation has been known to play an important role in the development of ALI [4]. LPS could induce the activation of NF-κB, which regulates the expression of inflammatory mediators [5]. In experimental animal models of ALI, elevated inflammatory cytokines, such as TNF-α and IL-1β, were observed in the BALF [6]. A large body of studies suggested that inhibition of these inflammatory cytokines could attenuate LPS-induced ALI [7, 8]. NF-κB has been reported to play an important role in the regulation of inflammatory mediator production. Previous studies showed that inhibition of NF-κB activation could attenuate LPS-induced ALI in mice [9]. NLRP3 inflammasome also played an important role in the development of ALI [10]. Consequently, an effective anti-inflammatory drug for the treatment of ALI was urgently needed.
Saikosaponin a (SSa), a triterpene saponin derived from Radix bupleuri, has been reported to have a wide range of pharmacological activities [11]. In vitro, SSa was found to inhibit LPS-induced TNF-α and IL-1β production in primary mouse macrophages [12]. SSa also inhibited LPS-induced inflammation and oxidative stress in the human umbilical vein endothelial cells [13]. In vivo, SSa has been reported to protect against experimental sepsis via inhibition of NF-κB activation [14]. SSa effectively attenuated neuropathic pain in CCI rats by inhibiting the activation of p38 MAPK and NF-κB signaling pathways [15]. However, whether SSa has protective effects against LPS-induced ALI has not been reported. In this study, we sought to investigate the therapeutic effects of SSa on LPS-induced ALI in mice.
MATERIALS AND METHODS
Materials
SSa (purity > 98%) was purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). LPS (Escherichia coli, O55:B5) was purchased from Sigma (St. Louis, MO, USA). ELISA kits for TNF-α and IL-1β were purchased from BioLegend (CA, USA). Antibodies used in this study were purchased from Cell Signaling Technology, Inc. (Beverly, MA, USA).
Animals
Male BALB/c mice were obtained from Medical Experimental Animal Center of Jinzhou Medical University (Jinzhou, China) and fed under specific pathogen-free conditions. The mice were given adequate food and water ad libitum. All animal experiments were performed in accordance with the Care and Use of Laboratory Animals established by the US National Institutes of Health. The mice were divided into five groups: control group, LPS group, LPS + SSa (5, 10, and 20 mg/kg) groups. The mice of LPS group received intratracheal of LPS (10 μg of LPS dissolved in 50 μl PBS). SSa (5, 10, and 20 mg/kg) was administered 1 h after LPS challenge. The doses of SSa used in this study were based on previous study [12]. Twelve hours after LPS treatment, the BALF and lung tissues were collected for subsequent experiments [16].
Histological Analyses of Lung Tissues
Lung tissues were collected and fixed in 10% buffered formalin. Then, the tissues were embedded in paraffin, cut into 5 μm sections, and stained with hematoxylin and eosin (H&E). Finally, the pathological changes of lung tissues were observed with an optical microscope (Olympus).
Lung Wet/Dry Ratio
Mice were sacrificed 12 h after LPS challenge. Then, the right lung was collected to obtain the “wet” weight. The lung was dried in an oven at 80 °C for 72 h and weighted to obtain the “dry” weight. Lung edema was assessed by calculating lung wet/dry (W/D) ratio.
MPO Assay
Twelve hours after LPS treatment, lung tissues were collected and homogenized. MPO activity in lung tissues was measured by test kits purchased from Nanjing Jiancheng Bioengineering Institute (China, Nanjing) according to the manufacturer’s instructions.
ELISA
Twelve hours after LPS treatment, the BALF were collected and the levels of TNF-α and IL-1ß in the BALF were measured by ELISA kits (BioLegend, CA, USA) in accordance with the manufacturer’s instructions.
Western Blot Analysis
Lung tissues were collected and homogenized in cold RIPA buffer to obtain proteins. The protein concentration was detected by BCA method. Equal amounts of protein were separated on 12% SDS-PAGE and electrotransferred to nitrocellulose membranes. The membranes were blocked with 3% nonfat milk in TBST for 2 h and incubated with primary antibodies overnight. Then, the membranes were washed with TBST for three times and incubated with HRP-conjugated secondary antibodies. Finally, the membranes were detected by chemiluminescence (ECL) Western blotting detection kit (Thermo, USA).
Statistical Analyses
All the data were analyzed by GraphPad prism 5.0 and expressed as the mean ± SD. Statistical significance was analyzed using one-way analysis of variance followed by post hoc Dunnett’s test. p < 0.05 was considered statistically significant.
RESULTS
SSa Attenuates LPS-Induced Lung Histopathological Changes
To investigate the protective effects of SSa on LPS-induced ALI, lung histopathological changes were detected by H&E staining. As shown in Fig. 1, LPS group exhibited interstitial edema, neutrophil infiltration, and increased alveolar wall thickness. However, treatment of SSa significantly attenuated LPS-induced lung injury.
Effects of SSa on histopathological changes in lung tissues in LPS-induced ALI mice. Representative histological changes of lung obtained from mice of different groups. a Control group. b LPS group. c LPS + SSa (5 mg/kg) group. d LPS + SSa (10 mg/kg) group. e LPS + SSa (20 mg/kg) group (hematoxylin and eosin staining, magnification ×200).
SSa Inhibits LPS-Induced MOP Activity
MPO has been known as a biomarker of neutrophil. In this study, the effects of SSa on LPS-induced MPO activity were detected. As shown in Fig. 2, compared with the control group, MPO activity obviously increased in LPS-treated group. Conversely, compared with LPS group, treatment of SSa 1 h after LPS exposure decreased MPO activity in a dose-dependent manner.
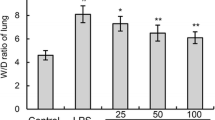
SSa Inhibits LPS-Induced Lung W/D Ratio
Lung edema was assessed by detecting lung W/D ratio. The effects of SSa on LPS-induced lung W/D ratio were detected in this study. As shown in Fig. 3, compared with the control group, lung W/D ratio obviously increased in LPS-treated group. Conversely, compared with LPS group, treatment of SSa 1 h after LPS exposure decreased lung W/D ratio in a dose-dependent manner.
SSa Inhibits LPS-Induced TNF-α and IL-1β Production in BALF
Inflammatory cytokines play a critical role in the development of lung injury. The effects of SSa on LPS-induced inflammatory cytokines TNF-α and IL-1β production were detected in this study. As shown in Fig. 4, compared with the control group, the levels of TNF-α and IL-1β obviously increased in LPS-treated group. Conversely, compared with LPS group, treatment of SSa 1 h after LPS exposure decreased TNF-α and IL-1β levels in a dose-dependent manner.
Effects of SSa on LPS-Induced NF-κB Activation
To investigate the anti-inflammatory mechanism of SSa, LPS-induced NF-κB activation was detected in this study. As shown in Fig. 5, compared with the control group, the phosphorylation of NF-κB-p65 and IκBα obviously increased in LPS-treated group. Conversely, compared with LPS group, treatment of SSa 1 h after LPS exposure decreased the phosphorylation of NF-κB-p65 and IκBα in a dose-dependent manner.
Effects of SSa on NLRP3, ASC, and Caspase-1 Expression
To further investigate the anti-inflammatory mechanism of SSa, the activation of NLRP3 inflammasome was detected in this study. As shown in Fig. 6, compared with the control group, the expression of NLRP3, ASC, and caspase-1 obviously increased in LPS-treated group. Conversely, compared with LPS group, treatment of SSa 1 h after LPS exposure decreased the expression of NLRP3, ASC, and caspase-1 in a dose-dependent manner.
DISCUSSION
LPS-induced ALI is characterized by the inflammation of lung tissues. In this study, our results demonstrated that SSa could inhibit LPS-induced ALI in mice. SSa significantly inhibited LPS-induced inflammatory cytokine production in the BALF. Moreover, SSa significantly inhibited LPS-induced NF-κB activation and NLRP3 expression. These results indicated that SSa was a potential agent for the treatment of ALI.
Inflammation has been identified as the major cause that leads to lung injury [17]. Studies showed that LPS-induced ALI was characterized by the inflammation of lung tissues [18]. A complex cytokine network mediates the inflammatory response in LPS-induced ALI. Inflammatory cytokines TNF-α and IL-1β increased significantly in BALF of LPS-induced ALI [19]. In the present study, we found that SSa dose dependently inhibited LPS-induced TNF-α and IL-1β production. The infiltration of neutrophils in the lung is an early step in the inflammatory process of ALI [20]. Studies showed that elimination of neutrophils could attenuate the severity of ALI [3, 21]. MPO activity is an effective measure of neutrophil infiltration into tissues [22]. In this study, we found that SSa significantly inhibited LPS-induced MPO activity. The results suggested that LPS-induced neutrophil infiltration was suppressed by treatment of SSa. Furthermore, LPS-induced lung histopathological changes were also inhibited by SSa. Taken together, these results suggested that SSa had protective effects against LPS-induced ALI.
IL-1β is an important inflammatory cytokine that involved in the pathogenesis of acute lung injury [23]. The maturation and secretion of IL-1β required two signaling pathways: NF-κB signaling pathway and NLRP3 inflammasome signaling pathway [24]. NF-κB, a critical signaling molecule, has been reported to play critical roles in the regulation of inflammatory mediators [25]. Activation of NF-κB by LPS regulated the expression of pro-IL-1β [26]. NLRP3 inflammasome, a key factor in innate immunity and senses soluble pathogen and danger-associated molecular patterns, played an important role in the maturation of IL-1β [27]. Activation of NLRP3 inflammasome leads to the activation of caspase-1, which induces the secretion and maturation of IL-1β [28]. Furthermore, previous study showed that inhibition of NLRP3 inflammasome could attenuate LPS-induced ALI [29]. To investigate the anti-inflammatory mechanism of SSa, NF-κB and NLRP3 inflammasome signaling pathways were detected. The results showed that SSa significantly inhibited LPS-induced NF-κB activation and NLRP3 inflammasome expression. These results suggested that SSa inhibited LPS-induced ALI by inhibiting NF-κB and NLRP3 signaling pathways.
In conclusion, our results showed that SSa exhibited protective effects in LPS-induced mice with ALI by inhibiting inflammatory response. The inhibitory effects were based on the inhibition of NF-κB and NLRP3 signaling pathways. SSa may be used as an anti-inflammatory agent for the treatment of inflammatory diseases, such as ALI.
References
Grichnik, K.P., and T.A. D'Amico. 2004. Acute lung injury and acute respiratory distress syndrome after pulmonary resection. Seminars in Cardiothoracic and Vascular Anesthesia 8: 317–334.
Asimakopoulos, G., P.L. Smith, C.P. Ratnatunga, and K.M. Taylor. 1999. Lung injury and acute respiratory distress syndrome after cardiopulmonary bypass. The Annals of Thoracic Surgery 68: 1107–1115.
Grommes, J., and O. Soehnlein. 2011. Contribution of neutrophils to acute lung injury. Molecular Medicine 17: 293–307.
Gando, S., T. Kameue, N. Matsuda, A. Sawamura, M. Hayakawa, and H. Kato. 2004. Systemic inflammation and disseminated intravascular coagulation in early stage of ALI and ARDS: role of neutrophil and endothelial activation. Inflammation 28: 237–244.
Guha, M., and N. Mackman. 2001. LPS induction of gene expression in human monocytes. Cellular Signalling 13: 85–94.
Shen, W., J. Gan, S. Xu, G. Jiang, and H. Wu. 2009. Penehyclidine hydrochloride attenuates LPS-induced acute lung injury involvement of NF-kappaB pathway. Pharmacological Research 60: 296–302.
Liu, S., G. Feng, G.L. Wang, and G.J. Liu. 2008. p38MAPK inhibition attenuates LPS-induced acute lung injury involvement of NF-kappaB pathway. European Journal of Pharmacology 584: 159–165.
Wang, B., X. Gong, J.Y. Wan, L. Zhang, Z. Zhang, H.Z. Li, and S. Min. 2011. Resolvin D1 protects mice from LPS-induced acute lung injury. Pulmonary Pharmacology & Therapeutics 24: 434–441.
Everhart, M.B., W. Han, T.P. Sherrill, M. Arutiunov, V.V. Polosukhin, J.R. Burke, R.T. Sadikot, J.W. Christman, F.E. Yull, and T.S. Blackwell. 2006. Duration and intensity of NF-kappaB activity determine the severity of endotoxin-induced acute lung injury. Journal of Immunology 176: 4995–5005.
Grailer, J.J., B.A. Canning, M. Kalbitz, M.D. Haggadone, R.M. Dhond, A.V. Andjelkovic, F.S. Zetoune, and P.A. Ward. 2014. Critical role for the NLRP3 inflammasome during acute lung injury. Journal of Immunology 192: 5974–5983.
Zhu, J., C. Luo, P. Wang, Q. He, J. Zhou, and H. Peng. 2013. Saikosaponin A mediates the inflammatory response by inhibiting the MAPK and NF-kappaB pathways in LPS-stimulated RAW 264.7 cells. Experimental and Therapeutic Medicine 5: 1345–1350.
Wei, Z.K., J.J. Wang, M.Y. Shi, W.J. Liu, Z.T. Yang, and Y.H. Fu. 2016. Saikosaponin a inhibits LPS-induced inflammatory response by inducing liver X receptor alpha activation in primary mouse macrophages. Oncotarget 7: 48995–49007.
Fu, Y.H., X.Y. Hu, Y.G. Cao, Z.C. Zhang, and N.S. Zhang. 2015. Saikosaponin a inhibits lipopolysaccharide-oxidative stress and inflammation in Human umbilical vein endothelial cells via preventing TLR4 translocation into lipid rafts. Free Radical Biology and Medicine 89: 7773–7785.
Zhao, H.Y., S.P. Li, H.S. Zhang, G. Wang, G.L. Xu, and H.B. Zhang. 2015. Saikosaponin A protects against experimental sepsis via inhibition of NOD2-mediated NF-kappa B activation. Experimental and Therapeutic Medicine 10: 823–827.
Zhou, X., H. Cheng, D.D. Xu, Q. Yin, L. Cheng, L. Wang, S.S. Song, and M.Y. Zhang. 2014. Attenuation of neuropathic pain by Saikosaponin a in a rat model of chronic constriction injury. Neurochemical Research 39: 2136–2142.
Lu, X., Y. Pu, W. Kong, X. Tang, J. Zhou, H. Gou, X. Song, H. Zhou, N. Gao, and J. Shen. 2017. Antidesmone, a unique tetrahydroquinoline alkaloid, prevents acute lung injury via regulating MAPK and NF-kappaB activities. International Immunopharmacology 45: 34–42.
Goodman, R.B., J. Pugin, J.S. Lee, and M.A. Matthay. 2003. Cytokine-mediated inflammation in acute lung injury. Cytokine & Growth Factor Reviews 14: 523–535.
Reutershan, J., I. Vollmer, S. Stark, R. Wagner, K.C. Ngamsri, and H.K. Eltzschig. 2009. Adenosine and inflammation: CD39 and CD73 are critical mediators in LPS-induced PMN trafficking into the lungs. FASEB Journal 23: 473–482.
Ricard, J.D., D. Dreyfuss, and G. Saumon. 2001. Production of inflammatory cytokines in ventilator-induced lung injury: a reappraisal. American Journal of Respiratory and Critical Care Medicine 163: 1176–1180.
Abraham, E. 2003. Neutrophils and acute lung injury. Critical Care Medicine 31: S195–S199.
Lee, W.L., and G.P. Downey. 2001. Neutrophil activation and acute lung injury. Current Opinion in Critical Care 7: 1–7.
Schierwagen, C., A.C. Bylund-Fellenius, and C. Lundberg. 1990. Improved method for quantification of tissue PMN accumulation measured by myeloperoxidase activity. Journal of Pharmacological Methods 23: 179–186.
Kolb, M., P.J. Margetts, D.C. Anthony, F. Pitossi, and J. Gauldie. 2001. Transient expression of IL-1beta induces acute lung injury and chronic repair leading to pulmonary fibrosis. The Journal of Clinical Investigation 107: 1529–1536.
Latz, E., T.S. Xiao, and A. Stutz. 2013. Activation and regulation of the inflammasomes. Nature Reviews. Immunology 13: 397–411.
Li, Q., and I.M. Verma. 2002. NF-kappaB regulation in the immune system. Nature Reviews. Immunology 2: 725–734.
Bauernfeind, F.G., G. Horvath, A. Stutz, E.S. Alnemri, K. MacDonald, D. Speert, T. Fernandes-Alnemri, J. Wu, B.G. Monks, K.A. Fitzgerald, et al. 2009. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. Journal of Immunology 183: 787–791.
Chen, G., M.H. Shaw, Y.G. Kim, and G. Nunez. 2009. NOD-like receptors: role in innate immunity and inflammatory disease. Annual Review of Pathology 4: 365–398.
Ghiringhelli, F., L. Apetoh, A. Tesniere, L. Aymeric, Y. Ma, C. Ortiz, K. Vermaelen, T. Panaretakis, G. Mignot, E. Ullrich, et al. 2009. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nature Medicine 15: 1170–1178.
Luo, Y.P., L. Jiang, K. Kang, D.S. Fei, X.L. Meng, C.C. Nan, S.H. Pan, M.R. Zhao, and M.Y. Zhao. 2014. Hemin inhibits NLRP3 inflammasome activation in sepsis-induced acute lung injury, involving heme oxygenase-1. International Immunopharmacology 20: 24–32.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Du, ZA., Sun, MN. & Hu, ZS. Saikosaponin a Ameliorates LPS-Induced Acute Lung Injury in Mice. Inflammation 41, 193–198 (2018). https://doi.org/10.1007/s10753-017-0677-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-017-0677-3