Abstract
Autoimmune diseases contain a large number of pathologies characterized by various factors that contribute to a breakdown in self-tolerance. Cytokine-mediated immunity plays an essential role in the pathogenesis of varieties of autoimmune diseases. Recent studies reveal that interleukin-35 (IL-35), a newly identified cytokine of IL-12 family, is implicated in the pathogenesis of autoimmune diseases, such as systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), systemic sclerosis (SSc), etc. In this review, we will discuss the biological features of IL-35 and summarize recent advances in the role of IL-35 in the development and pathogenesis of autoimmune diseases; the discoveries gained from these findings might translate into future therapies for these diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Autoimmune diseases are characterized by various factors that contribute to a breakdown in self-tolerance, that is, the ability of the immune system to effectively distinguish self from non-self and to refrain from attacking self. Autoimmune diseases include a broad spectrum of disorders, such as systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), systemic sclerosis (SSc), inflammatory bowel disease (IBD), idiopathic thrombocytopenic purpura (ITP), etc. Although significant progresses have been achieved in the development of approaches to the treatment of autoimmune diseases, the etiologies and pathogenesis of autoimmune diseases remain obscure. Numerous studies have revealed that some cytokines exert immunosuppressive roles in the development and pathogenesis of autoimmune diseases, such as IL-10 [19, 30, 56], TGF-β [36, 68], etc. Recently, the imbalance between Treg cells and Th17 cells has been considered as a new paradigm in the pathogenesis of autoimmune diseases, including SLE [55, 69], RA [41, 58], SSc [3], IBD [24, 35], ITP [66, 67], etc. As a newly identified cytokine of IL-12 family, IL-35 facilitates the propagation and optimally suppressive function of Treg cells [6, 9] and restricts the differentiation and function of Th17 cells [39, 59, 60]. These evidences illustrated that IL-35 is vitally associated with autoimmune diseases, and IL-35 may exert an effective immunosuppressive role in autoimmune diseases. In this review, we will discuss the biological features of IL-35 and summarize recent advances in the role of IL-35 in the development and pathogenesis of autoimmune diseases; the discoveries gained from these findings might translate into future therapies for these diseases.
IL-35 AND ITS IMMUNOLOGICAL FUNCTIONS
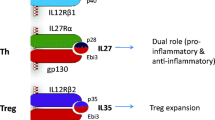
IL-35 is a newly identified heterodimeric cytokine belonging to the IL-12 family, which contains IL-12, IL-23, IL-27, and IL-35. All the four members are consisted of an α chain (p19, p28, or p35) and a β chain (p40 or Epstein-Barr virus induced gene 3 (EBI3)). For instance, IL-12 consists of p35 and p40 subunits, while p19 combines with p40 forming IL-23 and p28 combines with EBI3 forming IL-27. The latest recognized member, IL-35, composes of p35 and EBI3 [8, 40]. Similarly, the receptors of IL-12 family also possess the chain-sharing feature [11, 18]. The IL-12Rβ1 subunit couples with IL-12Rβ2 structuring the receptors for IL-12 and binds IL-23R together forming the receptors for IL-23. IL-27 and IL-35 share the gp130 subunit to structure their own receptors, gp130 and IL-27Rα structure, the receptor for IL-27, and that for IL-35 is gp130 and IL-12Rβ2 [7, 8, 40]. In addition, IL-35 can also signal through IL-12 Rβ2-IL-12 Rβ2 and gp130-gp130 homodimers [7]. It is notable that all the receptors of IL-35 enable suppression the proliferations of T cells, but the homodimers are incapable of mediating the generation of IL-35 induced regulatory T cells (iTr35) cells compared with heterodimeric one [7].
Despite the structural similarities with each other, the cytokines of IL-12 family have different cellular sources and immunological functions. IL-12, IL-23, and IL-27 are secreted by activated antigen-presenting cells (dendritic cells, monocyte/macrophages, B cells) in reaction to bacteria, intracellular parasites, etc. [16, 32, 42]. Both IL-12 and IL-23 primarily act as proinflammatory cytokines, respectively, promoting Th1 and Th17 cells development [16, 23, 25]. IL-12 promotes T and NK cells secreting interferon (IFN)-γ, thus enhancing the differentiation of Th1 cells and the cytotoxicity of cytotoxic T cells (CTL) and inhibiting the propagation of Th2 cells [23]. IL-23 mainly links to the development of pathogenic IL-17-producing Th17 cells, which further promotes the production of various proinflammatory cytokines such as tumor necrosis factor (TNF)-α, IL-6, etc. [25]. IL-27 possesses dual functions of proinflammatory and anti-inflammatory due to its roles in promoting Th1 cells propagation and IFN-γ production and restraining Th17 cells differentiation [44, 63, 64].
Unlike other IL-12 family members, IL-35 was reported to be secreted by regulatory T cells (Tregs) [6, 9], activated B cells [53], etc. Recently, the imbalance between Th17 cells and Treg cells has been considered as a new paradigm in the pathogenesis of autoimmune diseases, including SLE [55, 69], RA [41, 58], SSc [3], IBD [24, 35], ITP [66, 67], etc. IL-35 plays an essential role in promoting the optimally suppressive function of Treg cells in vitro and controlling homeostatic proliferation in vivo [9]. IL-35 could induce naive human or mouse T cells to differentiate into regulatory T cells (iTr35 cells), which maintain immunological self-tolerance exclusively via IL-35 rather than TGF-β or IL-10 [6]. Subsequently, IL-35 and iTr35 cells form a positive feedback cycle: IL-35 could elicit the propagation of iTr35 cells, and more iTr35 cells further secrete more IL-35 [6]. Massive studies supported that IL-35 could suppress Th17 differentiation and function, rather than TGF-β or IL-10 [39, 59, 60]. Therefore, IL-35 may exert an essential role in the balance between Th17 cells and Treg cells (Fig. 1). In addition, recent studies implied that IL-35-secreting B cells play a crucial role in the suppressive regulation of immunity, and IL-35 facilitates the differentiation of human B cells into Breg cells which secrete IL-35 and IL-10 [13, 33, 53, 57]. All these evidences indicate that IL-35 orchestrates a significant regulation in immune responses.
IL-35 plays an essential role in the balance between Th17 cells and Treg cells. IL-35 could induce naive human or mouse T cells to differentiate into regulatory T cells(iTr35 cells), and more iTr35 cells further secrete more IL-35, forming a positive feedback cycle. In addition, IL-35 could suppress the differentiation and function of Th17 cells. Therefore, IL-35 may exert an indispensable role in the balance between Th17 cells and Treg cells.
IL-35 IN AUTOIMMUNE DISEASES
Autoimmune diseases are characterized by the destruction, and the impaired function of tissues that are caused by an immune response in which abnormal antibodies are produced and attacking the body’s own cells and tissues. There is a wide spectrum of autoimmune diseases such as SLE, RA, SSc, IBD, and ITP. These diseases involve various molecules, cells, and tissues, which are targeted by the autoimmune responses [45]. Although the critical details of the etiologies of these diseases are not yet fully understood and the clinical features of these diseases are not equal with each other, current findings have revealed that IL-35 is implicated in the development and pathogenesis of autoimmune diseases, including SLE [5], RA [20, 38, 39], SSc [10], IBD [14, 28, 60], and ITP [62] (Table 1).
Systemic Lupus Erythematosus
Systemic lupus erythematosus (SLE) is a prototypic autoimmune disease that is characterized by the breakdown of immune tolerance of B and T cells to body’s own antigens and dysfunctions of multiple major organs, such as the kidney, skin, and blood vessels [37]. The etiology of SLE remains incompletely understood; it is generally thought to be caused by a combination of genetic and environmental factors [22]. Female MRL/lpr mice spontaneously develop severe autoimmune disease closely resembling human SLE and have been widely used as an experimental murine model of SLE [5, 12, 49, 54]. Recently, Cai et al. [5] demonstrated that compared with controls (phosphate-buffered saline (PBS)-treated MRL/lpr mice), IL-35-treated MRL/lpr mice got significant remissions of histopathology characteristics of lupus flare and nephritis. The plasma concentrations of proinflammatory cytokines (IFN-γ, TNF- α, IL-6, and IL-17A) were significantly decreased with IL-35 treatment, and the plasma concentrations of anti-inflammatory cytokines (IL-10 and IL-2) were significantly increased upon IL-35 treatment [5]. The messenger RNA (mRNA) expressions of Treg-regulated FoxP3, IL-35 subunit (p35 and EBI3), and soluble IL-35 receptor subunit (gp130 and IL-12Rβ2) of splenic and thymic cells in IL-35-treated MRL/lpr mice were significantly higher than that in PBS-treated MRL/lpr mice [5]. The Treg- related FoxP3 gene expression in IL-35-treated MRL/lpr mice was enhanced compared with that of PBS-treated MRL/lpr mice [5]. These in vivo results imply that IL-35 exerts an essentially immune regulatory role in SLE and provides a biochemical basis that IL-35 may act as an efficient therapeutic strategy for SLE.
Rheumatoid Arthritis
Rheumatoid arthritis (RA) is a chronic joint inflammatory disease with abnormal synovial hyperplasia and progressive destruction of cartilage and bone [34]. Although both clinical and basic scientific researches have attempted to determine the factors involved in the pathogenesis of this disease, the exact cause of RA is still unclear. Niedbala et al. [39] has illustrated that IL-35, in vitro, promotes the propagation of CD4+CD25+ T cells along with remarkable elevation of IL-10 levels and markedly restrains the differentiation of Th17 cells. In vivo, IL-35 significantly attenuated the synovial hyperplasia, cartilage, and bone erosion of collagen-induced arthritis (CIA) mice [39]. Compared with PBS-treated control mice, the frequency of IL-17-secreting spleen cells of IL-35-treated mice were significantly lower, whereas the serum levels of IL-10 in IL-35-treated mice were significantly higher [39]. Kochetkova et al. [20] verified that IL-35 markedly reduced the incidence of clinical symptoms of arthritis and attenuated the severity of synovial hyperplasia and bony destruction of CIA mice. Upon IL-35 treatment, the levels of IFN-γ and IL-17 in protected CIA mice were reduced by 4- and 5-fold, respectively. Recently, Nakano et al. [38] illustrated that compared with normal controls, the serum IL-35 levels of RA patients were significantly reduced. Moreover, serum IL-35 levels were significantly higher in patients with inactive RA vs active RA. There was a significant inverse correlation between serum IL-35 levels and the 28-joint DAS based on CRP (DAS28-CRP) in RA patients. Furthermore, recombinant IL-35 facilitated the function of natural Treg in vitro and restrained proinflammatory cytokines such as IL-17 and IFN-γ [38]. These findings suggest that IL-35 might suppress T cell activation during the peripheral immune responses of RA and thereby exert a potential role in the treatment of RA.
Systemic Sclerosis
Systemic sclerosis (SSc) is an autoimmune connective tissue disease characterized by a complex interaction of vascular injury, immune system activation, and fibrosis. Of the autoimmune connective tissue diseases, SSc has the highest case specific mortality rate, with half of patients dying from pulmonary or cardiac causes [4, 50]. SSc is divided into two subtypes, limited cutaneous SSc (lcSSc) and diffuse cutaneous SSc (dcSSc), based on the extent of skin involvement [26]. Currently, the etiology of SSc remains incompletely understood. Giovannetti et al. [15] revealed that increased Treg cell frequency in SSc patients was correlated with clinical phenotypes and clinical progression parameters. Moreover, elevated Treg frequency was also associated with interstitial lung disease and low carbon monoxide diffusing capacity in SSc patients [17]. Recently, Dantas et al. [10] reported that serum IL-35 levels were significantly higher in SSc patients, but there was no significant difference in serum IL-35 levels between lcSSc and dcSSc patients. Furthermore, IL-35 levels were higher in SSc patients with lung fibrosis than those without fibrosis [10]. Considering that Treg cells are the main source of IL-35 [6, 9], these results imply that IL-35 may play an indispensable role in the pathogenesis of SSc. However, further studies are required to clarify its potential utility as a serological biomarker and a new therapeutic target for the disease.
Inflammatory Bowel Diseases
Inflammatory bowel diseases (IBDs), including ulcerative colitis (UC) and Crohn’s disease (CD), that are chronic inflammatory disorders of unknown etiology, various genetic, environmental and immunologic factors have been proposed [47, 61]. Wirtz et al. [60] reported that compared with IL-27p28−/− (lacking IL-27 only) mice or control mice, EBI3−/− (lacking both IL-27 and IL-35) mice were more susceptible to spontaneous colitis, and their survival times were markedly shorter. In IL-35-treated experimental colitis mice, the mucosal levels of IFN-γ, IL-6, IL-17, and messenger RNA expression of T-bet and ROR-γt (transcription factors of Th1 and Th17, respectively) were dramatically restrained [60]. Li et al. [28] illustrated that compared with healthy controls, the serum levels of IL-35 were significantly declined in active UC patients and active CD patients. Moreover, there was a negative correlation between serum IL-35 levels and Mayo score in UC patients. Photomicrographs of immunostaining for IL-35 showed that IL-35 was expressed in infiltrating immune cells from UC patients and CD patients, whereas normal colon tissue from healthy controls had no IL-35 production [28]. There was a significant overexpression of Ebi3 and p35 mRNA in endoscopic specimens in both UC patients and CD patients. Additionally, a significant increase of IL-35 was also observed in inflamed mucosa of UC and CD patients [28]. Fonseca-Camarillo et al. [14] reported that the levels of IL-35 (EBI3) mRNA of colonic mucosa in active UC patients were significantly higher compared with inactive UC patients. And the percentage of CD20+/IL-35+ B cells was higher in active CD patients compared with non-inflamed colonic tissue [14]. These evidences from both animal models and human studies give a new insight that IL-35 might exert an essential role in the pathogenesis and development of IBD.
Idiopathic Thrombocytopenic Purpura
Idiopathic thrombocytopenic purpura (ITP) is an autoimmune disease characterized by bleeding disorder. Its pathogenesis involves both abnormal platelet destruction and impaired platelet propagation. Previous studies revealed that autoantibodies and cytotoxic T cells are implicated in the pathogenesis of ITP [43, 52]. Recent studies illuminated that inefficient production and functional defect of Treg cells were connected with the breakdown of immunologic tolerance in ITP patients [2, 31, 51, 65]. As a key immunosuppressive cytokine of Treg cells, the plasma IL-35 levels displayed a significantly positive correlation with platelet counts in active ITP patients, whereas the plasma levels of other immunosuppressive cytokines produced by Treg cells, such as TGF-β and IL-10, had no correlation with platelet counts in ITP patients [62]. Furthermore, compared with inactive ITP patients or healthy controls, the plasma levels of IL-35 in active ITP patients were significantly reduced. Similarly, the mRNA expression levels of P35 of peripheral blood mononuclear cells (PBMCs) in active ITP patients were reduced compared with inactive ITP patients or healthy controls [62]. Decreased IL-35 levels and its associations with platelet counts in ITP patients indicate that IL-35 may be involved in the development and pathogenesis of ITP.
IL-35 SERVES AS A POTENTIAL THERAPEUTIC AGENT FOR AUTOIMMUNE DISEASES
Due to its immunosuppressive roles in inflammation and autoimmunity, IL-35 may have promise as a potential therapeutic agent for autoimmune diseases. Understanding the specific mechanisms of IL-35 in autoimmune diseases, together with the knowledge on the capacity of current treatment strategy to target this process, may open a door to novel therapeutic options for autoimmune diseases. In fact, studies in animal models of several autoimmune diseases have yielded encouraging results. Upon IL-35 treatment, significant remissions of histopathology characteristics of lupus flare and nephritis were observed in MRL/lpr mice [5]; alleviations in synovial hyperplasia, cartilage and bone erosion, and fibroblast-like synoviocytes growth were seen in CIA mice [29, 39]; and mucosal levels of proinflammatory cytokines in colitis mice, such as IFN-γ, IL-6, and IL-17 were significantly decreased [60]. These evidences illuminated that IL-35 may serve as a promising therapeutic agent for autoimmune diseases.
THE PROSPECTIVE AND THE LIMITATION OF IL-35 IN CLINICAL APPLICATION
Since its suppressive activity in autoimmune diseases has been established in numerous reports in vitro and in vivo, IL-35 may act as a therapeutic agent for these diseases in clinical application. More recently, studies have explored the application of IL-35 in human and animal models. Kochetkova et al. reported that oral Escherichia coli colonization factor antigen I fimbriae can stimulate IL-35 and attenuate the inflammation and joint destruction in CIA mice by suppressing Th1 and Th17 cell responses [21]. Mesenchymal stem cells (MSCs) that can differentiate into cells of different lineages and possess the potent function of immune regulatory have recently emerged as promising cellular vehicles for potential clinical applications [46, 48]. In a study by Amari et al., human Wharton’s jelly-derived mesenchymal stem cells (hWJ-MSCs) were isolated and transduced with lentiviral particles harboring murine IL-35; the cells successfully secreted a high level of murine IL-35, which managed to inhibit CD4+ T cell proliferation, and enhanced the frequency of Treg cells [1]. Li et al. reported that adenovirus-mediated delivery of IL-35 gene can alleviate allergic airway inflammation in experimental asthma, and the adenovirus expressing IL-35 elevated the numbers of CD4+CD25+Foxp3+ Treg cells [27]. These results suggest that overexpressing IL-35 provides a useful approach for basic research on therapy for autoimmune disorders, which may be translated into clinical application.
Despite emerging evidence that IL-35 exerts a remarkable role in autoimmune diseases and the application of IL-35 in these diseases has already made significant progress, it is still too early to ensure the efficacy of this cytokine in clinical application. Most studies were based on animal experiments, and the specific mechanisms of these diseases were not involved, which may not be applicable to humans. Furthermore, the clinical features of autoimmune diseases are not equal with each other, and the causes of these diseases are miscellaneous, only as these take all the possible influencing factors into consideration, and can IL-35 exert as an efficient and safe therapeutic agent for autoimmune diseases.
CONCLUSION
Although much remains to be elucidated concerning the role of IL-35 in the etiology and pathogenesis of autoimmune diseases, a solid basis of data from in vitro and in vivo is now accumulating to support the therapeutic effect of IL-35 in autoimmune diseases. IL-35 promotes the propagation and suppressive function of Treg cells and restricts the differentiation and function of Th17 cells; IL-35 treatment significantly alleviates the histopathology characteristics and the plasma concentrations of proinflammatory cytokines in SLE model. Serum IL-35 level is inversely associated with disease activity of RA patients. Treg cell frequency is correlated with clinical phenotypes and clinical progression parameters in SSc patients. Plasma levels of IL-35 are negatively correlated with disease activity of IBD patients and positively correlated with platelet counts in active ITP patients. Given the correlations of IL-35 with clinical and histological markers of inflammation, promoter of IL-35 therefore might be useful for attenuating inflammation-related symptoms in autoimmune diseases. However, interventions for promoting IL-35 should be considered not only the advantageous effects but also the risk of potential deleterious consequences for the host. Furthermore, the causes of autoimmune diseases are miscellaneous, and several studies were based on in vitro experiments, which just elucidated typical symptoms of these diseases and the specific mechanisms of those diseases that were not involved. Therefore, further studies, especially in human systems, are required to clearly explore the immunosuppressive role and therapeutic benefits of IL-35 in autoimmune diseases.
References
Amari, A., M. Ebtekar, S.M. Moazzeni, M. Soleimani, L. Mohammadi Amirabad, M.T. Tahoori, and M. Massumi. 2015. In Vitro Generation of IL-35-expressing Human Wharton’s Jelly-derived Mesenchymal Stem Cells Using Lentiviral Vector. Iranian Journal of Allergy, Asthma, and Immunology 14(4): 416–426.
Aslam, R., Y. Hu, S. Gebremeskel, G.B. Segel, E.R. Speck, L. Guo, M. Kim, H. Ni, J. Freedman, and J.W. Semple. 2012. Thymic retention of CD4+CD25+FoxP3+ T regulatory cells is associated with their peripheral deficiency and thrombocytopenia in a murine model of immune thrombocytopenia. Blood 120(10): 2127–2132. doi:10.1182/blood-2012-02-413526.
Baraut, J., L. Michel, F. Verrecchia, and D. Farge. 2010. Relationship between cytokine profiles and clinical outcomes in patients with systemic sclerosis. Autoimmunity Reviews 10(2): 65–73. doi:10.1016/j.autrev.2010.08.003.
Bryan, C., Y. Howard, P. Brennan, C. Black, and A. Silman. 1996. Survival following the onset of scleroderma: results from a retrospective inception cohort study of the UK patient population. British Journal of Rheumatology 35(11): 1122–1126.
Cai, Z., C.K. Wong, J. Dong, M. Chu, D. Jiao, N.W. Kam, C.W. Lam, and L.S. Tam. 2015. Remission of systemic lupus erythematosus disease activity with regulatory cytokine interleukin (IL)-35 in Murphy Roths Large (MRL)/lpr mice. Clinical and Experimental Immunology 181(2): 253–266. doi:10.1111/cei.12639.
Collison, L.W., V. Chaturvedi, A.L. Henderson, P.R. Giacomin, C. Guy, J. Bankoti, D. Finkelstein, et al. 2010. IL-35-mediated induction of a potent regulatory T cell population. Nature Immunology 11(12): 1093–1101. doi:10.1038/ni.1952.
Collison, L.W., G.M. Delgoffe, C.S. Guy, K.M. Vignali, V. Chaturvedi, D. Fairweather, A.R. Satoskar, et al. 2012. The composition and signaling of the IL-35 receptor are unconventional. Nature Immunology 13(3): 290–299. doi:10.1038/ni.2227.
Collison, L.W., and D.A.A. Vignali. 2008. Interleukin-35: odd one out or part of the family? Immunological Reviews 226: 248–262.
Collison, L.W., C.J. Workman, T.T. Kuo, K. Boyd, Y. Wang, K.M. Vignali, R. Cross, D. Sehy, R.S. Blumberg, and D.A. Vignali. 2007. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature 450(7169): 566–569. doi:10.1038/nature06306.
Dantas, A.T., S.M. Goncalves, M.C. Pereira, R.S. Goncalves, C.D. Marques, M.J. Rego, R. Pitta Ida, A.L. Duarte, and M.G. Pitta. 2015. Increased IL-35 serum levels in systemic sclerosis and association with pulmonary interstitial involvement. Clinical Rheumatology 34(9): 1621–1625. doi:10.1007/s10067-015-3006-y.
Delgoffe, G.M., P.J. Murray, and D.A. Vignali. 2011. Interpreting mixed signals: the cell’s cytokine conundrum. Current Opinion in Immunology 23(5): 632–638. doi:10.1016/j.coi.2011.07.013.
Dieker, J., L. Hilbrands, A. Thielen, H. Dijkman, J.H. Berden, and J. van der Vlag. 2015. Enhanced activation of dendritic cells by autologous apoptotic microvesicles in MRL/lpr mice. Arthritis Research and Therapy 17: 103. doi:10.1186/s13075-015-0617-2.
Egwuagu, C.E., C.R. Yu, L. Sun, and R. Wang. 2015. Interleukin 35: Critical regulator of immunity and lymphocyte-mediated diseases. Cytokine and Growth Factor Reviews 26(5): 587–593. doi:10.1016/j.cytogfr.2015.07.013.
Fonseca-Camarillo, G., J. Furuzawa-Carballeda, and J.K. Yamamoto-Furusho. 2015. Interleukin 35 (IL-35) and IL-37: Intestinal and peripheral expression by T and B regulatory cells in patients with Inflammatory Bowel Disease. Cytokine 75(2): 389–402. doi:10.1016/j.cyto.2015.04.009.
Giovannetti, Antonello, Edoardo Rosato, Cristina Renzi, Angela Maselli, Lucrezia Gambardella, Anna Maria Giammarioli, Paolo Palange, et al. 2010. Analyses of T cell phenotype and function reveal an altered T cell homeostasis in systemic sclerosis. Clinical Immunology 137(1): 122–133. doi:10.1016/j.clim.2010.06.004.
Hunter, C.A. 2005. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nature Reviews Immunology 5(7): 521–531. doi:10.1038/nri1648.
Jiang, N., M. Li, and X. Zeng. 2014. Correlation of Th17 cells and CD4(+)CD25(+) regulatory T cells with clinical parameters in patients with systemic sclerosis. Chinese Medical Journal 127(20): 3557–3561.
Jones, L.L., and D.A.A. Vignali. 2011. Molecular interactions within the IL-6/IL-12 cytokine/receptor superfamily. Immunologic Research 51(1): 5–14. doi:10.1007/s12026-011-8209-y.
Kalampokis, I., A. Yoshizaki, and T. F. Tedder. 2013. IL-10-producing regulatory B cells (B10 cells) in autoimmune disease. Arthritis Res Ther 15 Suppl 1:S1. doi:10.1186/ar3907 ar3907
Kochetkova, I., S. Golden, K. Holderness, G. Callis, and D.W. Pascual. 2010. IL-35 stimulation of CD39+ regulatory T cells confers protection against collagen II-induced arthritis via the production of IL-10. Journal of Immunology 184(12): 7144–7153. doi:10.4049/jimmunol.0902739.
Kochetkova, I., T. Thornburg, G. Callis, K. Holderness, M. Maddaloni, and D.W. Pascual. 2014. Oral Escherichia coli colonization factor antigen I fimbriae ameliorate arthritis via IL-35, not IL-27. Journal of Immunology 192(2): 804–816. doi:10.4049/jimmunol.1302018.
Koutsokeras, T., and T. Healy. 2014. Systemic lupus erythematosus and lupus nephritis. Nature Reviews Drug Discovery 13(3): 173–174. doi:10.1038/nrd4227.
Langrish, C.L., B.S. McKenzie, N.J. Wilson, R. de Waal Malefyt, R.A. Kastelein, and D.J. Cua. 2004. IL-12 and IL-23: master regulators of innate and adaptive immunity. Immunology Reviews 202: 96–105. doi:10.1111/j.0105-2896.2004.00214.x.
Lee, S.Y., S.H. Lee, E.J. Yang, E.K. Kim, J.K. Kim, D.Y. Shin, and M.L. Cho. 2015. Metformin Ameliorates Inflammatory Bowel Disease by Suppression of the STAT3 Signaling Pathway and Regulation of the between Th17/Treg Balance. PloS One 10(9), e0135858. doi:10.1371/journal.pone.0135858.
Leng, R.X., H.F. Pan, G.M. Chen, C. Wang, W.Z. Qin, L.L. Chen, J.H. Tao, and D.Q. Ye. 2010. IL-23: a promising therapeutic target for systemic lupus erythematosus. Archives of Medical Research 41(3): 221–225. doi:10.1016/j.arcmed.2010.02.011.
LeRoy, E.C., C. Black, R. Fleischmajer, S. Jablonska, T. Krieg, T.A. Medsger Jr., N. Rowell, and F. Wollheim. 1988. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. Journal of Rheumatology 15(2): 202–205.
Li, Y., X. Pan, X. Peng, S. Li, Y. Zhou, X. Zheng, and M. Li. 2015. Adenovirus-mediated interleukin-35 gene transfer suppresses allergic airway inflammation in a murine model of asthma. Inflammation Research 64(10): 767–774. doi:10.1007/s00011-015-0858-1.
Li, Y., Y. Wang, Y. Liu, X. Zuo, and X. Lu. 2014. The possible role of the novel cytokines il-35 and il-37 in inflammatory bowel disease. Mediators of Inflammation 2014: 136329. doi:10.1155/2014/136329.
Li, Y., S. Wu, S. Jiang, T. Lin, L. Xia, H. Shen, and J. Lu. 2016. Interleukin-35 (IL-35) inhibits proliferation and promotes apoptosis of fibroblast-like synoviocytes isolated from mice with collagen-induced arthritis. Molecular Biology Reports 43(9): 947–956. doi:10.1007/s11033-016-4034-7.
Lim, J.Y., K.I. Im, E.S. Lee, N. Kim, Y.S. Nam, Y.W. Jeon, and S.G. Cho. 2016. Enhanced immunoregulation of mesenchymal stem cells by IL-10-producing type 1 regulatory T cells in collagen-induced arthritis. Science Reports 6: 26851. doi:10.1038/srep26851.
Liu, B., H. Zhao, M.C. Poon, Z. Han, D. Gu, M. Xu, H. Jia, R. Yang, and Z.C. Han. 2007. Abnormality of CD4(+)CD25(+) regulatory T cells in idiopathic thrombocytopenic purpura. European Journal of Haematology 78(2): 139–143. doi:10.1111/j.1600-0609.2006.00780.x.
Ma, X., and G. Trinchieri. 2001. Regulation of interleukin-12 production in antigen-presenting cells. Advances in Immunology 79: 55–92.
Mauri, C., and K. Nistala. 2014. Interleukin-35 takes the ‘B’ line. Nature Medicine 20(6): 580–581. doi:10.1038/nm.3594.
McInnes, I.B., and G. Schett. 2011. The pathogenesis of rheumatoid arthritis. New England Journal of Medicine 365(23): 2205–2219. doi:10.1056/NEJMra1004965.
McNamee, E.N., J.C. Masterson, M. Veny, C.B. Collins, P. Jedlicka, F.R. Byrne, G.Y. Ng, and J. Rivera-Nieves. 2015. Chemokine receptor CCR7 regulates the intestinal TH1/TH17/Treg balance during Crohn’s-like murine ileitis. Journal of Leukocyte Biology 97(6): 1011–1022. doi:10.1189/jlb.3HI0614-303R.
Mirshafiey, A., and M. Mohsenzadegan. 2009. TGF-beta as a promising option in the treatment of multiple sclerosis. Neuropharmacology 56(6–7): 929–936.
Mok, C.C., and C.S. Lau. 2003. Pathogenesis of systemic lupus erythematosus. Journal of Clinical Pathology 56(7): 481–490.
Nakano, S., S. Morimoto, S. Suzuki, H. Tsushima, K. Yamanaka, I. Sekigawa, and Y. Takasaki. 2015. Immunoregulatory role of IL-35 in T cells of patients with rheumatoid arthritis. Rheumatology (Oxford) 54(8): 1498–1506. doi:10.1093/rheumatology/keu528.
Niedbala, W., X.Q. Wei, B. Cai, A.J. Hueber, B.P. Leung, I.B. McInnes, and F.Y. Liew. 2007. IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells. European Journal of Immunology 37(11): 3021–3029. doi:10.1002/eji.200737810.
Ning-Wei, Z. 2010. Interleukin (IL)-35 is raising our expectations. Revista Medica De Chile 138(6): 758–766.
Niu, Q., B. Cai, Z.C. Huang, Y.Y. Shi, and L.L. Wang. 2012. Disturbed Th17/Treg balance in patients with rheumatoid arthritis. Rheumatology International 32(9): 2731–2736. doi:10.1007/s00296-011-1984-x.
O’Shea, J.J., and W.E. Paul. 2002. Regulation of T(H)1 differentiation—controlling the controllers. Nature Immunology 3(6): 506–508. doi:10.1038/ni0602-506.
Olsson, B., P.O. Andersson, M. Jernas, S. Jacobsson, B. Carlsson, L.M. Carlsson, and H. Wadenvik. 2003. T-cell-mediated cytotoxicity toward platelets in chronic idiopathic thrombocytopenic purpura. Nature Medicine 9(9): 1123–1124. doi:10.1038/nm921.
Pan, H.F., J.H. Tao, and D.Q. Ye. 2010. Therapeutic potential of IL-27 in systemic lupus erythematosus. Expert Opinion on Therapeutic Targets 14(5): 479–484. doi:10.1517/14728221003769911.
Pan, Hai-Feng, Xiang-Pei Li, Song Guo Zheng, and Dong-Qing Ye. 2013. Emerging role of interleukin-22 in autoimmune diseases. Cytokine & Growth Factor Reviews 24(1): 51–57. doi:10.1016/j.cytogfr.2012.07.002.
Pittenger, M.F., A.M. Mackay, S.C. Beck, R.K. Jaiswal, R. Douglas, J.D. Mosca, M.A. Moorman, D.W. Simonetti, S. Craig, and D.R. Marshak. 1999. Multilineage potential of adult human mesenchymal stem cells. Science 284(5411): 143–147.
Podolsky, D.K. 2002. Inflammatory bowel disease. New England Journal of Medicine 347(6): 417–429. doi:10.1056/NEJMra020831.
Rasmusson, I., O. Ringden, B. Sundberg, and K. Le Blanc. 2003. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation 76(8): 1208–1213. doi:10.1097/01.TP.0000082540.43730.80.
Renner, K., F.J. Hermann, K. Schmidbauer, Y. Talke, M. Rodriguez Gomez, G. Schiechl, J. Schlossmann, H. Bruhl, H.J. Anders, and M. Mack. 2015. IL-3 contributes to development of lupus nephritis in MRL/lpr mice. Kidney International 88(5): 1088–1098. doi:10.1038/ki.2015.196.
Rubio-Rivas, M., C. Royo, C.P. Simeon, X. Corbella, and V. Fonollosa. 2014. Mortality and survival in systemic sclerosis: systematic review and meta-analysis. Seminars in Arthritis and Rheumatism 44(2): 208–219. doi:10.1016/j.semarthrit.2014.05.010.
Sakakura, M., H. Wada, I. Tawara, T. Nobori, T. Sugiyama, N. Sagawa, and H. Shiku. 2007. Reduced Cd4+Cd25+ T cells in patients with idiopathic thrombocytopenic purpura. Thrombosis Research 120(2): 187–193. doi:10.1016/j.thromres.2006.09.008.
Semple, J.W. 2002. Immune pathophysiology of autoimmune thrombocytopenic purpura. Blood Reviews 16(1): 9–12. doi:10.1054/blre.2001.0172.
Shen, P., T. Roch, V. Lampropoulou, R.A. O’Connor, U. Stervbo, E. Hilgenberg, S. Ries, et al. 2014. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature 507(7492): 366–370. doi:10.1038/nature12979.
Shimomatsu, T., N. Kanazawa, N. Mikita, Y. Nakatani, H. J. Li, Y. Inaba, T. Ikeda, T. Kondo, and F. Furukawa. 2016. The effect of hydroxychloroquine on lupus erythematosus-like skin lesions in MRL/lpr mice. Modern Rheumatology:1–5. doi:10.3109/14397595.2016.1140711
Talaat, R.M., S.F. Mohamed, I.H. Bassyouni, and A.A. Raouf. 2015. Th1/Th2/Th17/Treg cytokine imbalance in systemic lupus erythematosus (SLE) patients: Correlation with disease activity. Cytokine 72(2): 146–153. doi:10.1016/j.cyto.2014.12.027.
Toriani-Terenzi, C., and E. Fagiolo. 2005. IL-10 and the cytokine network in the pathogenesis of human autoimmune hemolytic anemia. Annals of the New York Academy of Sciences 1051: 29–44. doi:10.1196/annals.1361.044.
Wang, R.X., C.R. Yu, I.M. Dambuza, R.M. Mahdi, M.B. Dolinska, Y.V. Sergeev, P.T. Wingfield, S.H. Kim, and C.E. Egwuagu. 2014. Interleukin-35 induces regulatory B cells that suppress autoimmune disease. Nature Medicine 20(6): 633–641. doi:10.1038/nm.3554.
Wang, W., S. Shao, Z. Jiao, M. Guo, H. Xu, and S. Wang. 2012. The Th17/Treg imbalance and cytokine environment in peripheral blood of patients with rheumatoid arthritis. Rheumatology International 32(4): 887–893. doi:10.1007/s00296-010-1710-0.
Whitehead, G.S., R.H. Wilson, K. Nakano, L.H. Burch, H. Nakano, and D.N. Cook. 2012. IL-35 production by inducible costimulator (ICOS)-positive regulatory T cells reverses established IL-17-dependent allergic airways disease. The Journal of Allergy and Clinical Immunology 129(1): 207–215. doi:10.1016/j.jaci.2011.08.009. e201-205.
Wirtz, S., U. Billmeier, T. McHedlidze, R.S. Blumberg, and M.F. Neurath. 2011. Interleukin-35 mediates mucosal immune responses that protect against T-cell-dependent colitis. Gastroenterology 141(5): 1875–1886. doi:10.1053/j.gastro.2011.07.040.
Xavier, R.J., D.K. Podolsk, and D.K. Podolsky. 2007. Unravelling the pathogenesis of inflammatory bowel disease. Nature 448(7152): 427–434. doi:10.1038/nature06005.
Yang, Y., M. Xuan, X. Zhang, D. Zhang, R. Fu, F. Zhou, L. Ma, et al. 2014. Decreased IL-35 levels in patients with immune thrombocytopenia. Human Immunology 75(8): 909–913. doi:10.1016/j.humimm.2014.06.019.
Yoshida, H., and Y. Miyazaki. 2008. Regulation of immune responses by interleukin-27. Immunology Reviews 226: 234–247. doi:10.1111/j.1600-065X.2008.00710.x.
Yoshimura, T., A. Takeda, S. Hamano, Y. Miyazaki, I. Kinjyo, T. Ishibashi, A. Yoshimura, and H. Yoshida. 2006. Two-sided roles of IL-27: induction of Th1 differentiation on naive CD4+ T cells versus suppression of proinflammatory cytokine production including IL-23-induced IL-17 on activated CD4+ T cells partially through STAT3-dependent mechanism. Journal of Immunology 177(8): 5377–5385.
Yu, J., S. Heck, V. Patel, J. Levan, Y. Yu, J.B. Bussel, and K. Yazdanbakhsh. 2008. Defective circulating CD25 regulatory T cells in patients with chronic immune thrombocytopenic purpura. Blood 112(4): 1325–1328. doi:10.1182/blood-2008-01-135335.
Yu, S., C. Liu, L. Li, T. Tian, M. Wang, Y. Hu, C. Yuan, L. Zhang, C. Ji, and D. Ma. 2015. Inactivation of Notch signaling reverses the Th17/Treg imbalance in cells from patients with immune thrombocytopenia. Laboratory Investigation 95(2): 157–167. doi:10.1038/labinvest.2014.142.
Zhou, L., F. Xu, C. Chang, Y. Tao, L. Song, and X. Li. 2016. Interleukin-17-producing CD4+ T lymphocytes are increased in patients with primary immune thrombocytopenia. Blood Coagulation and Fibrinolysis 27(3): 301–307. doi:10.1097/MBC.0000000000000423.
Zhou, X., N. Kong, H. Zou, D. Brand, X. Li, Z. Liu, and S.G. Zheng. 2011. Therapeutic potential of TGF-beta-induced CD4(+) Foxp3(+) regulatory T cells in autoimmune diseases. Autoimmunity 44(1): 43–50. doi:10.3109/08916931003782163.
Zhu, M., H. Mo, D. Li, X. Luo, and L. Zhang. 2013. Th17/Treg imbalance induced by increased incidence of atherosclerosis in patients with systemic lupus erythematosus (SLE). Clinical Rheumatology 32(7): 1045–1052. doi:10.1007/s10067-013-2237-z.
Acknowledgment
This work was supported by grants from the National Natural Science Foundation of China (81573222).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Guan, SY., Leng, RX., Khan, M.I. et al. Interleukin-35: a Potential Therapeutic Agent for Autoimmune Diseases. Inflammation 40, 303–310 (2017). https://doi.org/10.1007/s10753-016-0453-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-016-0453-9