Abstract
It is well known that CD4+CD25+Foxp3+Treg cells play an important role in the development of allergic rhinitis (AR); the defect of cell numbers and functions contribute to AR. Hydrogen has been proven effective in alleviating symptoms of AR. We herein aim to verify the protective effects of hydrogen on CD4+CD25+Foxp3+Treg cells in guinea pigs with AR and to explore the effect of hydrogen-rich saline (HRS) on CD4+CD25+Foxp3+Treg cells in animals with AR and investigate the underlying anti-inflammatory mechanism. Eighteen guinea pigs were randomly divided into three groups (control group/AR group/AR-HRS group). The guinea pigs were injected with hydrogen-rich saline (AR-HRS group) for 10 days after sensitization. The control group was injected with an equal volume of normal saline. The number of sneezes, degree of runny nose, and nasal-rubbing movements were scored. Peripheral blood eosinophil count was recorded. The proportions of Th1/Th2 of the peripheral blood and the CD4+CD25+Foxp3+T cells in the CD4+T cells of the spleen and peripheral blood were determined by flow cytometry. The content of interleukin (IL)-10 and transforming growth factor (TGF)-β in the serum was detected by enzyme-linked immunosorbent assay (ELISA). The protein and mRNA expression of Foxp3, IL-10, and TGF-β were determined by Western blot, immunofluorescence, and real-time PCR analysis, respectively. Scores of symptoms, number of eosinophils,and nasal mucosa damage were dramatically reduced after HRS treatment. HRS increased the expression of Foxp3, IL-10, TGF-β, and number of CD4+CD25+Foxp3+Treg cells, which were reduced in AR. HRS also revised the dysregulation of Th1/Th2 balance. Both the number and biological activity of CD4+CD25+Foxp3+Treg cells increased with up-regulation of Th1/Th2 after HRS administration. HRS could play a protective role in attenuating AR through improving the proportion and functions of CD4+CD25+Foxp3+Treg cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Allergic rhinitis (AR) is a chronic inflammatory airway disease, which is clinically identified as a symptomatic disorder of the nose induced by an immunoglobulin E (IgE)-mediated hypersensitivity reaction against allergens and involves mucosal inflammation driven by type 2 helper T (Th2) cells [1]. Nasal itching, sneezing, watery rhinorrhea, and nasal congestion are the cardinal symptoms of AR. With the change in the living environment, the incidence of AR is gradually increasing around the world, and AR has been shown to affect quality of life and productivity at school or work and cause severe financial losses [2]. Therefore, it is imperative to clarify fully the pathogenesis of AR and to develop a novel therapeutic strategy.
CD4+CD25+regulatory T (Treg) cells are a subset of thymus-derived CD4+T cell population that can promote self-immune tolerance and maintenance of immune balance and account for 5–10% of mature CD4+T cells. They can secrete anti-inflammatory cytokines, including transforming growth factor (TGF)-β and interleukin (IL)-10, at high levels. The forkhead box P3 (Foxp3) gene is a specific transcription factor, which plays a critical role in the development and functional maintenance of CD4+CD25+Treg cells [3]. In recent years, cumulative studies have found that CD4+CD25+Treg cells are involved in allergic inflammation and play an important role in balancing Th1/Th2 cell differentiation and that a defect in Treg function or reduced numbers of Treg cells trigger the development and progression of AR [4, 5].
Hydrogen has long been recognized as a physiological inert gas without biological activity. Recently, it has been demonstrated that hydrogen gas may protect cells and organs from damage in animal models such as those of ischemia and reperfusion, acute trauma, and inflammation [6]. Experimental data has indicated that hydrogen-rich saline (HRS) reduces airway inflammation in a murine model of asthma [7]. Furthermore, the latest studies show that HRS can attenuate the severity of AR in guinea pigs through potent antioxidation effect and inhibition of expressions of IL-4 and IL-13 [8, 9]. However, it has not been shown that the underlying mechanism of HRS in reduction of allergic inflammation in AR is via regulation of CD4+CD25+Foxp3+Treg cells. In the current study, AR model guinea pigs were induced with ovalbumin in order to investigate the effect of HRS on CD4+CD25+Foxp3+Treg cells and underlying anti-inflammatory mechanism.
MATERIALS AND METHODS
Material and Animal Models
Hydrogen gas (0.4 MPa) was dissolved in normal saline for at least 2 h to reach supersaturated level (>0.6 mmol/L) [10]. HRS in our experiment was obtained from the Second Military Medical University, Shanghai, China. Mature, healthy, and clean male guinea pigs weighing 220 ± 10 g were purchased from Shanghai Laboratory Animal Company (Shanghai, China) and bred in the Experimental Animal Science Center of our institute. All animal care and experimental procedures were approved by the Tongji University Institution Animal Care and Use Committee (2017-DW-002).
The AR models were prepared as follows: 18 guinea pigs were randomly divided into three groups (of six each), namely, control group, AR group, and AR-HRS group. The AR and AR-HRS groups were both sensitized with ovalbumin (OVA, Sigma, USA). Every guinea pig was sensitized by intraperitoneal injection with OVA (0.3 mg) mixed with aluminum adjuvant (30 mg) in 10 mL normal saline, which was administered every other day for seven times. From day 15, the guinea pigs were treated with 1% OVA aerosol five times to stimulate AR symptoms. Subsequently, each side of the nasal cavity was administered 20 μL of 2% OVA solution intranasally once a day for 5 days to maintain AR symptoms. The control group was administered normal saline instead of OVA in the same manner and dosage.
From day 25, intranasal challenge was carried out. In short, 20 μL of HRS was introduced into each nasal airway passage of guinea pigs for ten consecutive days. The control group was administered the same dosage of saline intranasally.
Evaluation of Nasal Symptoms
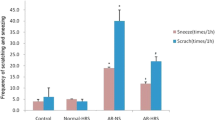
Nasal symptoms of guinea pigs were observed for 15 min after intranasal excitation and intervention for the final time. The frequencies of sneezes and runny nose were evaluated in accordance with the score system used by Narita et al. [11]. Sneezes were scored on a scale of 1 (1–3 sneezes) to 3 (≥ 11 sneezes).The score of runny nose degree was scored as follows:1 (flowing to anterior nostril), 2 (flowing through anterior nostril), and 3 (very severe rhinorrhea). Itchiness of the nose was scored as 1 (scratching a few times) or 2 (scratching continually).
Peripheral Blood Eosinophil Count
A peripheral venous blood sample was collected, and eosinophils were counted through automatic blood cell analyzer (Sysmex Medical Electronics Co. Ltd., Japan).The detectable range of blood eosinophil was counted in the same way, i.e., 0–10,000/μL.
Flow Cytometric Analysis
The proportion of CD4+CD25+Foxp3+Treg cells was tested by flow cytometric analysis. Briefly, single-cell suspension of the spleen and blood from the peripheral vein was labeled with anti-CD4-fluorescein isothiocyanate (FITC) and anti-CD25-allophycocyanin (APC) monoclonal antibodies (BD, USA). For intracellular staining of Foxp3, cells were fixed and permeabilized using the human Foxp3 buffer set according to the manufacturer’s instruction (BD, USA). Following this, the cells were stained with anti-Foxp3-phycoerythrin (PE) monoclonal antibodies (eBioscience, USA) for Treg detection at 4 °C for 30 min. In addition, the ratio of Th1/Th2 in peripheral blood was detected by flow cytometric analysis. The peripheral blood mononuclear cell was labeled with anti-CD4-fluorescein isothiocyanate and anti-IFN-γ antibody or anti-IL-4 antibody (BD, USA). Non-specific fluorescence was determined using an isotype-matched IgG as control. Data were acquired on a FACSCalibur (Beckman Coulter, USA) and analyzed using CellQuest software (BD, USA).
Serum Cytokine Measurements
The detection of serum contents of IL-10 and TGF-β was carried out by means of enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instruction. The lower limit of detection was 6.25 pg/mL for IL-10 and 75 pg/mL for TGF-β.
Quantitative Real-Time Polymerase Chain Reaction
RT-PCR was performed to determine the expression levels of Foxp3, IL-10, and TGF-β mRNAs in nasal mucosa. Total RNA was extracted according to the description in the TRIzol protocol, and cDNA was synthesized with a cDNA synthesis kit (Invitrogen, USA) according to manufacturer’s instructions. To determine the expression of Foxp3, IL-10, and TGF-β, we performed a fluorescent quantitative real-time RT-PCR assay. The primer sequences used were as follows: GAPDH forward 5′-ACCACAGTCCATGCCATCAC-3′ and reverse 5′-TCCACCACCCTGTTGCTGTA-3′; Foxp3 forward 5′-GCGCCCTCTGGACAGC-3′ and reverse 5′-TTCTTTCCTGGGCACACTGG-3′; IL-10 forward 5′-TAATAAGCTCCAAGACCAAG-3′ and reverse 5′-TAGAATGGGAACTGAGGTATC-3′; TGF-β forward 5′-AGGGCTACCATGCCAACTTC-3′ and reverse 5′-CCACGTAGTAGACGATGGGC-3′. All primers were synthesized by Shanghai Huada Biotech Company (Shanghai, China).The thermal cycling conditions were 2 min at 50 °C; 10 min at 95 °C, followed by 40 cycles of 95 °C for 15 s, 60 °C for 1 min, and 50 °C for 15 s; and finally, 60 °C for 15 s and 95 °C for 10 min. All PCR reactions were performed in triplicate. The expression of Foxp3, IL-10, and TGF-β mRNA was normalized to the expression of the GAPDH housekeeping gene using 2−△CT (comparative threshold cycle, CT) method.
Western Blot Analysis
The guinea pig nasal mucosa (10 mg), which was frozen in liquid nitrogen, was homogenized by phenylmethylsulfonyl fluoride (PMSF) lysis buffer and centrifuged at 12,000×g for 5 min at 4 °C. The quantity of protein was detected by the Bradford method (Bio-Rad). Subsequently, the protein was loaded per line and was separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and was then transferred to nitrocellulose membrane (Pierce, USA).The membranes were blocked by 5% non-fat milk (Yili, China) in TBST (tris-buffered saline containing 0.05% Tween-20) for 60 min and then incubated with rabbit anti-mouse Foxp3 (1.0 μg/mL; Boster Co., BA2913, Ltd., China) at 4 °C overnight, followed by TBST washing three times for 10 min each time at room temperature. HRP-labeled anti-rabbit IgG (Beyotime Co., A0208, Ltd., China) with a 1:1000 dilution was then used for 1–2 h at room temperature and washed two times in TBST for 10 min each. The second batch of images was quantified using Quantity One software (Bio-Rad Laboratories, USA).Moreover, glyceraldehyde-3-phosphate dehydrogenase (GAPDA) was employed as an internal control for protein normalization. The methods for IL-10 and TGF-β were similar to those for Foxp3.The experiments were performed in duplicate.
Immunofluorescence Assay
Tissue from nasal mucosa was fixed in 10% buffered formalin. Immunohistochemical staining was performed on formalin-fixed and paraffin-embedded 4-μm sections. The tissue sections were deparaffinized and permeabilized in precooled acetone at − 20 °C for 15 min. Slides were washed in PBS. Normal goat serum (10% in PBS) was used to block non-specific antibody binding. The slides were incubated for 1.5 h at room temperature with anti-IL-10 rat monoclonal antibody (1:100, Boster Co., BA0980–1, Ltd., China).Slides were washed twice in PBS and incubated for 30 min at room temperature with goat anti-rabbit IL-10 IgG/Cy3 (1:500, Bioss, bs-0295G-Cy3, China).Slides were washed twice in PBS and once in distilled water and then allowed to dry. Fluorescence microscope was applied for observation and photography. The methods for TGF-β were the same as those for IL-10.
Statistical Analysis
Statistical analyses were performed using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA).All data were expressed as mean ± standard deviation (SD). Statistical analyses of data were performed using ANOVA for multiple comparison among groups. The least significant difference (LSD) method was used for multiple pair-wise comparisons of data, which followed normal distribution. Dunnett’s T3 test was performed for data that did not follow normal distribution. The correlation coefficient was generated by using Pearson’s rank correlation. Statistical significance was defined as p < 0.05.
RESULTS
Effect of HRS on Nasal Symptoms in Guinea Pigs
The number of sneezes, degree of runny nose, and nasal rubbing movements were recorded in 15 min after sensitization and HRS treatment, and the nasal symptoms were presented as scores (Table 1).The score of the normal control group was found to be low. However, the score of the AR group was significantly higher than that of the AR-HRS group (p < 0.05), which suggests that HRS may alleviate inflammatory symptoms of AR.
Blood Eosinophil Count
Similarly, eosinophil count in AR group was significantly higher than that in the control group ((0.41 ± 0.05) × 109/L vs. (0.25 ± 0.04) × 109/L; p < 0.05) and significantly lower than that in AR-HRS group ((0.32 ± 0.03) × 109 /L; p < 0.05) (Table 1).
Comparison of CD4+IFN-γ+Th1 Cells and CD4+IL-4+Th2 Cells in Peripheral Blood Mononuclear Cells
In comparison to the control group, the number of CD4+IFN-γ+Th1 cells and the proportion of Th1/Th2 in the AR group were significantly reduced with the up-regulation of CD4+IL-4+Th2 cells. HRS reduced the count of CD4+IL-4+Th2 cells, thereby improving the level of CD4+IFN-γ+Th1 cells and the proportion of Th1/Th2 in the peripheral blood (p < 0.05, Table 2 and Fig. 1 (1a, 1b, 1c)).
Analysis of CD4+IFN-γ+Th1 cells, CD4+IL-4+Th2 cells, and CD4+CD25+Foxp3+Treg cells of peripheral blood mononuclear cell and CD4+CD25+Foxp3+Treg cells of the spleen tissue by FCM in control group (a), AR group (b), and AR-HRS group (c), where 1 and 2 refer to peripheral blood and 3 refers to the spleen tissue. Q1+Q2 represents the CD4+IFN-γ+Th1 cells in 1a, 1b, and 1c, while Q2+Q3 in 1a, 1b, and 1c represents CD4+IL-4+Th2 cells. AR allergic rhinitis, HRS hydrogen-rich saline.
Proportion of CD4+CD25+Foxp3+Treg Cells in Each Group
PBMCs and splenocyte tissue single-cell suspensions in each group were examined for the prevalence of CD4+CD25+Foxp3+Treg cells. As shown in Table 1 and Fig. 1 (1a, 1b, 1c), the frequency of CD4+CD25+Foxp3+Treg cells was markedly reduced in the AR group when compared to the other two groups (p < 0.05, Table 2 and Fig. 1 (2a, 2b, 2c, 3a, 3b, 3c)).Owing to HRS, the level of CD4+CD25+Foxp3+Treg cells in the peripheral blood of the AR-HRS group is higher than that of the AR group (p < 0.05, Table 2 and Fig. 1). However, there was no significant difference between the AR group and AR-HRS group in the splenocyte tissue (p > 0.05).
Serum Level of IL-10 and TGF-β
ELISA was performed to detect the levels of CD4+CD25+Foxp3+Treg cell effector cytokines IL-10 and TGF-β in the plasma in all groups. The results showed that the expression of IL-10 and TGF-β was significantly decreased in AR group, whereas HRS increased the plasma levels of IL-10 and TGF-β in AR-HRS group (p < 0.05, Table 3).
Expression of Foxp3, IL-10, and TGF-β mRNA Levels of Nasal Mucosa in Each Group
The mRNA expression of Foxp3, IL-10, and TGF-β was detected by RT-PCR analysis. As shown in Fig. 2, the expression of Foxp3, IL-10, and TGF-β mRNAs was markedly lower in the AR group in nasal mucosa. The expression of IL-10 and TGF-β mRNAs was significantly higher in the AR-HRS group treated with HRS. HRS may have increased the mRNA expression of Foxp3 in the AR-HRS group (p < 0.05).
Expression of Foxp3, IL-10, and TGF-β mRNA in nasal mucosa. Lanes from left to right are products of control group, AR group, and AR-HRS group, respectively. There was a decrease in IL-10, TGF-β, and Foxp3 mRNA in AR group compared with that of the control group, which increased after HRS treatment. AR allergic rhinitis, HRS hydrogen-rich saline. *p < 0.05 vs.control group; #p < 0.05 vs.AR group.
Western Blot Results of Foxp3, IL-10, and TGF-β Expressions in Nasal Mucosa Among the Three Groups
Protein expression of Foxp3, IL-10, and TGF-β in inflamed local tissues was detected by Western blot. It was found that the expression level of Foxp3, IL-10, and TGF-β proteins was lower in the AR group as compared to the control group and AR-HRS group and that HRS increased the protein expression in the nasal mucosa (Fig. 3a, b, p < 0.05).
Western blot analysis of Foxp3, IL-10, and TGF-β proteins of nasal mucosa in the following groups: control group, AR group, and AR-HRS group. Western blot analysis showed that the relative expression of IL-10, TGF-β, and Foxp3 proteins in the AR group was lower in comparison with that in the control group, whereas expression was much higher in the AR-HRS group. AR allergic rhinitis, HRS hydrogen-rich saline. *p < 0.05 vs. control group; #p < 0.05 vs.AR group.
Assessing IL-10 and TGF-β Expressions by Immunofluorescence
As shown in Fig. 4, IL-10 and TGF-β expressions in the control group were more than those in the AR group, and after HRS treatment, the levels of IL-10 and TGF-β were increased significantly in the AR-HRS group.
Correlation Analysis in AR-HRS Group Between CD4+CD25+Foxp3+Treg Cells in Peripheral Blood and Their Effector Cytokines IL-10, TGF-β, and Eosinophil in Serum
Correlation analysis of Pearson revealed that the frequency of peripheral blood CD4+CD25+Foxp3+Treg cells was positively correlated to plasma IL-10 (Fig. 5a; r = 0.51, p < 0.05) and TGF-β levels (Fig. 5b; r = 0.53, p < 0.05).However, it was negatively related to eosinophil in serum (Fig. 5c; r = − 0.906, p < 0.05).
Correlation analysis of Pearson revealed that the frequency of peripheral blood CD4+CD25+Foxp3+Treg cells was positively correlated with plasma IL-10 (a) and TGF-β (b) levels and the frequency of CD4+CD25+Foxp3+Treg cells/CD4+T cells was negatively correlated with the eosinophil count in peripheral blood (c).
DISCUSSION
Hydrogen is a colorless gas and is the lightest element. In 2007, researchers from Japan reported that 2% hydrogen gas possesses antioxidant and anti-apoptotic properties that protect the brain against I/R injury [10]. Subsequently, an increasing number of scholars focused on hydrogen research in biology and medical fields. Bucholz proved that hydrogen plays a critical role in alleviating inflammation [12]. HRS was prepared by dissolving hydrogen gas (0.4 MPa) in normal saline for at least 2 h to reach supersaturated level (> 0.6 mmol/L) [10]. Experimental and clinical data have indicated that the effect of inhaling hydrogen or drinking HRS is parallel [13]. However, HRS is safer and more convenient than hydrogen gas, which is in favor of clinical application. In addition, previous observations suggest that HRS, as an interesting anti-allergic treatment, could alleviate respiratory symptoms of asthma and AR [7,8,9]. Therefore, HRS plays an important role in preventing airway inflammation. In our study, the symptoms of AR, which consist of sneezing, rhinorrhea, and nasal itching, significantly took a turn for the better along with the declined number of eosinophils on HRS treatment. It is shown that HRS could promote eosinophil apoptosis and reduce the growth of eosinophil, which is parallel to the mechanism of corticosteroid in inhibiting allergic inflammation [14, 15]. Thus, HRS may be an effective therapy for AR in animals.
Sakaguchi et al. observed that CD4+CD25+Treg cells were shown in the peripheral blood and spleen in people and animals, such as murine animals [16]. CD4+CD25+Treg cells can be divided into two major subsets, natural Treg (nTreg), which develops in the thymus, and adjusted Treg (aTreg), which develops in the periphery from naïve T cells in response to signals, such as low immunogenic doses of antigen, commensal microbes, or lymphopenia [17]. Several lines of evidence have shown that CD4+CD25+Treg cells could not only inhibit the activation of allergic inflammatory cells such as eosinophil and mast cells, but could also reduce production of IgE [18]. Besides, CD4+CD25+Treg cells are actively engaged in negative control of inflammation and allergy, which is necessary to sustain normal immune responses [19]. Furthermore, as CD4+CD25+Treg cells play a key role in airway allergic disorders such as AR and asthma, the declined number and function of CD4+CD25+Treg cells may result in airway allergic diseases [20]. The animal experiments manifested that the number of CD4+CD25+Foxp3+Treg cells in the peripheral blood and splenocyte tissue was significantly reduced in comparison with the normal control group in the AR murine model [21]. Moreover, clinical research also draws the same conclusion. Compared to control group, the proportion of CD4+CD25+Foxp3+Treg cells is lower in the nasal mucosa and peripheral blood of AR patients [4, 22]. It has been proven that specific immunotherapy may improve the symptoms of AR by means of increasing CD4+CD25+Foxp3+Treg cell numbers [23].
In this present study, levels of CD4+CD25+Foxp3+Treg cells in the peripheral blood and splenocyte tissue decreased after OVA sensitization and increased after HRS treatment. Therefore, we argue that HRS could increase the proportion of CD4+CD25+Foxp3+Treg cells. By comparing the number of CD4+CD25+Foxp3+Treg cells in the peripheral blood and splenocyte tissue, we realized that the impact on the change of the CD4+CD25+Foxp3+Treg cells in the peripheral blood after HRS treatment was evident. A higher concentration of HRS, applied to the nasal mucosa, might have led to a greater influence on the peripheral blood than on spleen tissue. It is possible that the effect of HRS on CD4+CD25+Foxp3+Treg cells had dependence on organs. HRS could protect the nasal mucosa and peripheral blood but induces the activation of CD4+effect T cells in the splenocyte tissue with lower expression of Foxp3 [24]. Although no significant difference in the proportion of CD4+CD25+Foxp3+Treg cells after HRS treatment was found in the splenocyte tissue between the AR group and AR-HRS group, there was an increasing trend. Our study suggested that HRS could strengthen the function of inhibition by means of improving the number of CD4+CD25+Foxp3+Treg cells, which contributed to the treatment of AR.
Apart from this, CD4+CD25+Foxp3+Treg cells could suppress allergen-specific Th1 and Th2 cell responses, which play an important role in the physiological immune response to allergens [25]. From the viewpoint of Th1 and Th2 cells, current research indicates that HRS could revise the dysregulation of Th1/Th2, increase the number of Th1 cells, and decrease that of Th2, which presented opposite changes in the AR group. The experiment revealed that HRS could efficiently treat AR by down-regulation of Th2 and inhibition of Th2 cell response in AR.
Anti-inflammatory cytokines, such as IL-10 and TGF-β, are produced by CD4+CD25+Foxp3+Treg cells, which exert their function in controlling inflammation and protecting tissues and organs from inflammatory injury partly via decreasing IL-4 and IL-5 of Th2 cells and ameliorating IgE [26, 27]. Studies have reported that IL-10 not only reduced the function and activity of eosinophil and suppressed the inflammatory medium generated by monocytes and macrophages, but also reduced the effect of antigen-presenting cells [21, 28]. TGF-β could convert naive CD4+CD25-T cells into CD4+CD25+anergic T cells in the periphery, involving the induction of Foxp3 expression, and then boost the number of CD4+CD25+Foxp3+Treg cells [29]. However, the inhibitory function of CD4+CD25+Foxp3+Treg cells could be blocked by the antibodies of IL-10 and TGF-β [30]. The findings of Frew et al. had demonstrated that CD4+CD25+Treg cells could inhibit allergic inflammatory reactions relying on production of IL-10 and TGF-β [31].
From the point of view of cytokines, serum IL-10 and TGF-β levels in the AR group were dramatically reduced compared with healthy controls, implying the secretion disorder of CD4+CD25+Foxp3+Treg cells, which is in line with previous studies [32, 33]. As serum contents of IL-10 and TGF-β were evidently up-regulated after HRS treatment, HRS could play an anti-inflammatory role through acceleration of secretion of inhibitory cytokines. Furthermore, our study suggested that the frequency of peripheral blood CD4+CD25+Foxp3+Treg cells was positively correlated to plasma IL-10 and TGF-β levels. There may exist a synergistic effect between CD4+CD25+Foxp3+Treg cells and their cytokines. However, no correlation between IL-10 and TGF-β may be related with the fact that CD4+CD25+Foxp3+Treg cells are not the only cells that could secrete them [24, 34]. In addition, we measured IL-10 and TGF-β mRNA expressions in local inflamed sites and found that protein production was improved in nasal mucosa after HRS induction. Interestingly, the transcription of IL-10 and TGF-β increased in the AR-HRS group. Thus, our data suggested that IL-10 and TGF-β play an important role in HRS-mediated anti-inflammatory mechanism. In short, a decline in the number of CD4+CD25+Foxp3+Treg cells and their inhibitory cytokines is involved in the pathogenesis of AR. HRS is a novel treatment strategy for AR in animals. On the one hand, HRS may increase the proportion of CD4+CD25+Foxp3+Treg cells in order to heighten contact inhibition. On the other hand, HRS may also increase secretion of proteins such as IL-10 and TGF-β by CD4+CD25+Foxp3+Treg cells. Both these alterations aid allergic reaction inhibition and AR treatment.
Foxp3 has not only been shown to be the specific molecule marker of CD4+CD25+Foxp3+Treg cells, but also regulates differentiation in the thymus and function in the peripheral blood [35]. At the same time, Foxp3 mRNA levels could directly reflect the amount and function of CD4+CD25+Foxp3+Treg cells, which in turn reflects the activity level [35]. Foxp3 gene defect could reduce the inhibitory effect of CD4+CD25+Foxp3+Treg cells, leading to inflammatory syndrome in people and murine animals [36]. In a study carried out by Lee, the mRNA expression of Foxp3 detected by RT-PCR was significantly decreased in AR patients [37]. Some scholars argued that the katabatic expression of Foxp3 mRNA was related to the functional deficiency of CD4+CD25+Treg cells [38]. In the present study, the expression of Foxp3 mRNA is significantly decreased in nasal mucosa in the AR group, which is in accordance with the report by Xu [22]. The declined expression of Foxp3 mRNA gave rise to functional shortcomings of CD4+CD25+Treg cells in AR individuals, and the expression of Foxp3 mRNA increased after HRS treatment. Evidence showed that up-regulation of Foxp3 mRNA could improve the function of CD4+CD25+Treg cells, which is beneficial in inhibition of allergic inflammation [39]. Through analysis of the contents of protein in every group of nasal mucosa, we found that Foxp3 protein was the least in the AR group. Foxp3 protein content was increased significantly after HRS treatment. Our study indicated that the change in Foxp3 expression is directly related to the number of CD4+CD25+Treg cells. The study went one step further in suggesting that HRS could promote the expression of Foxp3, which in turn improves the number and/or function of CD4+CD25+Treg cells and thereby aids in treatment of AR in animals.
In conclusion, our study has showed, for the first time, that HRS can be used in effective treatment of AR guinea pigs by improving the proportion and enhancing the function of CD4+CD25+Foxp3+Treg cells, facilitating the production of serum contents of IL-10 and TGF-β, and promoting expression of Foxp3. Moreover, HRS could reverse the imbalance of Th1/Th2, which plays a critical role in antigen-specific Th2-mediated allergic response. However, further investigations should be carried out to confirm the results and clarify more precise mechanisms.
References
Hellgren, J., A. Cervin, S. Nordling, A. Bergman, and L.O. Cardell. 2010. Allergic rhinitis and the common cold—high cost to society. Allergy 65 (6): 776–783.
Haque, M., K. Fino, F. Lei, X. Xiong, and J. Song. 2014. Utilizing regulatory T cells against rheumatoid arthritis. Frontiers in Oncology 4 (2): 209.
Huang, X., Y. Chen, F. Zhang, Q. Yang, and G. Zhang. 2014. Peripheral Th17/Treg cell-mediated immunity imbalance in allergic rhinitis patients. Brazilian Journal of Otorhinolaryngology 80 (2): 152–155.
Frew, A.J. 2008. Sublingual immunotherapy. New England Journal of Medicine 358 (21): 2259–2264.
Zheng, X.F., X.J. Sun, and Z.F. Xia. 2011. Hydrogen resuscitation, a new cytoprotective approach. Clinical and Experimental Pharmacology & Physiology 38 (3): 155–163.
Xiao, M., T. Zhu, T. Wang, and F.Q. Wen. 2013. Hydrogen-rich saline reduces airway remodeling via inactivation of NF-kappaB in a murine model of asthma. European Review for Medical & Pharmacological Sciences 17 (8): 1033–1043.
Yu, S., C. Zhao, N. Che, L. Jing, and R. Ge. 2017. Hydrogen-rich saline attenuates eosinophil activation in a guinea pig model of allergic rhinitis via reducing oxidative stress. Journal of Inflammation 14 (1): 1.
Zhao, C., S. Yu, J. Li, W. Xu, and R. Ge. 2017. Changes in Il-4 and Il-13 expression in allergic-rhinitis treated with hydrogen-rich saline in guinea-pig model. Allergologia Et Immunopathologia International Journal for Clinical & Investigate Allergology & Clinical Immunology 45: 350–355.
Cai, J., Z. Kang, K. Liu, W. Liu, R. Li, J.H. Zhang, X. Luo, and X. Sun. 2009. Neuroprotective effects of hydrogen saline in neonatal hypoxia—ischemia rat model. Brain Research 1256: 129–137.
Ohsawa, I., M. Ishikawa, K. Takahashi, M. Watanabe, K. Nishimaki, K. Yamagata, K. Katsura, Y. Katayama, S. Asoh, and S. Ohta. 2007. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nature Medicine 13 (6): 688–694.
Narita, S.I., K. Asakura, and A. Kataura. 1996. Effects of thromboxane A2 receptor antagonist (bay u 3405) on nasal symptoms after antigen challenge in sensitized guinea pigs. International Archives of Allergy and Immunology 109 (2): 161–166.
Buchholz, B.M., D.J. Kaczorowski, R. Sugimoto, R. Yang, Y. Wang, T.R. Billiar, K.R. McCurry, A.J. Bauer, and A. Nakao. 2008. Hydrogen inhalation ameliorates oxidative stress in transplantation induced intestinal graft injury. American Journal of Transplantation 8 (10): 2015–2024.
Nakashima-Kamimura, N., T. Mori, I. Ohsawa, S. Asoh, and S. Ohta. 2009. Molecular hydrogen alleviates nephrotoxicity induced by an anti-cancer drug cisplatin without compromising anti-tumor activity in mice. Cancer Chemotherapy and Pharmacology 64 (4): 753–761.
Vignola, A.M., G. Chiappara, R. Gagliardo, M. Gjomarkaj, A. Merendino, L. Siena, J. Bousquet, and G. Bonsignore. 2000. Apoptosis and airway inflammation in asthma. Apoptosis 5 (5): 473–485.
Druilhe, A., S. Letuve, and M. Pretolani. 2003. Glucocorticoid-induced apoptosis in human eosinophils: mechanisms of action. Apoptosis 8 (5): 481–495.
Sakaguchi, S., N. Sakaguchi, M. Asano, M. Itoh, and M. Toda. 1995. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. The Journal of Immunology 155 (3): 1151–1164.
Lehtimäki, S., and R. Lahesmaa. 2013. Regulatory T cells control immune responses through their non-redundant tissue specific features. Frontiers in Immunology 4: 294.
Braga, M., C. Quecchia, E. Cavallucci, L. Di Giampaolo, C. Schiavone, C. Petrarca, and M. Di Gioacchino. 2011. T regulatory cells in allergy. International Journal of Immunopathology and Pharmacology 24 (1 Suppl): 55S–64S.
Taylor, A., J. Verhagen, C.A. Akdis, and M. Akdis. 2005. T regulatory cells and allergy. Microbes and Infection 7 (7): 1049–1055.
Robinson, D.S., M. Larché, and S.R. Durham. 2004. Tregs and allergic disease. Journal of Clinical Investigation 114 (10): 1389–1397.
Han, D., L. Xi, E. Fan, Y. Li, and L. Zhang. 2009. Expression of the Foxp3 gene in spleen mononuclear cells of a mouse model with allergic rhinitis. ORL 71 (6): 317–322.
Xu, G., Z. Mou, H. Jiang, L. Cheng, J. Shi, R. Xu, Y. Oh, and H. Li. 2007. A possible role of CD4+ CD25+ T cells as well as transcription factor Foxp3 in the dysregulation of allergic rhinitis. The Laryngoscope 117 (5): 876–880.
Radulovic, S., M.R. Jacobson, S.R. Durham, and K.T. Nouri-Aria. 2008. Grass pollen immunotherapy induces Foxp3-expressing CD4+ CD25+ cells in the nasal mucosa. Journal of Allergy and Clinical Immunology 121 (6): 1467–1472.
Zhou, H.T., X.Z. Huang, X.Z. Wu, W.Y. Chen, and X.B. Wu. 2013. Bone marrow-derived dendritic cells rather than spleen-derived dendritic cells can generate regulatory T cells. Journal of Experimental Hematology 21 (4): 1015–1020.
Sakaguchi, S., K. Wing, Y. Onishi, P. Prieto-Martin, and T. Yamaguchi. 2009. Regulatory T cells: how do they suppress immune responses? International Immunology 21 (10): 1105–1111.
Larché, M., C.A. Akdis, and R. Valenta. 2006. Immunological mechanisms of allergen-specific immunotherapy. Nature Reviews. Immunology 6 (10): 761–771.
Ozdemir, C., M. Akdis, and C.A. Akdis. 2009. T regulatory cells and their counterparts: masters of immune regulation. Clinical & Experimental Allergy 39 (5): 626–639.
Liu, K.S., X.Q. Fan, L. Zhang, Q.N. Wen, J.H. Feng, F.C. Chen, J.M. Luo, and W.B. Sun. 2014. Effects of recombinant human interleukin-10 on Treg cells, IL-10 and TGF-β in transplantation of rabbit skin. Molecular Medicine Reports 9 (2): 639–644.
Chen, W., W. Jin, N. Hardegen, K.J. Lei, L. Li, N. Marinos, G. McGrady, and S.M. Wahl. 2003. Conversion of peripheral CD4+ CD25-naive T cells to CD4+ CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. Journal of Experimental Medicine 198 (12): 1875–1886.
Luo, C.Y., L. Wang, C. Sun, and D.J. Li. 2011. Estrogen enhances the functions of CD4+ CD25+ Foxp3+ regulatory T cells that suppress osteoclast differentiation and bone resorption in vitro. Cellular and Molecular Immunology 8: 50–58.
Guzman-Flores, J.M., and D.P. Portales-Perez. 2012. Mechanisms of suppression of regulatory T-cells (Treg). Gaceta Medica de Mexico 149 (6): 630–638.
König, K., C. Klemens, K. Eder, M. San Nicoló, S. Becker, M.F. Kramer, and M. Gröger. 2015. Cytokine profiles in nasal fluid of patients with seasonal or persistent allergic rhinitis. Allergy, Asthma & Clinical Immunology 11 (1): 26.
Anderson, A.E., K.J. Mackerness, M. Aizen, V.A. Carr, D. Nguyen, F.D. Pre, S.R. Durham, and D.S. Robinson. 2009. Seasonal changes in suppressive capacity of CD4+ CD25+ T cells from patients with hayfever are allergen-specific and may result in part from expansion of effector T cells among the CD25+ population. Clinical & Experimental Allergy 39 (11): 1693–1699.
Tan, C., M.K. Aziz, J.D. Lovaas, B.P. Vistica, G. Shi, E.F. Wawrousek, and I. Gery. 2010. Antigen-specific Th9 cells exhibit uniqueness in their kinetics of cytokine production and short retention at the inflammatory site. The Journal of Immunology 185 (11): 6795–6801.
Marson, A., K. Kretschmer, G.M. Frampton, E.S. Jacobsen, J.K. Polansky, K.D. MacIsaac, S.L. Stuart, E. Fraenkel, H. von Boehmer, and R.A. Young. 2007. Foxp3 occupancy and regulation of key target genes during T-cell stimulation. Nature 445 (7130): 931–935.
Hori, S., T. Nomura, and S. Sakaguchi. 2003. Control of regulatory T cell development by the transcription factor Foxp3. Science 299 (5609): 1057–1061.
Lee, S.M., B. Gao, M. Dahl, K. Calhoun, and D. Fang. 2009. Decreased FoxP3 gene expression in the nasal secretions from patients with allergic rhinitis. Otolaryngology—Head and Neck Surgery 140 (2): 197–201.
Okamoto, A., K. Fujio, T. Okamura, and K. Yamamoto. 2011. Regulatory T-cell-associated cytokines in systemic lupus erythematosus. BioMed Research International 2011 (4): 463412.
O'garra, A., and P. Vieira. 2004. Regulatory T cells and mechanisms of immune system control. Nature Medicine 10 (8): 801–805.
Acknowledgements
This project was sponsored by the grants from the doctoral program of the Ministry of Education (20130072120022) and Western Medicine guide project of Shanghai City (17411970500).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The research was approved by the Tongji University Institution Animal Care and Use Committee (2017-DW-002).
Conflict of Interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Xu, F., Yu, S., Qin, M. et al. Hydrogen-Rich Saline Ameliorates Allergic Rhinitis by Reversing the Imbalance of Th1/Th2 and Up-Regulation of CD4+CD25+Foxp3+Regulatory T Cells, Interleukin-10, and Membrane-Bound Transforming Growth Factor-β in Guinea Pigs. Inflammation 41, 81–92 (2018). https://doi.org/10.1007/s10753-017-0666-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-017-0666-6