Abstract
In this study, the antioxidant activity of crocin via experimental and theoretical methods was investigated. In order to induce oxidative stress, 30-min renal ischemia and 24-h reperfusion were used in male Wistar rats. Oxidative stress was assessed by measuring tissue malondialdehyde (MDA) and ferric reducing/antioxidant power (FRAP). The results showed that following ischemia/reperfusion, the level of MDA was increased and FRAP decreased. Both of these changes were alleviated by crocin administration. The bond dissociation enthalpy and ionization potential values as enthalpies of mechanism of antioxidant activity of crocin were calculated by density functional theory method. According to obtained results, the novel structures of crocin with higher antioxidant activity for synthesis were proposed. Results indicated that NH2, OMe, and F substituents can improve the antioxidant activity of crocin. The crocin delivery via carbon and boron nitride nanotubes and nanocones was investigated. The results confirm that the calculated adsorption and free Gibbs energies of crocin on the surface of studied nanostructures were negative meaningfully, so these processes were exothermic and experimentally possible from the energetic viewpoint.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Carotenoids are a class of natural pigments that play an important role in plant health, and in human nutrition, they have an important role as antioxidant agents. Antioxidant potential of carotenoids reduced risk of development of various types of cancer. Chromatographic methods have been used for the study of carotenoids usually, that synergistic effects and large molecular size of carotenoids were limitations concerning factors [1,2,3]. Computational chemistry can solve the important problems in biology and experimental investigation of antioxidant activity of carotenoids. So, computational studies were very useful in order to understand the antioxidant mechanisms of carotenoids. Crocin (structure shown in Fig. 1) is the main carotenoid that is regarded as an effective antioxidant, and it is protecting cells and a drug for quenching various free radicals [4, 5].
There are two important antioxidant mechanisms that crocin can deactivate free radicals (R●). In the first mechanism (hydrogen atom transfer (HAT)), crocin directly transfers hydrogen atom (H●) to free radicals and yield a non-free radical ([crocin] + R● ➜ [crocin] ● + RH). For this mechanism, the bond dissociation enthalpy (BDE) represents the reaction enthalpy of crocin with free radical, and BDE is an important parameter to investigate the antioxidant activity of crocin.
In the second mechanism (single electron transfer (SET)), crocin transfers single electron to free radical and deactivate free radical ([crocin] + R● ➔ [crocin]+● + R−). For this mechanism, crocin acts as an electron donor and then yield a crocin in radical cation form that reaction enthalpy of this mechanism present via ionization potential (IP) [6,7,8,9,10,11,12].
Nanotechnology has an important role in the development and treatment of diseases. The high ratio in surface to mass and high ability to adsorb and carry various drugs were important and unique features of nanoparticles [13,14,15,16,17]. Since their discovery, nanotubes and nanocones have opened up new fields in science and technology because of its unique properties. The bio-compatibility properties of nanotubes and nanocones suggest that they are emerging as nanostructures for drug delivery and biomedical applications. The interactions between nanotubes and nanocones with various drugs proved that nanotubes and nanocones can be used as a smooth nanostructure channel for transporting biological and useful drugs [18,19,20,21,22].
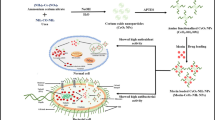
In this study, the antioxidant activity of crocin via theoretical method and in vivo experimental technique was investigated, and the effects of various substituents on the antioxidant activity of crocin were assessed. According to calculated results, novel crocin derivatives with lower BDE and IP values and higher antioxidant activity to synthesis were proposed. Drugs to treat sickness is being attached to nanostructures, so in order to identify the suitable nanostructure with high potential for crocin delivery, the crocin delivery via various nanotubes and nanocones was investigated.
Finally, the main aims of this study are (1) investigation of the antioxidant activity of crocin in vivo, (2) analysis of the antioxidant properties of crocin via theoretical method, (3) proposal of the novel crocin derivatives with higher antioxidant activity, and (4) assessment of the crocin delivery via various nanotubes and nanocones.
MATERIAL AND METHODS
Experimental Method
For induction of oxidative stress, renal ischemia and reperfusion (I/R) were used in male Wistar rats. Experimental animals were intraperitoneally (i.p.) injected with 0.5 ml of normal saline (Sham and I/R groups) or various doses of crocin (100, 200, or 400 mg/kg) in normal saline (n = 7 for each group). Thirty minutes after saline or crocin injection and following anesthesia with diethyl ether, rats in I/R and crocin-receiving groups underwent half-an-hour bilateral renal ischemia followed by 24 h of reperfusion. While in the sham group, only sham operation was done and spent equivalent reperfusion period. After 24-h reperfusion period, animals were sacrificed and the right kidneys were used to determine oxidative stress by measuring malondialdehyde (MDA) and ferric reducing/antioxidant power (FRAP). For this purpose, frozen kidney samples were weighed and rapidly homogenized in ice-cold phosphate buffer saline (PBS). As previously described in detail [23,24,25], the renal tissue MDA levels, as a final product of lipid peroxidation, and the FRAP, representing a direct measure of total antioxidant activities of all defense mechanisms, were determined using a spectrophotometer (Spectrolab, Newbury, UK).
Computational Details
In this paper, structure of crocin derivatives and their radical and radical cation forms were geometry optimized. The structure of CNT (10, 0), BNNT (10, 0), CNC (with disclination angles of 300°), BNNC (with disclination angles of 300°), and their complexes with crocin derivatives were geometry optimized. All the calculations were performed using the DFT/B3PW91 method and 6-311G (d, p) basis set within the GAMESS package [26,27,28,29,30]. The BDE and IP values of crocin derivatives were obtained by following equations:
where H ([crocin]+•) and H ([crocin]•) were the enthalpy of crocin cation radical and crocin radical forms, respectively. The adsorption energies (Ead) and free Gibbs energies (Gad) of crocin derivatives on the studied nanostructures were obtained by following equations:
where E (nanostructure/crocin) and G (nanostructure/crocin) were the adsorption energies and free Gibbs energies of complexes of crocin derivatives with studied nanostructures, respectively.
RESULTS AND DISCUSSION
Experimental in vivo Antioxidant Activity of Crocin
As shown in Table 1, kidney MDA levels in the sham group was about 55.46 ± 2.23 nmol/g of kidney weight which significantly increased in the I/R group (P < 0.001). Applying crocin at doses of 100 or 400 mg/kg could significantly decrease the tissue MDA level in I/R+crocin groups (P < 0.001), but they still had significantly higher MDA level than the sham group (P < 0.01). However, crocin at the dosage of 200 mg/kg was more effective.
Likewise, applying I/R led to a significant decrease in FRAP level in the kidney tissue compared to that of the sham group (P < 0.001). Administration of crocin significantly increased FRAP level in the kidney tissue in a dose-dependent manner (100 mg/kg, P < 0.01; 200 and 400 mg/kg, P < 0.001). But there was no significant difference among groups receiving different doses of crocin.
Antioxidant Activity of Crocin and Proposed Novel Crocin Derivatives
In recent years, the knowledge of BDE and IP has been accumulated in order to develop both experimental and quantum chemical techniques. In previous studies, BDE and IP of various antioxidants have been investigated [31,32,33,34].
The calculated BDE of various C–H and O–H bonds of crocin is reported in Table 2. Results show that the average of BDE values of C–H bonds was lower than corresponding values of O–H bonds ca 6.23 kcal/mol. The calculated data in Table 2 show that BDE values of C–H (2) and C–H (13) bonds were 79 and 79.2 kcal/mol, respectively. Results show that C–H (2) and C–H (13) have the lowest BDE values.
The BDE of C–H (2) and C–H (13) bonds was lower than the average of BDE values of C–H bonds ca 4.5 and 4.7 kcal/mol, respectively. Results show that highest BDE values were related to O–H (15) and O–H (22) bonds and mentioned values were 91.4 and 91.6 kcal/mol, respectively. The IP value of crocin (159.7 kcal/mol) is reported in Table 2. Results show that the IP value of crocin was higher than the BDE value of crocin 80.5 kcal/mol. The optimized structure of crocin and its radical and radical cation forms are shown in Fig. 2.
In previous studies, the substituent effects on the antioxidant activity of various antioxidants were investigated, and results show that antioxidant derivatives with substituted electron donating groups reduced the BDE and IP values and so increased the antioxidant activity of studied antioxidant [31,32,33,34].
In this study, the effect of NH2, OMe, and F substituents on BDE and IP values of crocin was investigated. The hydrogen atoms of carbon (12) were replaced with NH2, OMe, and F, respectively, and so the three new crocin derivatives (NH2-crocin, OMe-crocin, and F-crocin) were produced. The BDE and IP values of NH2-crocin, OMe-crocin, and F-crocin were calculated and are reported in Table 3.
The BDE and IP values of NH2-crocin were lower than the corresponding values of crocin ca 7.7 and 12.1 kcal/mol, respectively. The OMe and F substituent decrease the BDE value of crocin ca 3.9 and 4.4 kcal/mol, respectively. The IP values of OMe-crocin and F-crocin were lower than the corresponding values of crocin ca 7.6 and 8.4 kcal/mol, respectively. Results show that the average of IP values of the studied crocin derivatives was higher than the corresponding BDE values ca 77.2 kcal/mol.
In this study, the dependencies of BDE and IP values of the studied crocin derivatives were investigated, and IP values are plotted against BDE in Fig. 3. Results show that, calculated IP and BDE values have linear dependencies. Obtained linear equation was important in order to predict IP values for the studied crocin derivatives from their BDE values.
The calculated EHOMO and ELUMO of the studied crocin derivatives are reported in Table 3. The description of HOMO and LUMO in crocin and its radical and radical cation forms are shown in Fig. 4. Results indicated that EHOMO and ELUMO values of crocin were −5.21 and −2.83 eV, respectively. The absolute value of EHOMO of NH2-crocin and OMe-crocin was lower than the corresponding value of crocin ca 0.19 and 0.10 eV, respectively. The OMe and F substituent increase the absolute value ELUMO of crocin ca 0.05 and 0.08 eV, respectively.
The EHLG (HOMO–LUMO gap) of the studied crocin derivatives are reported in Table 3. Calculated EHLG values of the studied crocin derivatives ranged from 1.00 to 1.19 eV. The obtained EHLG values of the studied crocin derivatives decrease in this order: crocin > F-crocin > OMe-crocin > NH2-crocin. The EHLG of NH2-crocin and OMe-crocin was lower than the corresponding value of crocin ca 0.19 and 0.07, respectively. Therefore, EHLG values show that crocin has lower reactivity than substituted crocin and also NH2-crocin has the highest reactivity and lowest EHLG value.
Crocin Delivery with Carbon and Boron Nitride Nanostructures
In this study, the adsorption of crocin and NH2-crocin on surfaces of carbon nanotube (CNT (10, 0)), carbon nanocone (CNC), boron nitride nanotube (BNNT (10, 0)), and boron nitride nanocone (BNNC) was investigated. The optimized structures of complexes of crocin with the studied nanostructures are shown in Fig. 5. The calculated Ead and Gad values of crocin on surfaces of CNT (10, 0), BNNT (10, 0), CNC, and BNNC are reported in Table 4.
Results show that Ead of crocin on surfaces of CNT (10, 0) and BNNT (10, 0) was −0.521 and −0.543 eV, respectively. The absolute values of Ead of crocin on surfaces of CNC and BNNC were lower than the corresponding values of BNNT (10, 0) ca 0.031and 0.013 eV, respectively. Results indicated that BNNT (10, 0) and CNC have the highest and lowest absolute values of Ead for crocin adsorption. Results show that absolute values of Gad of crocin on surfaces of CNT (10, 0), BNNT (10, 0), CNC, and BNNC were lower than the corresponding absolute value of Ead ca 0.105 eV.
In this section, also the Ead and Gad of NH2-crocin on surfaces of CNT (10, 0), BNNT (10, 0), CNC, and BNNC were investigated and are summarized in Table 4. Results show that Ead and Gad of NH2-crocin on surface of CNT (10, 0) were negative than the corresponding values for crocin ca 0.042 and 0.041 eV, respectively. The Ead of NH2-crocin on surface of BNNT (10, 0) and BNNC was negative than the corresponding values for crocin ca 0.046 and 0.048 eV, respectively. Also, results show that NH2 increases the absolute values of Ead and Gad of crocin on surface of CNC ca 0.043 and 0.040 eV, respectively.
CONCLUSION
In this study, the reaction enthalpies of antioxidant action of crocin via DFT/B3PW91 as theoretical method were investigated. Also, the antioxidant activity of crocin was assessed in vivo by measuring MDA and FRAP levels in the renal tissue after ischemia and reperfusion. The effects of NH2, OMe, and F substituents on antioxidant activity of crocin were investigated. The potential of carbon and boron nitride nanotubes and nanocones for crocin delivery via theoretical scales was investigated. The results of in vivo study showed that crocin can alleviate oxidative stress induced by renal ischemia and reperfusion. The results of the theoretical study show that replacing substituents in crocin may be a good strategy for improving the antioxidant activity and sensitivity of crocin toward nanostructure surfaces. Results indicated that NH2-crocin derivatives with lower BDE and IP values have higher antioxidant activity than crocin. Results show that boron nitride nanotube can effectively interact with the studied crocin derivatives, and so their adsorptions are exothermic and experimentally possible from the energetic viewpoint. The calculated BDE values of the studied crocin have linear dependences with corresponding IP values that can be useful to predicate BDE from IP and vice versa.
Abbreviations
- PBS:
-
phosphate buffer saline
- MDA:
-
malondialdehyde
- FRAP:
-
ferric reducing/antioxidant power
- DFT:
-
density functional theory
- PW91:
-
Perdew–Wang 91
- HOMO:
-
highest occupied molecular orbital
- LUMO:
-
lowest unoccupied molecular orbital
- HLG:
-
HOMO–LUMO gap
- CNC:
-
carbon nanocone
- BNNC:
-
boron nitride nanocone
- CNT:
-
carbon nanotube
- BNNT:
-
boron nitride nanotube
- BDE:
-
bond dissociation enthalpy
- IP:
-
ionization potential
- HAT:
-
hydrogen atom transfer
- SET:
-
single electron transfer
References
Karrer, P., F. Benz, R. Morf, and H. Raudnitz. 1932. Helvetica Chimica Acta 15: 1399.
Isler, O. 1979. Pure and Applied Chemistry 51: 447–462.
Hosseinzadeh, H., T. Ziaee, and A. Sadeghi. 2008. Phytomedicine 15: 491–495.
Laabich, A., and G.P. Vissvesvaran. 2006. Investigative Ophthalmology & Visual Science 47: 3156–3163.
Papandreou, M.A., C.D. Kanakis, and M. Margarity. 2006. Journal of Agricultural and Food Chemistry 54: 8762–8768.
Shinji, S., T. Ochiai, H. Shimeno, H. Saito, and K. Abe. 2007. Journal of Natural Medicines 61: 102–111.
Edge, R., D.J. McGarvey, and T.G. Truscott. 1997. Journal of Photochemistry and Photobiology. B 41: 189–200.
Woodall, A.A., S.W.M. Lee, and R.J. Weesie. 1997. Biochimica et Biophysica Acta 1336: 33–42.
Guo, J., Y. Luo, and F. Himo. 2002. Chemical Physics Letters 366: 73–81.
Chen, Y., H. Zhang, C. Zhao, L. Cai, Y. Liu, and L. Jia. 2008. Food Chemistry 109: 484–492.
Najafi, M., M. Najafi, and H. Najafi. 2012. Bulletin of the Korean Chemical Society 33: 3343–3348.
Najafi, M., M. Najafi, and H. Najafi. 2012. Bulletin of the Korean Chemical Society 33: 3607–3617.
Iijima, S. 1991. Nature 354: 56–58.
Kong, J., N.R. Franklin, C. Zhou, and M.G. Chapline. 2000. Science 287: 622–625.
Collins, P.G., K. Bradley, and M. Ishigami. 2000. A. Science 287: 1801–1804.
Snow, E.S., F.K. Perkins, and J.A. Robinson. 2006. Chemical Society Reviews 35: 790–798.
Kauffman, D.R., and A. Star. 2008. Ang. Chemistry International 47: 6550–6570.
Russo, G.L., M. Russo, and I. Castellano. 2014. Marine Drugs 12 (7): 4069–4085.
Sieliwoniuk, E.N., and J. Malejko. Food Chemistry 176: 175–183.
Veloso, M.V., A.G. Souza Filho, and Filho Mendes. Journal Chemistry Physics Letters: 430–471.
Meng, L., X. Zhang, Q. Lu, Z. Fei, and P.J. Dyson. 2012. Biomaterials 33: 1689–1698.
Cuevas-Glory, L., and E. Ortiz-Vázquez. 2015. Mexico. Food Chemistry 166: 17–22.
Mahmoudzadeh, L., H. Najafi, S. Changizi-Ashtiyani, and Z. Mohamadi Yarijani. 2016. Nephrology.
Najafi, H., M.R. Firouzifar, O. Shafaat, S. Changizi Ashtiyani, and N. Hosseini. 2014. Iranian Journal of Kidney Diseases 8: 293–298.
Najafi, H., S.M. Owji, E. Kamali-Sarvestani, and S.M. Shid Moosavi. 2016. Experimental Physiology 101 (7): 913–931.
Máté, S., and J. Nie. 2010. Surface Science 604: 654.
Oubal, M., S. Picaud, M. Rayez, and J. Rayez. 2010. Surface Science 604: 1666.
Ferro, Y., A. Allouche, F. Marinelli, and C. Bros. 2004. Sets Surface Science 559: 158.
Grimme, S. 2006. Journal of Computational Chemistry 27: 1787.
Schmidt, M., et al. 1993. Journal of Computational Chemistry 14: 1347.
Najafi, M., M. Najafi, and H. Najafi. 2013. Journal of Theoretical and Computational Chemistry 12: 1250116.
Najafi, M., M. Najafi, and H. Najafi. 2013. Bulletin of the Chemical Society of Japan 86: 497–509.
Najafi, M., M. Najafi, and H. Najafi. 2013. Computational & Theoretical Chemistry 999: 34–42.
Najafi, M., M. Najafi, and H. Najafi. 2012. Canadian Journal of Chemistry 90: 915–926.
Acknowledgments
The experimental results described in this paper were part of research project No 91077. The authors would like to thank the Vice-Chancellery for research affairs of Kermanshah University of Medical Sciences for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Najafi, H., Yarijani, Z.M. & Najafi, M. Theoretical and Experimental in vivo Study of Antioxidant Activity of Crocin in Order to Propose Novel Derivatives with Higher Antioxidant Activity and Their Delivery via Nanotubes and Nanocones. Inflammation 40, 1794–1802 (2017). https://doi.org/10.1007/s10753-017-0623-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-017-0623-4