Abstract
Cannabinoid receptor type 2 (CB2) agonists display potential analgesic effects in acute and neuropathic pain. However, its complex cellular and molecular mechanisms in bone cancer pain remain unclear. And less relevant reports concerned its time-dependent effects on the long-lasting modifications of behavior, spinal inflammatory cytokines levels, astrocytes activity induced by bone cancer pain. A rat model of bone cancer pain induced by intra-tibia inoculation of Walker 256 mammary gland carcinoma cells was utilized. Pain behaviors at different time points were assessed by ambulatory pain scores and paw withdrawal mechanical threshold (PWMT). Pro-inflammatory cytokines, such as interleukin (IL)-1β, IL-6, IL-18, and tumor necrosis factor alpha (TNF-α), were quantitated by Western blots. Glial activity was assessed by immunohistochemistry. Intra-tibia inoculation of Walker 256 mammary gland carcinoma cells induced progressive bone cancer pain; a long-term up-regulation of IL-1β, IL-6, IL-18, and TNF-α; and the activation of glia in spinal cord. Activation of microglia was first evident on day 4 after surgery and reached to a peak on day 7 while activation of astrocytes was on day 10. A single intrathecal injection of JWH-015 attenuated bone cancer induced spontaneous pain and mechanical allodynia, reduced the expression of pro-inflammatory cytokines, and inhibited the activity of astrocytes. All the modifications were transient and peaked at 24 h after JWH-015 administration. Furthermore, the protective effects of JWH-015 were reversed in the presence of CB2-selective antagonist AM630. Overall, our results provided evidences for the persistent participation of inflammation reaction in the progression of bone cancer pain, and demonstrated that JWH-015 reduced the expression of IL-1β, IL-6, IL-18, and TNF-α and inhibited astrocytes activation in a time-dependent manner, thereby displaying an analgesic effect.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Every year in the USA, nearly 200,000 women are diagnosed with breast cancer [1]. Breast cancer typically metastasizes to bone [2], and one of the most common and devastating complications in cancer patients with bone metastases is bone pain [3, 4]. However, the currently available therapeutics for chronic pain is ineffective or display limited efficacy due to several unwanted side effects (somnolence, analgesic tolerance, etc.) [5]. In the last 30 years, palliative care and therapeutics approved for use in the advanced stages of breast cancer have not improved. Therefore, alternative therapies must be developed to improve the quality of life of cancer patients.
Recent studies suggest a protective role of the cannabinoid signaling system in acute and chronic pain [6, 7]. To date, CB1 and CB2 receptors—two cannabinoid receptors—have been identified. CB1 receptors are highly expressed in the central nervous system (CNS) [8–11]. CB2 receptors were originally believed to be located on immune cells [12]; recently, CB2 receptors were identified in the dorsal root ganglia, spinal cord, and supraspinal structures [13–17]. The expressions of these two cannabinoid receptors may be found along pain pathways [6, 18]. CB1 receptors agonists produce antinociceptive effects accompanied with psychotropic side effects, whereas CB2 receptors agonists have not been reported to be psychotropic. Given that the underlying molecular mechanisms have not been fully elucidated, enthusiasm for treatment with CB2 receptors agonists has been tempered. Several studies have demonstrated that the endocannabinoid signaling system mediated the neurotransmission and neuroinflammation in nervous system [19, 20]. In particular, CB2 receptors expressed in microglial regulated the immunomodulator production [21, 22]. Emerging evidence indicates that glia and glia-derived pro-inflammatory cytokines, such as IL-1β, IL-6, and TNF-α, in the spinal cord play a fatal role in the pathogenesis of neuropathic pain [23, 24]. In our previous studies, we noted that the astrocytes and microglia in the spinal dorsal horn were activated after spinal cord injury [25, 26]. Intrathecal injection of JWH-015 could attenuate remifentanil-induced postoperative hyperalgesia via inhibiting the activation of spinal glia in a rat model [26]. Recently, the pro-inflammatory cytokine IL-18 appears to be a potent player in the immunological regulatory pathways of cytotoxicity as well as neuroinflammatory and neurodegenerative processes [27–30]. In addition, increased IL-18R in astrocytes, but not neurons or microglia in the spinal cord, are involved in the mechanisms underlying nerve injury [31].
Therefore, the present study aimed to evaluate the time-dependent effects of CB2-selective agonist on the long-lasting modifications of behavior, spinal inflammatory cytokines levels, and astrocytes activity in bone cancer pain model, and provide a potential therapeutic alternative for bone cancer pain.
MATERIAL AND METHODS
All procedures were approved by the Medical College of Nanjing University Animal Care and Use Committee (Nanjing, China) and in agreement with the ethical guidelines of the National Institutes of Health and the International Association for the Study of Pain [32].
Animals
The experiments were performed on female Sprague Dawley rats (60–80 g body weight for ascites passaging; 160–180 g body weight for surgery). All of the animals were obtained from the Laboratory Animal Center of Drum Tower Hospital and maintained in a temperature-controlled (21 ± 1 °C) room under a controlled 12-h light/dark cycle with lights on at 6:00 a.m. Rats were housed four per cage. Food and water were freely available in the vivarium. Rats (160–180 g) were randomly divided into four groups according to the following experimental conditions: [1] sham-operated group, [2] tumor-bearing group with intrathecal JWH-015 injection, [3] tumor-bearing group with intrathecal AM630 and JWH-015 injection, and [4] tumor-bearing group with intrathecal dimethyl sulfoxide (DMSO) injection.
Preparation of Walker 256 Rat Mammary Gland Carcinoma Cells
The ascetic cancer cells (0.5 ml; 2 × 107 cells/ml; Shanghai Research Center of Biomedical Engineering, China) were injected into the Sprague Dawley rats (60–80 g) by intraperitoneal injection. Then, 6–7 days later, the ascitic fluid was extracted. The cancer cells were isolated by centrifugation at 1500 rpm for 3 min. The precipitate was thrice washed with 10 ml normal saline and re-centrifuged at 1500 rpm. Using a hemocytometer, the final precipitate was diluted with normal saline to achieve a cell density of 1 × 105/μl. Before the cell suspension was injected into rats, it was maintained on ice [33].
Bone Cancer Pain Model
All of the animals were briefly anesthetized with pentobarbital sodium (50 mg/kg, i.p.). Rats were placed with their abdominal sides up. Then, the left leg of the rat was shaved, and the skin was disinfected with 10 % povidone–iodine solution. A superficial incision was made in skin overlying the lower third of the left tibia, and the muscles were separated to expose the tibia. A 23-gauge needle was used to perforate the bone cortex, and 5 μl Walker 256 rat mammary gland carcinoma cells (1 × 105/μl) were injected into the left tibia cavity using a 25-μl microsyringe. Sham-operated animals received 5 μl of normal saline treatment. The microsyringe was kept in place for 2 min to prevent the cells from spilling from the injection track. Afterward, the injection site was sealed with bone wax followed by copious irrigation with normal saline, and the wound was closed in layers with 3–0 silk thread (Ethicon, USA) [33, 34]. No significant motor impairment after implantation was demonstrated in this study.
Drug Treatments
JWH-015 ((2-methyl-1-propyl-1H-indol-3-yl)-1-naphtha-lenylmethanone) and AM630 (6-iodo-2-methyl-1-(2-(4-morpholinyl) ethyl)-1H-indol-3-yl) were purchased from Sigma, USA. Then, JWH-015 and AM630 were dissolved in DMSO to achieve 10 μg/10 μl and 15 μg/10 μl solution separately. On the tenth day after inoculation of the tibia with Walker 256 rat mammary gland carcinoma cells, animals received intrathecal administration of JWH-015 [35]. AM630 was injected 30 min before JWH-015. Control groups included only vehicle-treated animals.
Analysis of Bone Cancer Pain
Before every test, rats were allowed to acclimatize for 30 min. Room humidity and temperature remained stable for all experiments. Animals were tested for spontaneous pain and mechanical allodynia in a blinded fashion. Spontaneous pain and mechanical allodynia behaviors were measured 1 day before surgery (baseline); 4, 7, and 10 days following surgery; and 2, 6, 24, 48, and 72 h after a single dose of drug.
Analysis of Ambulatory Pain
The rats were placed in individual transparent boxes (50 × 50 × 40 cm) and allowed to walk across the box freely. The limping behavior of the ipsilateral limb was observed for 2 min and compared with the contralateral limb. Ambulatory pain scores were recorded using the following scale: 0 = normal, 1 = slight limping, 2 = extent between 1 and 3, 3 = marked limping, and 4 = avoidance of use of limb.
Mechanical Allodynia Test
Mechanical allodynia was assessed by Von Frey filaments (Stoelting, Wood Dale, IL) using the Chaplan up-down method [36]. The withdrawal threshold of the paw ipsilateral to the site of tumor or normal saline inoculation was exclusively measured. Filaments with values ranging from 2 to 15 g were applied perpendicularly to plantar surface of the hind paw for 6–8 s until the filament bent. A cut-off point was set at a filament bending force of 15 g to prevent tissue damage. A positive response meant brisk withdrawal or paw lifting. This analysis was performed five times per filament with an interval of at least 5 min between each test. The lowest force leading to three or more positive responses was defined as the paw withdrawal mechanical threshold (PWMT).
Western Blotting
After deep anesthesia, the lumbosacral enlargement of the spinal cord was quickly removed and stored in liquid nitrogen for further study. Tissue samples were extracted by (KeyGen Biotech, Nanjing, China) homogenization in ice-cold lyses buffer (0.1 % sodium lauryl sulfate, 0.5 % sodium deoxycholate, 1 % Nonidet P-40 in phosphate buffered saline) with freshly added protease inhibitors (Roche Diagnostics, Shanghai, China). The samples were then incubated on ice for 30 min and centrifuged at 13,000 rpm for 20 min. The supernatants of each sample were collected. The concentration of protein was determined using BCA Protein Assay Kit. Samples with equal amounts of proteins were separated by SDS-PAGE (8 %). Proteins were subsequently transferred to polyvinylidene fluoride membranes (Carlsbad, CA) and then blocked with 5 % skim milk in phosphate buffered saline (PBS) with 0.1 % Tween 20 for 2 h at room temperature. After blocking, the membranes were incubated overnight at 4 °C with primary antibodies for IL-18 (1: 800; Santa Cruz; sc-7954; USA), IL-6 (1:1000, Abcam; ab6672; USA), IL-1β (1:1000; Abcam; ab9722; USA), TNF-α (1: 800; Abcam; ab66579; USA), and β-actin (1: 4000; Abcam; ab52614; USA). On the following day, the membranes were washed with PBST buffer and incubated with horseradish peroxidase-coupled secondary antibody (1:5000; Jackson; 115-005-003; USA) at a dilution of 1:5000 in blocking buffer for 2 h at room temperature. Immunoblots were visualized in ECl solution for 1 min followed by X-ray film exposure for 1–10 min. The films were scanned, and immunoreactivity was analyzed by using ImageJ software.
Spinal Glial Activity Assay
After deep anesthesia with pentobarbital sodium (100 mg/kg, i.p.), rats were perfused transcardially with normal saline, followed by 4 % paraformaldehyde in 0.1 M phosphate buffer at pH 7.4. The L3–L5 spinal cord segments were removed and cryoprotected in 30 % sucrose for 48–72 h at 4 °C and then for frozen section. The frozen spinal cord was serially sectioned into 20-μm-thick sections using a sliding microtome. Segments were placed in PBS and sequentially blocked with a solution including 10 % BSA in Tris-buffered saline-Tween. To label the microglia and astrocytes, the segments were separately incubated in the polyclonal OX-42 primary antibody (1:200, Chemicon, Temecula, CA) and polyclonal glial fibrillary acidic protein (GFAP; 1:300; Cell Signaling; 3670; USA) primary antibody overnight at 4 °C. After washing with PBS, the spinal cord segments were separately exposed to goat anti-rabbit secondary antibody (1:500; Abcam; ab150077; USA) and donkey anti-mouse secondary antibody (1:500; Invitrogen; A21202; USA) conjugated to Alexa Fluor 488. Segments were mounted on glass slides, air-dried, and covered with a coverslip using Aquamount (Fisher Scientific, Ottawa, Canada). Images were taken at ×200 magnification by using the Leica TCS SP2 multiphoton confocal microscope (×20/0.45, Leica Microsystems, Wetzlar, Germany). Images were randomly selected for further analysis. Fluorescence mean densities of these images were analyzed by Image-Pro Plus software.
Statistical Analysis
All values are presented as mean ± SD (standard deviation). To determine overall differences at each time point in behavioral tests, repeated measures was performed. One-way ANOVA was used to analyze the data from Western blot experiments. Statistical comparisons between treatment groups were analyzed using ANOVA. Pairwise comparisons were performed using the Student’s t test, and multiple comparisons between groups were performed using Bonferroni’s multiple comparison test. P < 0.05 indicated statistical significance.
RESULTS
Intrathecal Injection of JWH-015 Attenuated Bone Cancer Pain in a Time-Dependent Manner
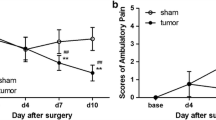
Compared with sham group, there was a trend for lower PWMT and higher ambulatory pain scores in the group with tibia Walker 256 rat mammary gland carcinoma cells inoculation (Fig. 1a, b). At days 7 and 10, significant differences were presented (P < 0.01), and ambulatory pain and mechanical allodynia were elicited. Then, tumor-bearing rats received different treatments at day 10. In contrast to vehicle-treated rats, JWH-015-treated rats developed a time-dependent reduction in bone cancer-related pain behaviors (Fig. 1c, d). This was manifested by an increase in the PWMT and a decrease of ambulatory pain scores. In addition, the antinociceptive effects were exclusively observed 6–48 h after JWH-015 administration, and the most efficacious production of analgesia was at 24 h after injection. However, the analgesic effects of JWH-015 were completely prevented by pretreatment with CB2 receptor antagonist AM630.
The effects of intrathecal administration of JWH-015 on PWMT and ambulatory pain scores (n = 8 for each group). JWH-015 was injected intrathecally 10 days after surgery. PWMT (a, c) and ambulatory pain scores (b, d) were observed 1 day before surgery (baseline); 4, 7, and 10 days after surgery; and 2, 6, 24, 48, and 72 h after different treatments. # P < 0.05 vs. baseline, ## P < 0.01 vs. baseline, ### P < 0.001 vs. baseline; *P < 0.05 vs. sham group, **P < 0.01 vs. sham group, ***P < 0.001 vs. sham group; △ P < 0.05 vs. day 10, △△ P < 0.01 vs. day 10.
Activation of Glia and Long-Lasting Modifications of Spinal IL-6, IL-18, IL-1β, and TNF-α Expression in Tumor-Bearing Rats
To elucidate the mechanism underlying bone cancer pain, we analyzed the activity of glia and expressions of IL-6, IL-18, IL-1β, and TNF-α in the spinal cord. The activation of microglia was first evident on day 4 and reached a peak on day 7 after intra-tibia inoculation of Walker 256 mammary gland carcinoma cells (Fig. 2). On day 10, the activity of microglia decreased under the level on day 4. The activation of astrocytes was first evident on day 10 (Fig. 3). This suggested an acute role of microglial in bone cancer pain. And astrocytes contributed to the maintenance of bone cancer pain. In addition, increases in IL-6, IL-18, IL-1β, and TNF-α levels were first evident at day 4 after tibia inoculation with cancer cells and continued to day 10 (Fig. 4). In contrast, there were no differences observed in the sham group during the various time points.
The activity of spinal microglia in tumor-bearing rats (n = 5 for each group). Immunostaining of the green reaction product for spinal OX-42 before surgery (a), 4 days (b), 7 days (c), and 10 days (d) after surgery were shown in the left panel. The immunofluorescence densities of OX-42 in different groups were shown in the right panel. Scale bar = 100 μm. Magnification ×200. On day 4, microglia was significantly activated in tumor-bearing rats. And the activation of microglia reached a peak on day 7. However, the activity of microglia on day 10 was lower than the level of day 4. *P < 0.05 vs. baseline.
The activity of spinal astrocytes in tumor-bearing rats (n = 5 for each group). Immunostaining of the green reaction product for spinal GFAP before surgery (a), 4 days (b), 7 days (c), and 10 days (d) after surgery were shown in the left panel. The immunofluorescence densities of GFAP in different groups were shown in the right panel. Scale bar = 100 μm. Magnification ×200. Spinal astrocytes were only significantly activated on day 10 after intra-tibia inoculation of Walker 256 mammary gland carcinoma cells. These were no difference on day 4 and day 7. *P < 0.05 vs. baseline.
Western blot and statistical analysis revealed the expressions of IL-6, IL-18, IL-1β, and TNF-α in the spinal cord of the tumor-bearing and sham groups (n = 5 for each group). In the tumor-bearing group, the expressions of all the proteins gradually increased over time and were significantly up-regulated on 4, 7, and 10 days after surgery. No corresponding change was observed in the sham group rats. *P < 0.05 vs. sham group.
Intrathecal Injection of JWH-015 Inhibited Up-regulation of IL-6, IL-18, IL-1β, and TNF-α in a Time-Dependent Manner
The following experiments were designed to assess the effects of intrathecal administration of JWH-015 on IL-6, IL-18, IL-1β, and TNF-α expression in the spinal cord. We found an intrathecal injection of JWH-015 evoked a time-dependent response (Fig. 5). JWH-015-treated group displayed the down-regulation of bone cancer pain-induced proteins since 6 h after injection (P < 0.05) and peaked at 24 h after injection (P < 0.01). However, at 72 h, all the protein levels returned to the levels before treatment (P > 0.05). Conversely, pretreatment of selective antagonists AM630 completely blocked the downward trend induced by JWH-015. Vehicles administered alone could not modify the changes induced by bone cancer pain.
Western blot and statistical analysis showed the expressions of IL-6, IL-18, IL-1β, and TNF-α in the spinal cord of the different groups (n = 5 for each group). The expressions of all of the proteins were analyzed before treatment as well as 6, 24, and 72 h after treatment. In JWH-015-treated group, all of the proteins were significantly reduced at 6 h. However, at 72 h, protein levels for all targets had returned to pre JWH-015-treated levels. The effects were prevented by pretreatment with AM630. No corresponding changes were observed in the DMSO-treated group rats. *P < 0.05 vs. day 10, **P < 0.01 vs. day 10.
Time-Dependent Effect of JWH-015 on Activated Astrocytes
Astrocytes are the most abundant cells in the CNS, and previous studies have demonstrated that astrocytes play a key role in the maintenance of neuropathic pain [37]. In our experiment, we also found astrocytic activation followed the microglial response, and astrocytes might maintain the state of bone cancer pain. Then, we went on to investigate the stimulatory effect of JWH-015 on the activities of astrocytes using immunostaining (Fig. 6). At 10 days after tibia inoculation with Walker 256 rat mammary gland carcinoma cells, a significant increase in the staining density of GFAP was observed in the dorsal horn (P < 0.05). This increase was inhibited by JWH-015. However, this change was only statistically significant at 24 h after JWH-015 treatment and could be completely prevented by pretreatment with AM630. As time passed, the staining density returned to normal levels (approximately equal to the level before JWH-015 treatment). No corresponding changes were observed in the vehicle-treated group.
JWH-015 inhibited the activation of astrocytes induced by bone cancer pain. Immunostaining of the green reaction product for GFAP in the ipsilateral dorsal horn before surgery (a), 10 days after surgery (b), 6 h (c), 24 h (d), and 72 h (e) at different groups. Scale bar = 100 μm. Magnification ×200. On day 10, the astrocytes were activated in the tumor-bearing rats, but this activation was inhibited by JWH-015. JWH-015 displayed a time-dependent effect on astrocytes, which was only significant 24 h after JWH-015 treatment and could be prevented by pretreatment with AM630. No corresponding changes were observed in the DMSO-treated group rats. *P < 0.05 vs. day 10, **P < 0.01 vs. day 10.
DISCUSSION
Many epithelial-derived cancers, such as breast, prostate, sarcoma, and others, typically metastasize to bone [3]. Once bone metastases occur, excruciating pain strongly reduces the quality of life [38, 39]. We chose the animal model of bone cancer pain model based on tibia injection of Walker 256 rat mammary gland carcinoma cells because this model was frequently utilized to mirror the characteristics of clinical bone cancer pain.
Historically, the cannabinoid system has been known to possess the pharmacological property of analgesia in human studies and a variety of animal models of acute and chronic pain [40–44]. Therefore, we chose the frequently used CB2 selective agonist JWH-015 as our pharmacological tool. However, it was also noted that JWH-015 at a dose of 150 mg/kg/day could trigger apoptosis in immune cells, and that was in a dose-dependent fashion [45]. Another article showed that intraperitoneal injection of JWH-133 (analogue of JWH-015 used for animal studies) at a dose of 1 mg/kg/day could inhibit growth, migration, and invasion of NSCLC cells in a mouse model [46]. And based on our previous studies [47], low dose of JWH-015 10 μg/10 μl was chosen for intrathecal injection.
Glial cells are activated in reaction to the stimuli related to invading pathogens, ischemia, and trauma. Recent studies have demonstrated that gliosis and inflammation are vital mediators of the changes in nociceptive sensitivity [48–50]. Here, we demonstrated that upon induction of bone pain, the expressions of IL-6, IL-18, IL-1β, and TNF-α in the spinal cord were increased over time. Acute treatment with the CB2 receptor agonist JWH-015 attenuated bone pain and induced an anti-inflammatory phenotype in a time-dependent manner. This observation is partially consistent with our previous work [47]. Interestingly, the initial time of effect on pro-inflammatory cytokines and astrocytes was inconsistent. Changes of pro-inflammatory cytokines were first evident at 6 h after JWH-015 treatment, while changes of astrocytes activity were significant at 24 h after treatment. The possible reason is that the temporal patterns of pro-inflammatory cytokines release are different from astrocytes and microglia. In our experiment, microglia was initially activated on day 4 and astrocytes were subsequently activated on day 10. It has shown that the role of minocycline, a nonselective inhibitor of microglia, in reducing the established late-phase neuropathic pain is limited [51]. This suggested microglia might contribute to the induction of a chronic pain state while astrocytes were involved in the maintenance of bone cancer pain. In chronic neuropathic pain, microglia is initially activated and subsequently releases many substances, like microglia-derived inflammatory factors, that lead to the activation of nearby microglia, astrocytes, and neurons [52, 53]. This leads to the production of pro-inflammatory cytokines, such as IL-1β, IL-6, and TNF-α. In addition, the activation of IL-18R in astrocytes by IL-18 in microglia induces the up-regulation of NF-κB [31]. The pro-inflammatory cytokines released from activated glial cells including IL-1β, IL-6, and TNF-α exert their functions, at least partially, through the activation of NF-κB. In turn, NF-κB regulates the transcription of these pro-inflammatory cytokines [54]. These processes and productions can mediate the interaction between microglia and astrocytes and contribute to pain facilitation [54, 55]. Although the CB2 selective agonist inhibited the up-regulation of these pro-inflammatory cytokines and did ameliorate mechanical allodynia and ambulatory pain, it did not normalize these responses to pain, and the effects were reversed 72 h after JWH-015 administration. Therefore, the analgesia observed was transient and partial. This finding was partly consistent with other studies [56]. These observations suggest that the CB2 signal is just one of the major underlying mechanisms in bone cancer pain; other mechanisms may be involved. For example, CB2 receptors and the chemokine receptor (CCR) 2 are responsible for tactile allodynia after nerve injury, and these receptors are expressed in spinal microglia [57]. Recent studies have demonstrated that 5-HT3 receptor agonists can induce hyperalgesia and allodynia in the pain model with L5 spinal nerve ligation. One of mechanisms underlying is a neuron-chemokine fractalkine-microglia–astrocyte-neuron-N-methyl-d-aspartic acid receptor (NMDA) signal cascade in the spinal cord [58]. In our previous studies, we reported that the up-regulation of the NR2B subunits of NMDA receptor contribute to bone cancer pain in mice [59], and intrathecal administration of JWH-015 decreases NR2B mRNA expression and attenuates bone cancer pain [47]. So we hypothesize that in bone cancer pain, there may be a microglia to astrocyte signal via IL-18 and then astrocyte to neuron signal via pro-inflammatory cytokines, like IL-1β, TNF-α, etc., which may increase the level of NR2B or enhance phosphorylation of NMDA receptors in the spinal cord, and ultimately lead to behavioral hyperalgesia. Since the underlying mechanisms of JWH-015 in bone cancer pain are complex, further studies need to elucidate. Some studies also find that bone tumors induce the up-regulation of spinal COX-2 mRNA and protein without the involvement of CB1 or CB2 receptors. In addition, a cyclo-oxygen-ase (COX)-2 inhibitor increase the PWMT in a dose-dependent fashion, but the COX-2 inhibitor was less potent than the CB2 receptor agonist [60].
In conclusion, the present study demonstrated that pro-inflammatory system was continuously activated during bone cancer pain. JWH-015 might elicit its analgesic effects via time-dependent modulation of pro-inflammatory cytokines IL-6, IL-18, IL-1β, and TNF-α as well as astroglial activity in the spinal cord.
Abbreviations
- CB1 :
-
Cannabinoid receptor type 1
- CB2 :
-
Cannabinoid receptor type 2
- IL:
-
Interleukin
- TNF-α:
-
Tumor necrosis factor alpha
- PWMT:
-
Paw withdrawal mechanical threshold
- CNS:
-
Central nervous system
- DMSO:
-
Dimethyl sulfoxide
- PBS:
-
Phosphate buffered saline
- GFAP:
-
Glial fibrillary acidic protein
- SD:
-
Standard deviation
- CCR:
-
Chemokine receptor
- COX:
-
Cyclo-oxygen-ase
- NMDA:
-
N-methyl-d-aspartic acid receptor
References
Hernandez, B.Y., M.D. Green, K.D. Cassel, A.M. Pobutsky, V. Vu, and L.R. Wilkens. 2010. Preview of Hawaii cancer facts and Figures 2010. Hawaii Medical Journal 69: 223–224.
Coleman, R.E. 2006. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clinical Cancer Research: an Official Journal of the American Association for Cancer Research 12: 6243s–6249s.
DeNardo, D.G., M. Johansson, and L.M. Coussens. 2008. Immune cells as mediators of solid tumor metastasis. Cancer Metastasis Reviews 27: 11–18.
Luger, N.M., D.B. Mach, M.A. Sevcik, and P.W. Mantyh. 2005. Bone cancer pain: from model to mechanism to therapy. Journal of Pain and Symptom Management 29: S32–S46.
O’Connor, J.P., and T. Lysz. 2008. Celecoxib, NSAIDs and the skeleton. Drugs of Today 44: 693–709.
Davis, M.P. 2014. Cannabinoids in pain management: CB1, CB2 and non-classic receptor ligands. Expert Opinion on Investigational Drugs 23: 1123–1140.
Gilron, I., and A.H. Dickenson. 2014. Emerging drugs for neuropathic pain. Expert Opinion on Emerging Drugs 19: 329–341.
Devane, W.A., F.A. Dysarz 3rd, M.R. Johnson, L.S. Melvin, and A.C. Howlett. 1988. Determination and characterization of a cannabinoid receptor in rat brain. Molecular Pharmacology 34: 605–613.
Tsou, K., S. Brown, M.C. Sanudo-Pena, K. Mackie, and J.M. Walker. 1998. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience 83: 393–411.
Gaffuri, A.L., D. Ladarre, and Z. Lenkei. 2012. Type-1 cannabinoid receptor signaling in neuronal development. Pharmacology 90: 19–39.
Harvey-Girard, E., A.C. Giassi, W. Ellis, and L. Maler. 2013. Expression of the cannabinoid CB1 receptor in the gymnotiform fish brain and its implications for the organization of the teleost pallium. The Journal of Comparative Neurology 521: 949–975.
Klein, T.W., C. Newton, K. Larsen, L. Lu, I. Perkins, L. Nong, and H. Friedman. 2003. The cannabinoid system and immune modulation. Journal of Leukocyte Biology 74: 486–496.
Solas, M., P.T. Francis, R. Franco, and M.J. Ramirez. 2013. CB2 receptor and amyloid pathology in frontal cortex of Alzheimer’s disease patients. Neurobiology of Aging 34: 805–808.
Moreno-Martet, M., F. Espejo-Porras, J. Fernandez-Ruiz, and E. de Lago. 2014. Changes in endocannabinoid receptors and enzymes in the spinal cord of SOD1(G93A) transgenic mice and evaluation of a Sativex((R)) -like combination of phytocannabinoids: interest for future therapies in amyotrophic lateral sclerosis. CNS Neuroscience & Therapeutics 20: 809–815.
Lanciego, J.L., P. Barroso-Chinea, A.J. Rico, L. Conte-Perales, L. Callen, E. Roda, V. Gomez-Bautista, I.P. Lopez, C. Lluis, J.L. Labandeira-Garcia, and R. Franco. 2011. Expression of the mRNA coding the cannabinoid receptor 2 in the pallidal complex of Macaca fascicularis. Journal of Psychopharmacology 25: 97–104.
Gong, J.P., E.S. Onaivi, H. Ishiguro, Q.R. Liu, P.A. Tagliaferro, A. Brusco, and G.R. Uhl. 2006. Cannabinoid CB2 receptors: immunohistochemical localization in rat brain. Brain Research 1071: 10–23.
Onaivi, E.S., H. Ishiguro, J.P. Gong, S. Patel, A. Perchuk, P.A. Meozzi, L. Myers, Z. Mora, P. Tagliaferro, E. Gardner, A. Brusco, B.E. Akinshola, Q.R. Liu, B. Hope, S. Iwasaki, T. Arinami, L. Teasenfitz, and G.R. Uhl. 2006. Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Annals of the New York Academy of Sciences 1074: 514–536.
Rahn, E.J., and A.G. Hohmann. 2009. Cannabinoids as pharmacotherapies for neuropathic pain: from the bench to the bedside. Neurotherapeutics: the Journal of the American Society for Experimental NeuroTherapeutics 6: 713–737.
Fernandez-Ruiz, J. 2009. The endocannabinoid system as a target for the treatment of motor dysfunction. British Journal of Pharmacology 156: 1029–1040.
Marrs, W.R., J.L. Blankman, E.A. Horne, A. Thomazeau, Y.H. Lin, J. Coy, A.L. Bodor, G.G. Muccioli, S.S. Hu, G. Woodruff, S. Fung, M. Lafourcade, J.P. Alexander, J.Z. Long, W. Li, C. Xu, T. Moller, K. Mackie, O.J. Manzoni, B.F. Cravatt, and N. Stella. 2010. The serine hydrolase ABHD6 controls the accumulation and efficacy of 2-AG at cannabinoid receptors. Nature Neuroscience 13: 951–957.
Pertwee, R.G., A.C. Howlett, M.E. Abood, S.P. Alexander, V. Di Marzo, M.R. Elphick, P.J. Greasley, H.S. Hansen, G. Kunos, K. Mackie, R. Mechoulam, and R.A. Ross. 2010. International union of basic and clinical pharmacology. LXXIX. Cannabinoid receptors and their ligands: Beyond CB(1) and CB(2). Pharmacological Reviews 62: 588–631.
Atwood, B.K., and K. Mackie. 2010. CB2: a cannabinoid receptor with an identity crisis. British Journal of Pharmacology 160: 467–479.
Watkins, L.R., E.D. Milligan, and S.F. Maier. 2001. Glial activation: a driving force for pathological pain. Trends in Neurosciences 24: 450–455.
Guo, W., H. Wang, M. Watanabe, K. Shimizu, S. Zou, S.C. LaGraize, F. Wei, R. Dubner, and K. Ren. 2007. Glial-cytokine-neuronal interactions underlying the mechanisms of persistent pain. Journal of Neuroscience : the Official Journal of the Society for Neuroscience 27: 6006–6018.
Sun, Y.E., L. Peng, X. Sun, J. Bo, D. Yang, Y. Zheng, C. Liu, B. Zhu, Z. Ma, and X. Gu. 2012. Intrathecal injection of spironolactone attenuates radicular pain by inhibition of spinal microglia activation in a rat model. PLoS One 7: e39897.
Sun, Y., W. Zhang, Y. Liu, X. Liu, Z. Ma, and X. Gu. 2014. Intrathecal injection of JWH015 attenuates remifentanil-induced postoperative hyperalgesia by inhibiting activation of spinal glia in a rat model. Anesthesia and Analgesia 118: 841–853.
Bossu, P., A. Ciaramella, F. Salani, D. Vanni, I. Palladino, C. Caltagirone, and G. Scapigliati. 2010. Interleukin-18, from neuroinflammation to Alzheimer’s disease. Current Pharmaceutical Design 16: 4213–4224.
Sutinen, E.M., T. Pirttila, G. Anderson, A. Salminen, and J.O. Ojala. 2012. Pro-inflammatory interleukin-18 increases Alzheimer’s disease-associated amyloid-beta production in human neuron-like cells. Journal of Neuroinflammation 9: 199.
Bossu, P., D. Cutuli, I. Palladino, P. Caporali, F. Angelucci, D. Laricchiuta, F. Gelfo, P. De Bartolo, C. Caltagirone, and L. Petrosini. 2012. A single intraperitoneal injection of endotoxin in rats induces long-lasting modifications in behavior and brain protein levels of TNF-alpha and IL-18. Journal of Neuroinflammation 9: 101.
Karavelioglu, E., Y. Gonul, S. Kokulu, O. Hazman, F. Bozkurt, A. Kocak, and O. Eser. 2014. Anti-inflammatory and antiapoptotic effect of interleukine-18 binding protein on the spinal cord ischemia-reperfusion injury. Inflammation 37: 917–923.
Miyoshi, K., K. Obata, T. Kondo, H. Okamura, and K. Noguchi. 2008. Interleukin-18-mediated microglia/astrocyte interaction in the spinal cord enhances neuropathic pain processing after nerve injury. Journal of Neuroscience : the Official Journal of the Society for Neuroscience 28: 12775–12787.
Zimmermann, M. 1983. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16: 109–110.
Mao-Ying, Q.L., J. Zhao, Z.Q. Dong, J. Wang, J. Yu, M.F. Yan, Y.Q. Zhang, G.C. Wu, and Y.Q. Wang. 2006. A rat model of bone cancer pain induced by intra-tibia inoculation of Walker 256 mammary gland carcinoma cells. Biochemical and Biophysical Research Communications 345: 1292–1298.
Medhurst, S.J., K. Walker, M. Bowes, B.L. Kidd, M. Glatt, M. Muller, M. Hattenberger, J. Vaxelaire, T. O’Reilly, G. Wotherspoon, J. Winter, J. Green, and L. Urban. 2002. A rat model of bone cancer pain. Pain 96: 129–140.
Mestre, C., T. Pelissier, J. Fialip, G. Wilcox, and A. Eschalier. 1994. A method to perform direct transcutaneous intrathecal injection in rats. Journal of Pharmacological and Toxicological Methods 32: 197–200.
Dixon, W.J. 1980. Efficient analysis of experimental observations. Annual Review of Pharmacology and Toxicology 20: 441–462.
Ji, R.R., Y. Kawasaki, Z.Y. Zhuang, Y.R. Wen, and I. Decosterd. 2006. Possible role of spinal astrocytes in maintaining chronic pain sensitization: review of current evidence with focus on bFGF/JNK pathway. Neuron Glia Biology 2: 259–269.
Rubens, R.D. 1998. Bone metastases—the clinical problem. European Journal of Cancer 34: 210–213.
Solomayer, E.F., I.J. Diel, G.C. Meyberg, C. Gollan, and G. Bastert. 2000. Metastatic breast cancer: clinical course, prognosis and therapy related to the first site of metastasis. Breast Cancer Research and Treatment 59: 271–278.
Romero-Sandoval, A., N. Nutile-McMenemy, and J.A. DeLeo. 2008. Spinal microglial and perivascular cell cannabinoid receptor type 2 activation reduces behavioral hypersensitivity without tolerance after peripheral nerve injury. Anesthesiology 108: 722–734.
Martin Fontelles, M.I., and C. Goicoechea Garcia. 2008. Role of cannabinoids in the management of neuropathic pain. CNS Drugs 22: 645–653.
Elikkottil, J., P. Gupta, and K. Gupta. 2009. The analgesic potential of cannabinoids. Journal of Opioid Management 5: 341–357.
Karst, M., S. Wippermann, and J. Ahrens. 2010. Role of cannabinoids in the treatment of pain and (painful) spasticity. Drugs 70: 2409–2438.
Thaler, A., A. Gupta, and S.P. Cohen. 2011. Cannabinoids for pain management. Advances in Psychosomatic Medicine 30: 125–138.
Lombard, C., M. Nagarkatti, and P. Nagarkatti. 2007. CB2 cannabinoid receptor agonist, JWH-015, triggers apoptosis in immune cells: potential role for CB2-selective ligands as immunosuppressive agents. Clinical Immunology 122: 259–270.
Preet, A., Z. Qamri, M.W. Nasser, A. Prasad, K. Shilo, X. Zou, J.E. Groopman, and R.K. Ganju. 2011. Cannabinoid receptors, CB1 and CB2, as novel targets for inhibition of non-small cell lung cancer growth and metastasis. Cancer Prevention Research 4: 65–75.
Gu, X., F. Mei, Y. Liu, R. Zhang, J. Zhang, and Z. Ma. 2011. Intrathecal administration of the cannabinoid 2 receptor agonist JWH015 can attenuate cancer pain and decrease mRNA expression of the 2B subunit of N-methyl-D-aspartic acid. Anesthesia and Analgesia 113: 405–411.
Austin, P.J., and G. Moalem-Taylor. 2010. The neuro-immune balance in neuropathic pain: involvement of inflammatory immune cells, immune-like glial cells and cytokines. Journal of Neuroimmunology 229: 26–50.
Kawasaki, Y., L. Zhang, J.K. Cheng, and R.R. Ji. 2008. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. Journal of Neuroscience : the Official Journal of the Society for Neuroscience 28: 5189–5194.
Liou, J.T., F.C. Liu, C.C. Mao, Y.S. Lai, and Y.J. Day. 2011. Inflammation confers dual effects on nociceptive processing in chronic neuropathic pain model. Anesthesiology 114: 660–672.
Raghavendra, V., F. Tanga, and J.A. DeLeo. 2003. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. The Journal of Pharmacology and Experimental Therapeutics 306: 624–630.
Sung, B., G. Lim, and J. Mao. 2003. Altered expression and uptake activity of spinal glutamate transporters after nerve injury contribute to the pathogenesis of neuropathic pain in rats. Journal of Neuroscience : the Official Journal of the Society for Neuroscience 23: 2899–2910.
Ji, R.R., and M.R. Suter. 2007. p38 MAPK, microglial signaling, and neuropathic pain. Molecular Pain 3: 33.
Meunier, A., A. Latremoliere, E. Dominguez, A. Mauborgne, S. Philippe, M. Hamon, J. Mallet, J.J. Benoliel, and M. Pohl. 2007. Lentiviral-mediated targeted NF-kappaB blockade in dorsal spinal cord glia attenuates sciatic nerve injury-induced neuropathic pain in the rat. Molecular Therapy : the Journal of the American Society of Gene Therapy 15: 687–697.
Ledeboer, A., M. Gamanos, W. Lai, D. Martin, S.F. Maier, L.R. Watkins, and N. Quan. 2005. Involvement of spinal cord nuclear factor kappaB activation in rat models of proinflammatory cytokine-mediated pain facilitation. The European Journal of Neuroscience 22: 1977–1986.
Vincenzi, F., M. Targa, C. Corciulo, M.A. Tabrizi, S. Merighi, S. Gessi, G. Saponaro, P.G. Baraldi, P.A. Borea, and K. Varani. 2013. Antinociceptive effects of the selective CB2 agonist MT178 in inflammatory and chronic rodent pain models. Pain 154: 864–873.
Abbadie, C., J.A. Lindia, A.M. Cumiskey, L.B. Peterson, J.S. Mudgett, E.K. Bayne, J.A. DeMartino, D.E. MacIntyre, and M.J. Forrest. 2003. Impaired neuropathic pain responses in mice lacking the chemokine receptor CCR2. Proceedings of the National Academy of Sciences of the United States of America 100: 7947–7952.
Guo, W., K. Miyoshi, R. Dubner, M. Gu, M. Li, J. Liu, J. Yang, S. Zou, K. Ren, K. Noguchi, and F. Wei. 2014. Spinal 5-HT3 receptors mediate descending facilitation and contribute to behavioral hypersensitivity via a reciprocal neuron-glial signaling cascade. Molecular Pain 10: 35.
Gu, X., J. Zhang, Z. Ma, J. Wang, X. Zhou, Y. Jin, X. Xia, Q. Gao, and F. Mei. 2010. The role of N-methyl-D-aspartate receptor subunit NR2B in spinal cord in cancer pain. European Journal of Pain 14: 496–502.
Cui, J.H., J. Ju, and M.H. Yoon. 2013. Pharmacology of cannabinoid receptor agonists and a cyclooxygenase-2 inhibitor in rat bone tumor pain. Pharmacology 92: 150–157.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (81070892, 81171047, 81171048, and 81371207) and a grant from the Department of Health of Jiangsu Province of China (XK201140, RC2011006).
Conflict of Interest
The authors declare that they have no conflicts of interest.
Authors’ Contributions
All of the authors read and approved the final manuscript. CEL made substantial contributions to the experiments. YL was mainly involved in the pain behavioral tests and the spinal astrocyte activity assay. BS and YZ performed the surgical procedure, administration of drugs, and Western blotting studies. YES and BLH were responsible for statistical analyses. All of these individuals participated in drafting the manuscript. XPG and ZLM conceived the idea, designed the study, and helped to revise the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Cui’e Lu, Yue Liu, Bei Sun and Yu’e Sun contributed equally to this work.
Rights and permissions
About this article
Cite this article
Lu, C., Liu, Y., Sun, B. et al. Intrathecal Injection of JWH-015 Attenuates Bone Cancer Pain Via Time-Dependent Modification of Pro-inflammatory Cytokines Expression and Astrocytes Activity in Spinal Cord. Inflammation 38, 1880–1890 (2015). https://doi.org/10.1007/s10753-015-0168-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-015-0168-3