Abstract
SUV39H1, the histone methyltransferase (HMTase) of histone H3 lysine 9 trimethylation (H3K9me3), is a known transcriptional repressor of inflammatory genes. The effect of SUV39H1 on inflammatory gene promoters under high-glucose stimulation in vascular smooth muscle cells (VSMCs), macrophages, and cardiomyocytes has been studied, but how SUV39H1 functions in renal tubules under diabetic conditions is unclear. Renal biopsy specimens of ten diabetic nephropathy (DN) subjects and seven non-DN minimal change diseases (MCD) subjects were collected. SUV39H1, IL-6, and MCP-1 expression in renal tissues were measured using immunohistochemical, while SUV39H1, H3K9me3, IL-6, and MCP-1 in human proximal tubular epithelial cells (HK-2) under varying glucose conditions were assayed by Western blot and ELISA. SUV39H1 was overexpressed in HK-2 cells; the regulation of SUV39H1 and H3K9me3 on NF-κB, IL-6, MCP-1, caspase 3, and apoptosis was measured. SUV39H1 was expressed more in diabetic human renal tubules. HK-2 cells with high glucose up-regulated IL-6 and MCP-1 in a dose- and time-dependent manner, and SUV39H1 expression was reduced with greater glucose and prolonged stimulation. Expression of H3K9me3 was synchronized with SUV39H1. Moreover, overexpression of SUV39H1 in high glucose environment was accompanied with increased H3K9me3 and decreased inflammation and apoptosis. SUV39H1 dysregulation may be involved in DN progression. Overexpression of SUV39H1 may reduce renal inflammation and apoptosis via epigenetic modulation, thus plays a protective role in DN.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Currently, it is estimated that approximately 382 million people worldwide suffer from diabetes mellitus (DM), and this number is expected to rise to 592 million by 2035 [1]. Approximately 40% of those with DM progress to diabetic nephropathy (DN), which is a chief cause of end-stage renal disease (ESRD) [2]. Clinically, renin-angiotensin system inhibitors and glucose control, blood pressure reduction, and weight loss are used to treat DN but with limited benefit. Exact mechanisms responsible for the pathogenesis of DN are various [3]. In 1991, Hasegawa first suggested that inflammation may contribute to DN [4]. Studies suggest that expression of inflammatory cytokines in the blood, urine, and kidneys of DN subjects was greater than for normal controls, and inflammation was positively correlated with renal function [5,6,7]. Furthermore, increased apoptosis may result in abnormal renal structure and function [8]. Tubulointerstitial fibrosis is generally considered a final common pathway for DN. Apoptosis is regularly observed in fibrotic tissue, and mounting evidence confirms that apoptosis contributes to the fibrotic process [9].
Epigenetics is the study of heritable changes in gene expression that occur without alterations in underlying DNA sequences. These gene expression changes can be passed on between parent and progeny. Recent studies to examine the potential role of epigenetic mechanisms of DN progression [10] suggest that even without high-glucose stimulation, epigenetic modification and related histone modification enzymes in the promoter region of pathological DN genes continue to change, resulting in aberrant genes expression [11, 12].
The expression of SUV39H1 (the HMTase of H3K9me3) changes after epigenetic regulation may be used to measure epigenetic modification. Previous studies have examined the expression of SUV39H1 at inflammatory gene promoter regions under high-glucose stimulation in VSMCs, macrophages, and cardiomyocytes [11, 13, 14]. However, relatively little is known regarding changes in SUV39H1 expression in renal biopsy tissues of DN subjects and high-glucose-induced HK-2 cells. It is the extent of tubulointerstitial injury that determines renal function deterioration and ultimately plays a vital role in the pathogenesis and progression of DN [15]. Thus, we investigated whether SUV39H1 was involved in a high-glucose-mediated inflammatory response and apoptosis in HK-2 cells.
MATERIALS AND METHODS
Subject Identification
All subjects were diagnosed by renal biopsy at the Department of Nephrology, Second Xiangya Hospital, Central South University in 2015. Ten (10) DN patients were selected as DN group; seven (7) cases of non-DN minimal change disease (MCD) were selected as control group. They are described in Table 1. Subjects with cancer, liver disease, or other primary or secondary kidney diseases were excluded. Blood pressure, blood sugar, glycosylated hemoglobin (HbA1c), proteinuria, urine sediment red blood cell (RBC) count, serum creatinine, urea nitrogen, and other relative indexes of DN were recorded before treatment. Informed consent was obtained from all individual participants included in the study.
Cell Culture
Human proximal tubular epithelial cells line, HK-2, were cultured in DMEM plus F12 (1:1) medium (both obtained from Gibco, USA) containing 7-mmol/l glucose (normal glucose, NG) supplemented with 1% penicillin (100 U/ml)-streptomycin (100 μg/ml) (Gibco, USA) and 10% FBS (Gibco, USA) in a 37 °C, 5% CO2 incubator. Fresh medium was added daily until cells reached 70% confluence. Experiments were then conducted. For glucose concentration experiments, cells were cultured with 7, 15, 30, 45, and 60 mmol/l for 24 h. For time-point experiments, cells were incubated in 30-mmol/l glucose for 0, 8, 12, 24, and 48 h. A high-mannitol (Sigma, USA) medium, containing 7-mmol/l glucose and 53-mmol/L mannitol served as the osmotic control for 60-mmol/l glucose.
Transient Transfection
Cells were cultured in 6-well plates. When cells reached 50% confluence, SUV39H1 overexpression plasmids or non-specific plasmids (Genechem, China) were transfected into cells using Lipofectamine 2000 reagent (Gibco, USA) according to the manufacturer’s protocol. After 5 h of transfection, cells were incubated in normal glucose medium (NG) for 24 h and then stimulated with/without 30-mmol/l glucose for 24 h. The grouping was as follows: 7-mmol/l NG, 30 mmol/l HG, HG + Ncontrol, and HG + SUV39H1 (HG, high glucose; HG + Ncontrol, negative control plasmid transfected group; HG + SUV39H1, SUV39H1 overexpression plasmid transfected group). Target molecule expression was mainly measured using Western blot.
Immunohistochemistry
Renal biopsy specimens were obtained from study subjects after acquiring written informed consent in accordance with the Ethics Committee. Tissue samples were fixed in formalin and embedded in paraffin, and then 4-μm slices were deparaffinized with xylene and graded ethanol. Antigen was retrieved using heated citrate buffer (pH 6.0). Endogenous peroxidase activity was quenched with 3% hydrogen peroxide. After blocking for 30 min with normal goat serum (ZSGB-BIO, China), tissue sections were sequentially incubated with primary anti-SUV39H1 (1:100, Aviva System, USA), anti-IL-6 (1:100, CST, USA), anti-MCP-1 (1:100, Abcam, USA), normal rabbit serum (1:100, ZSGB-BIO, China), normal mouse serum (1:100, ZSGB-BIO, China), and PBS at 4 °C overnight. Slides were thoroughly washed in PBS buffer, and then HRP-conjugated anti-rabbit IgG or anti-mouse IgG secondary antibodies (1:200, LiankeBio, China) were applied for 30 min at room temperature. Signals were developed with a DAB Peroxidase Substrate Kit (LiankeBio, China). Sections were counterstained with hematoxylin and then studied under a light microscope.
Western Blot
Cells were resuspended in RIPA/PMSF buffer (100:1, LiankeBio, China) and lysed for 30 min on ice. Protein was measured in cell lysate using a BCA Reagent kit (Beyotime, China). Samples (30 μg) were separated using 12% SDS-PAGE and transferred onto PVDF membranes (Millipore, Germany). Membranes were blocked in 5% BSA for 1 h and respectively incubated with the following primary antibodies: anti-SUV39H1 (1:500, Aviva System, USA), anti-H3K9me3 (1:500, Abcam, USA), anti-NF-κB p65 (1:800, Proteintech, USA), anti-IL-6 (1:1000, CST, USA), anti-MCP-1 (1:800, Abcam, USA), anti-caspase 3 (1:800, CST, USA), and anti-β-actin (1:1000, Abcam, USA) overnight at 4 °C. PBST solution was used to wash membranes three times. After incubating with anti-rabbit IgG or anti-mouse IgG secondary antibodies (1:5000, LiankeBio, China) for 1 h at room temperature, membranes were visualized using ECL. Protein band intensity was quantified using a Kodak densitometer and Photoshop C5S software.
Total RNA Extraction and Quantitative Real-time PCR
Total RNA from cultured HK-2 cells was extracted using the TRIzol Reagent (Invitrogen, Carlsbad, CA). cDNA was synthesized from 1 μg of total RNA using PrimeScript™ RT reagent kit with gDNA Eraser (TaKara, Japan) according to the manufacturer’s instructions. Quantitative RT-PCR was performed on an ABI Prism 7300 real-time thermocycler (Applied Biosystems, USA) using the SYBR Premix Ex Taq™ II (TaKara, Japan). Data were normalized to β-actin expression by the comparative quantification method (2−ΔΔCt). Primer information for PCR was as follows:
SUV39H1, Forward (5′–3′) ATGTGATGAGGGGCGGATTG,
Reverse (5′–3′) GGGTCAGCCAGTCTGTCATT;
IL-6, Forward (5′-3′) TGCAATAACCACCCCTGACC,
Reverse (5′–3′) ATTTGCCGAAGAGCCCTCAG;
MCP-1, Forward (5′–3′) CCTTCATTCCCCAAGGGCTC,
Reverse (5′–3′) CTTCTTTGG GACACTTGCTGC;
β-actin, Forward (5′–3′) CCTGGCACCCAGCACAAT,
Reverse (5′–3′) GGGCCGGACTCGTCATAC.
ELISA
Concentrations of IL-6 and MCP-1 in the supernatants were quantified using commercial ELISA kit (CUSABIO, China) according to manufacturer’s instructions repeated three times. The detection sensitivity range was 7.8–500 pg/ml for IL-6 and 31.25–2000 pg/ml for MCP-1.
Flow Cytometry
Cells were stained with annexin V-fluorescein isocyanate (FITC) and propidium iodide (PI) according to the manufacturer’s instructions of apoptosis detection kit (BD Pharmingen, USA). Fluorescence was measured using a FACSJazz Flow Cytometer (BD, USA) and data were analyzed with FlowJo software (Treestar). Cells positive for annexin V-FITC were considered to be apoptotic.
Statistical Analysis
Statistical comparisons were performed using SPSS19.0 software and Prism GraphPad 5.0 software. All data are means ± SD. Statistical significance was established with one-way ANOVA analysis followed by independent samples t tests (p < 0.05 was considered statistically significant).
RESULTS
Clinical Characteristics of the Study Subjects
DN patients were older individuals with increased blood sugar levels, HbA1c (p < 0.05). The serum creatinine and urea nitrogen level of DN were higher than those of control group (p < 0.05). There was no significant difference in gender ratio, blood pressure, blood lipids, uric acid, urinary sediment RBC count, and proteinuria between the two groups (p > 0.05). Table 1 depicts study subjects.
Expression of SUV39H1 and Pro-inflammatory Mediators in Human Renal Tissues
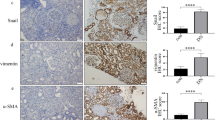
Expression of SUV39H1, IL-6, and MCP-1 in kidney tissue paraffin section from 17 participants (ten DN subjects; seven MCD controls) measured using IHC appears in Fig. 1. SUV39H1 expression was mainly located in renal proximal tubules, and its expression was greater in renal tubules of DN subjects (Fig. 1a). Quantitatively, SUV39H1 staining intensity increased ~ 4-fold in DN subjects (Fig. 1b). Pro-inflammatory mediators, IL-6 and MCP-1, were also mainly expressed in renal proximal tubules and they were more prominent in DN subjects (Fig. 1). Thus, DN subjects have more severe inflammation, and SUV39H1 may be associated with the pathogenesis of DN.
Expression of SUV39H1 and pro-inflammatory mediators in human renal tissues. a IHC of SUV39H1, IL-6, and MCP-1 staining increased in renal proximal tubules of DN compared with controls (scale bar = 100 μm, magnification ×200). b Semi-quantification of SUV39H1, IL-6, and MCP-1 staining. Values presented are means ± SD, **p < 0.01, ***p < 0.001 versus control.
Dynamic Change of SUV39H1, H3K9me3, IL-6, and MCP-1 in Response to Glucose Stimulation in HK-2 Cells
Glucose concentration and time-point experiments were conducted to further understand dynamic alteration of SUV39H1, H3K9me3, and inflammation under diabetic condition. Data show that glucose up-regulated expression of IL-6 and MCP-1 in dose- and time-dependent manner (Western blot and ELISA; Figs. 2 and 3), which indicated that high glucose can induce inflammation in the human kidney.
SUV39H1, H3K9me3, IL-6, and MCP-1 expression in HK-2 cells stimulated by different concentrations of glucose. a Western blot, glucose-induced IL-6 and MCP-1 protein expression increase in a dose-dependent manner. SUV39H1 and H3K9me3 in HK-2 cells increased then decreased with increasing glucose. b Quantification of average band density calculated from three independent experiments. c ELISA, glucose increased secretion of IL-6, MCP-1 in dose-dependent manner.*p < 0.05, **p < 0.01, ***p < 0.001 versus 7-mM NG group. ##p < 0.01 versus 60-mM HG. Values are means ± SD. NG, normal glucose; HG, high glucose; mM, mmol/l.
SUV39H1, H3K9me3, IL-6, and MCP-1expression in HK-2 cells stimulated with HG for different periods. a Western blot; HG-induced IL-6 and MCP-1 expression increased in a time-dependent manner. SUV39H1 and H3K9me3 in HK-2 cells increased then decreased with increased glucose stimulation time. b Quantification of average band density calculated from three independent experiments. c ELISA, glucose increased secretion of IL-6 and MCP-1 in time-dependent manner. *p < 0.05, **p < 0.01, ***p < 0.001 versus 0H group. ##p < 0.01 versus 16H group. Values are means ± SD. HG, high glucose; H, hour.
Western blot data show that SUV39H1 expression in HK-2 cells increased then decreased as glucose increased. Compared to 7-mM (mmol/l) NG group, SUV39H1 expression gradually increased in 15- and 30-mM HG groups (p < 0.05) and then began to decline when concentration reached 45 mmol/l. Finally, in the 60-mM HG group, it significantly decreased compared to the 30-mM HG group (p < 0.01; Fig. 2a and b). In time-point experiments, medium containing 30-mmol/l glucose was used to stimulate HK-2 cells for different time periods. Compared to 0H (hour) group, SUV39H1 expression increased in 8H group (p < 0.05) and peaked in 16H group (p < 0.05). After 24 h stimulation, SUV39H1 decreased and this decrease was more pronounced in 48H group (Fig. 3a and b). Expression of SUV39H1 in HK-2 cells increased and subsequently decreased in response to prolonged stimulation and this mimicked kinetic changes observed in the concentration experiments. Expression of H3K9me3 increased then decreased in both concentration and time-point experiments and this was synchronized with its methyltransferase SUV39H1 (Figs. 2a and b and 3a and b). There was no significant difference between the mannitol group and controls with regard to expression of SUV39H1, H3K9me3, IL-6, and MCP-1.
SUV39H1 Can Partially Reversed Inflammation and Apoptosis in High-glucose-induced HK-2 Cells
After 24 h of 30-mmol/l glucose stimulation, secretion of IL-6, MCP-1, caspase 3 and apoptosis significantly increased in the HG group compared with that of the NG group (Fig. 4). Transfection of SUV39H1 overexpression plasmids into HK-2 cells (confirmed with qRT-PCR and Western blot) showed (Fig. 4a and b) that plasmids were efficient in up-regulating expression of SUV39H1 in HK-2 cells. Overexpression of SUV39H1 catalyzed three times the amount of H3K9me3 compared to HG + Ncontrol group (p < 0.01). In Fig. 4a, overexpression of SUV39H1 efficiently down-regulated the gene expression of inflammatory cytokines. By Western blot, ELISA, and flow cytometry, the regulatory effect of SUV39H1 on renal inflammation and apoptosis under high-glucose ambience was further examined (Fig. 4b, c, d, and e). IL-6, MCP-1, caspase 3, and apoptosis were measured in transfected cells (HG + SUV39H1 and HG + Ncontrol groups). All indices in SUV39H1 transfected cells were markedly reduced compared to HG + Ncontrol groups, suggesting that high-glucose-induced renal inflammation and apoptosis could be partially abolished by concomitant transfection of SUV39H1 overexpression plasmids. NF-κB, as an essential nuclear transcription factor, is commonly activated by various stimuli including obesity, oxidative stress, and hyperglycemia. In our study, we observed that NF-κB p65 was notably enhanced in HG group and this was followed by up-regulation of IL-6 and MCP-1. However, in SUV39H1 transfected cells, NF-κB p65 was not significantly affected by SUV39H1 overexpression (Fig. 4b and c).
SUV39H1 could partially reverse inflammation and apoptosis in high-glucose-induced HK-2 cells. a Gene expression of SUV39H1, IL-6, and MCP-1 before and after transfection was assessed by qRT-PCR; overexpression of SUV39H1 efficiently down-regulated the gene expression of IL-6 and MCP-1. b Western blot of changes of protein expression before/after transfection. Compared to NG group, SUV39H1, H3K9me3 and NF-κB p65, IL-6, MCP-1, and cleaved-caspase 3 expressions elevated in HG. After transfection of SUV39H1 overexpression plasmids, SUV39H1 and H3K9me3 further increased; IL-6, MCP-1, and cleaved-caspase 3 markedly reduced as compared with HG + Ncontrol group; NF-κB p65 was not significantly change with SUV39H1 overexpression. c Quantification of average band density calculated from three independent experiments. d ELISA, secretion of IL-6, and MCP-1 increased after HG stimulation compared to NG group. Pro-inflammatory effect of HG can be partly eliminated by SUV39H1 overexpression which performed as secretion of IL-6 and MCP-1 decreased in HG + SUV39H1 relative to HG + Ncontrol group. e Flow cytometry for apoptosis; data corresponded to caspase 3 protein expression after transfecting SUV39H1 overexpression plasmids. Data are means ± SD. *p < 0.05, **p < 0.01, ***p < 0.001 versus HG + NControl group. #p < 0.05, ##p < 0.01, ###p < 0.001 versus NG group. HG + NControl, negative control plasmid transfected group; HG + SUV39H1, SUV39H1 overexpression plasmid transfected group.
DISCUSSION
Abbreviation of Su(var)3-9-related HMTase is Suv39h1 in mice, but SUV39H1 in human, and Suv39h1 in mice [11]. Suv39h1 was initially considered to be a major locus of controlling the position-effect variegation (PEV) of Drosophila, and its function as the H3K9me3 HMTase was first shown for the human SUV39H1 protein in 2000 [16]. SUV39H1 is localized in the centromeric region of the chromosome and forms complexes with the heterochromatin protein-1 (HP-1) to catalyze H3K9me3 during metaphase of cell division [17]. HP-1 can further induce methylation and chromatin condensation. Also, SUV39H1 is involved in the composition of histone deacetylase (HDACs) complex, which in turn, removes the acetyl from H3K9 site to promote K9 methylation [18].
Several studies suggest a role for SUV39H1 and H3K9me3 in inflammatory gene repression among different cell types under diabetic conditions. In VSMCs derived from type 2 diabetic mice, H3K9me3 and Suv39h1 were significantly decreased at the promoters of key inflammatory genes, and this was accompanied by increased inflammation [11]. Macrophages cultured in high-glucose ambience, and myocardial ischemia-reperfusion injury under diabetic condition also had similar characteristics [11, 13, 14]. These results suggested that the altered function of HMTase might be an essential event in diabetic vascular complications. Other than functioning as a regulator of inflammation, Suv39h1 may have anti-apoptotic effects in mice cardiac cells [14]. In our study, we may have defined another important role for SUV39H1 in HK-2 cells.
Renal tubules are the main position of reabsorbing metabolites (especially glucose and protein) in urine. A study [19] showed that proximal tubules drive an inflammatory response that causes not only tubulointerstitial fibrosis but also glomerulosclerosis. We found that high glucose up-regulated the expression of IL-6 and MCP-1 in a dose- and time-dependent manner in HK-2 cells. This suggests that high glucose can trigger inflammation in human proximal tubular epithelial cells (PTECs). The underlying mechanisms responsible for this phenomenon may involve several pathways. There is experimental evidence supporting the critical role of NF-κB activation in the pathogenesis of renal inflammation and human DN [20, 21]. NF-κb p65 is the most abundant and best characterized member of this family. In our study, we observed that p65 was enhanced by high glucose, and this was accompanied by up-regulation of inflammation. Furthermore, under high-glucose exposure, histone modifications such as a marked histone deacetylation may play an important role in regulating the inflammatory and apoptotic responses via modulating p65 [22]. We attempted to identify the role of SUV39H1 plays in high-glucose-induced inflammation and apoptosis.
SUV39H1 was mainly localized at the human renal tubules, and its expression in the DN group was greater than in controls. However, our results are inconsistent with studies which showed that expression of Suv39h1 was decreased in VSMCs and myocardial I/R injury in diabetic mice compared to controls [11, 14]. In view of this result, we suggest that different species and tissues may have varied expression patterns of SUV39H1, and we speculate that the length of the diabetic course may affect expression of SUV39H1.
In regard to the dynamic changes of SUV39H1 and H3K9me3 in HK-2 cells under high-glucose condition, we noted that SUV39H1 expression in HK-2 cells increased initially and then decreased with greater glucose and longer stimulation, so as the change of H3K9me3. We noted that, in human kidney, inflammation could also act as a regulator of SUV39H1 in high-glucose environment, not just the result of high glucose and SUV39H1. Besides, other studies have recently confirmed that TNF-α induced inflammatory gene expression was more striking in cultured db/db VSMCs as compared to control db/+ VSMCs. Suv39h1 in promoter regions of inflammatory genes (IL-6, MCP-1, MCSF) also experienced dramatic decrease and remained persistently low for 60 min in db/db VSMCs, while that of control group displayed an upward trend [11]. Thus, normally, inflammatory stimulation increased SUV39H1 to inhibit the expression of inflammatory genes via negative feedback. However, this mechanism may be impaired in diabetic condition and cause more inflammation. Thus, during the initial stage of high-glucose stimulation, IL-6 and MCP-1 secretion in HK-2 cells increased via inflammatory signaling pathways, such as NF-κB. Then via negative feedback, this inflammatory state up-regulates SUV39H1 expression to catalyze H3K9me3, which is subsequently enriched in the promoter region of inflammatory genes and inhibits their transcription and expression to reduce inflammation. Later, over time and with greater glucose, inhibitory effects of high glucose on SUV39H1 are obvious—that the negative feedback loop is broken. These series of events reduced SUV39H1 and H3K9me3 and further increased expression of IL-6 and MCP-1 (Fig. 5). Our collected DN subjects were mainly in the early stage of DN; the negative feedback mechanism may play the leading role in increasing SUV39H1 expression in the renal tubules among these patients.
Schematic representation of dynamic change of SUV39H1 in high-glucose ambience. During early stage of high-glucose stimulation, secretion of IL-6/MCP-1 increased. Via negative feedback, inflammation induced the expression of SUV39H1, and this catalyzed H3K9me3 to inhibit transcription of inflammatory genes. With increased glucose concentration and prolonged stimulation, the inhibitory effect of high glucose on SUV39H1 became more obvious and the negative feedback was impaired. These changes results decreased expression of SUV39H1 and up-regulated expression of IL-6 and MCP-1.
SUV39H1 gene transfection data suggest that overexpression of SUV39H1 in high-glucose environment may partially abolish inflammation and apoptosis in HK-2 cells via increasing H3K9me3 expression. Besides this direct regulation, other studies have indicated that SUV39H1 may also indirectly alleviate inflammation by reducing activation of NF-κB signaling pathways [14]. Though, according to our results, SUV39H1 regulated inflammation mainly by epigenetic modulation rather than activation of NF-κB.
Our study extends the repressive regulation of SUV39H1 on inflammation and apoptosis to human PTECs and we highlight a negative feedback mechanism between inflammation and SUV39H1 in high-glucose ambience. More studies are needed to address the signaling pathways used by inflammatory cytokines to induce production of SUV39H1 under normal conditions, and how high glucose inhibits SUV39H1 expression.
References
Guariguata, L., D.R. Whiting, I. Hambleton, J. Beagley, U. Linnenkamp, and J.E. Shaw. 2014. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Research and Clinical Practice 103: 137–149.
Cooper, M.E. 2012. Diabetes: treating diabetic nephropathy-still an unresolved issue. Nature Reviews. Endocrinology 8: 515–516.
Kanwar, Y.S., L. Sun, P. Xie, F.Y. Liu, and S. Chen. 2011. A glimpse of various pathogenetic mechanisms of diabetic nephropathy. Annual Review of Pathology 6: 395–423.
Hasegawa, G., K. Nakano, M. Sawada, K. Uno, Y. Shibayama, K. Ienaga, and M. Kondo. 1991. Possible role of tumor necrosis factor and interleukin-1 in the development of diabetic nephropathy. Kidney International 40: 1007–1012.
Suzuki, D., M. Miyazaki, R. Naka, T. Koji, M. Yagame, K. Jinde, M. Endoh, Y. Nomoto, and H. Sakai. 1995. In situ hybridization of interleukin 6 in diabetic nephropathy. Diabetes 44: 1233–1238.
Navarro, J.F., C. Mora, M. Maca, and J. Garca. 2003. Inflammatory parameters are independently associated with urinary albumin in type 2 diabetes mellitus. American Journal of Kidney Diseases 42: 53–61.
Moriwaki, Y., T. Yamamoto, Y. Shibutani, E. Aoki, Z. Tsutsumi, S. Takahashi, H. Okamura, M. Koga, M. Fukuchi, and T. Hada. 2003. Elevated levels of interleukin-18 and tumor necrosis factor-alpha in serum of patients with type 2 diabetes mellitus: relationship with diabetic nephropathy. Metabolism 52: 605–608.
Wei, Q., G. Dong, J.K. Chen, G. Ramesh, and Z. Dong. 2013. Bax and Bak have critical roles in ischemic acute kidney injury in global and proximal tubule-specific knockout mouse models. Kidney International 84: 138–148.
Johnson, A., and L.A. DiPietro. 2013. Apoptosis and angiogenesis: an evolving mechanism for fibrosis. The FASEB Journal 27: 3893–3901.
Kato, M., and R. Natarajan. 2014. Diabetic nephropathy—emerging epigenetic mechanisms. Nature Reviews. Nephrology 10: 517–530.
Villeneuve, L.M., M.A. Reddy, L.L. Lanting, M. Wang, L. Meng, and R. Natarajan. 2008. Epigenetic histone H3 lysine 9 methylation in metabolic memory and inflammatory phenotype of vascular smooth muscle cells in diabetes. Proceedings of the National Academy of Sciences of the United States of America 105: 9047–9052.
Keating, S.T., and A. El-Osta. 2013. Glycemic memories and the epigenetic component of diabetic nephropathy. Current Diabetes Reports 13: 574–581.
Li, M.F., R. Zhang, T.T. Li, M.Y. Chen, L.X. Li, J.X. Lu, and W.P. Jia. 2016. High glucose increases the expression of inflammatory cytokine genes in macrophages through H3K9 methyltransferase mechanism. Journal of Interferon & Cytokine Research 36: 48–61.
Yang, B., J. Yang, J. Bai, P. Pu, J. Liu, F. Wang, and B. Ruan. 2014. Suv39h1 protects from myocardial ischemia-reperfusion injury in diabetic rats. Cellular Physiology and Biochemistry 33: 1176–1185.
Zhu, X., X. Xiong, S. Yuan, L. Xiao, X. Fu, Y. Yang, C. Tang, L. He, F. Liu, and L. Sun. 2016. Validation of the interstitial fibrosis and tubular atrophy on the new pathological classification in patients with diabetic nephropathy: a single-center study in China. Journal of Diabetes and its Complications 30: 537–541.
Schotta, G., A. Ebert, and G. Reuter. 2003. SU(VAR)3-9 is a conserved key function in heterochromatic gene silencing. Genetica 117: 149–158.
Lachner, M., D. O'Carroll, S. Rea, K. Mechtler, and T. Jenuwein. 2001. Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature 410: 116–120.
Vaute, O., E. Nicolas, L. Vandel, and D. Trouche. 2002. Functional and physical interaction between the histone methyl transferase Suv39H1 and histone deacetylases. Nucleic Acids Research 30: 475–481.
Grgic, I., G. Campanholle, V. Bijol, C. Wang, V.S. Sabbisetti, T. Ichimura, B.D. Humphreys, and J.V. Bonventre. 2012. Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney International 82: 172–183.
Sanz, A.B., M.D. Sanchez-Nino, A.M. Ramos, J.A. Moreno, B. Santamaria, M. Ruiz-Ortega, J. Egido, and A. Ortiz. 2010. NF-kappaB in renal inflammation. Journal of the American Society of Nephrology 21: 1254–1262.
Mezzano, S., C. Aros, A. Droguett, M.E. Burgos, L. Ardiles, C. Flores, H. Schneider, M. Ruiz-Ortega, and J. Egido. 2004. NF-kappaB activation and overexpression of regulated genes in human diabetic nephropathy. Nephrology, Dialysis, Transplantation 19: 2505–2512.
Zhou, L., D.Y. Xu, W.G. Sha, L. Shen, G.Y. Lu, X. Yin, and M.J. Wang. 2015. High glucose induces renal tubular epithelial injury via Sirt1/NF-kappaB/microR-29/Keap1 signal pathway. Journal of Translational Medicine 13: 352.
Funding
This work was supported by research grants (81373227, 81400721, and 81470947) from the National Natural Science Foundation of China. It was also supported by a research fund from Natural Science Foundation of Hunan Province (Grant No. 2016JJ3173).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Wang, J., Yan, W., Peng, X. et al. Functional Role of SUV39H1 in Human Renal Tubular Epithelial Cells Under High-glucose Ambiance. Inflammation 41, 1–10 (2018). https://doi.org/10.1007/s10753-017-0657-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-017-0657-7