Abstract
Among the known Toll-like receptors (TLRs), Toll-like receptor 2 (TLR2) is a key sensor for detecting Staphylococcus aureus invasion. But the function of TLR2 during S. aureus infection in different cell populations is unclear. Two different cell subtypes were chosen to study the interaction of S. aureus with TLR2 because macrophages are extremely different from one compartment to another and their capacity to respond to live bacteria or bacterial products differs from one site to another. The contribution of TLR2 to the host innate response against acute live S. aureus infection and heat-killed S. aureus (HKSA) using anti-TLR2 antibody in murine peritoneal macrophages and resident fresh bone marrow cells has been investigated here. TLR2 blocking before infection induces the release of interleukin (IL)-10 by macrophages thereby inhibiting excessive production of oxidants by activating antioxidant enzymes. TLR2-blocked peritoneal macrophages showed impaired release of tumor necrosis factor-alpha (TNF-α), interferon-gamma (IFN-γ) and IL-6 in response to both live and heat-killed S. aureus infection except bone marrow cells. TLR2-mediated free radical production and killing of S. aureus were modulated by TLR2 blocking in peritoneal macrophages and resident bone marrow cells. This study supported that S. aureus persists in resident bone marrow cells in a state of quiescence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Staphylococcus aureus is a major pathogen that causes a variety of diseases ranging from minor skin infections to severe systemic complications such as bacteraemia, septic shock, septic arthritis and osteomyelitis often with fatal results [1]. S. aureus, while generally considered an extracellular pathogen, is one such bacterium that has the ability to invade and survive within different cell types, both phagocytic and non-phagocytic cells [2]. By ‘hiding’ inside the host cells, S. aureus may elude host defences and most antibiotic treatments and may be responsible for chronic and recurrent bone infections by shifting the balance of cell repertoire. S. aureus possesses a number of potential virulence factors including surface-associated and secreted proteins, which especially cause inflammatory disease [3, 4]. Several staphylococcal cell wall components (e.g. peptidoglycan and lipoteichoic acid) have been extensively studied to date [5–8] in several infectious disease models. However, bacterial pathogenicity in the case of S. aureus is multi-factorial since a single virulence factor is not sufficient to cause a staphylococcal infection [9, 10]. Therefore, it is important to compare the host cell response after exposure to the whole live S. aureus as well as purified staphylococcal cell wall component or using heat-killed S. aureus (HKSA).
Protection from primary staphylococcal infection is mainly dependent on innate rather than adaptive immune responses [11]. In the innate immune response, Toll-like receptors (TLRs), which are predominantly expressed in cells involved in inflammatory responses, play pivotal roles in the host defence against microbial pathogens by recognizing pathogen-associated molecular patterns (PAMPs) and activating intracellular signalling pathways [12]. There are lines of evidence that show that Toll-like receptor 2 (TLR2) is responsible for the immunity against S. aureus. Among the known TLRs, TLR2 is a key sensor for detecting S. aureus invasion. There is increasing evidence that show that TLR2 found on macrophages is involved in the detection of S. aureus PAMPs and acts with other co-receptors to mediate phagocytosis of the bacterium [13]. The products of S. aureus function as agonists for TLR2 [9]. Furthermore, TLR2 induces the synthesis of proinflammatory mediators to rapidly activate the innate immune system [14, 15]. The absence of TLR2 has been shown to provide protective effects to the host organisms in certain models suggesting that TLR2 has not only beneficial [16] but also detrimental roles in host innate response against S. aureus infections [17]. Results of either single or dual TLR blockade before or upon acute bacterial infection have been shown to play a central role of TLR2 in sensing bacterial challenge in vivo and the capacity to protect from shock upon subsequent or synchronous antibiotic therapy [18]. Another interesting field worthy of study in susceptibility to infection is the ability developed by many virulent strains of pathogens to evade immunity through TLRs of different cell subtypes. Certain pathogens used TLR-based strategies to evade host defence [19]. Such is the case with the bacteria Mycobacterium tuberculosis, Yersinia enterocolitica, Yersenia pestis and Yerseinia pseudotuberculosis and fungi such as Candida albicans and Aspergillus fumigates which activate a TLR2-mediated mechanism to induce an anti-inflammatory pattern that downmodulates the microbicidal function of primary leukocytes [20]. This regulation by TLR2 seemed to be beneficial to bacteria and dependent on the strain of bacteria, as TLR2-deficient mouse macrophages were reported to have a greater capacity to kill S. aureus organisms compared to wild-type macrophages [21–23]. The activation of TLR signalling with these infections promotes sustained efforts to develop a novel strategy to block these pathways for a variety of infectious diseases [24, 25]. TLR2- or MyD88-deficient peritoneal macrophages produced significantly less proinflammatory cytokines in response to heat-killed S. aureus. TLR2-deficient mice were hypersusceptible to S. aureus and more slowly cleared subcutaneously inoculated S. aureus than wild-type mice, and peritoneal macrophages derived from the TLR2-deficient mice were insensitive to lipoteichoic acid and heat-killed S. aureus [26]. Live but not heat-killed Treponema denticola suppress expression of human beta defensin 3 from gingival epithelial cells via inhibition of TLR2 signalling [27]. Taken together, studies on the differential induction of inflammatory cytokines and reactive oxygen species in fresh bone marrow cells and peritoneal macrophages of Swiss mice by S. aureus and contribution of TLR2 to make countermeasures against infection are needed. Thus, it appears that even though S. aureus is recognized by macrophages through TLR2, S. aureus may utilize TLR2 as part of its survival mechanism. This makes TLR2 a very intriguing molecule to study when trying to understand the survival mechanism of S. aureus in primary macrophages. Currently, there is no study on the differential induction of inflammatory cytokines and reactive oxygen species in resident fresh bone marrow cells and peritoneal macrophages after acute S. aureus infection and contribution of TLR2 in this response. The interruption of TLR-mediated recognition by immune cells is a reasonable strategy for immune evasion of bacteria, since the recognition of bacteria and bacterial components via TLRs plays a pivotal role in the initiation of antibacterial innate immunity. There have been only a few reports with respect to interruption of TLR function in primary immune cells by bacteria. Therefore, the modulatory activity of live and killed S. aureus on TLR2 in peritoneal and resident bone marrow cells may follow a different mechanism.
We have recently reported on cellular events and intracellular survival of S. aureus during infection of murine macrophages of Swiss albino mice [28]. The roles of TLRs in the sensing of oxidants by cells and tissues in vitro have been investigated [29]. TLR2 was required for oxidant-induced inflammation in vivo. Therefore, it seems that as in higher vertebrates, S. aureus has evolved a diversified array of antioxidant tools, both enzymatic and non-enzymatic, to resist immune-mediated oxidative attack [30]. Catalase has been proposed to be a potential virulence factor in many bacterial pathogens, because its activity might protect them from the reactive oxygen species (ROS) generated by eukaryotic cells [31]. Thus, this enzyme has been demonstrated to be an essential factor for the intracellular survival of bacteria [32]. There have been only a few reports with respect to regulation of TLR function in fresh bone marrow cells or peritoneal macrophages of Swiss albino mice by bacteria. Therefore, the modulatory activity of live and killed S. aureus on TLR2 in peritoneal and resident bone marrow cells may follow a different mechanism. The present comparative study was performed to figure out the involvement of reactive oxygen intermediates and cytokines in TLR2-dependent bacteriostasis of S. aureus in murine peritoneal macrophages and resident bone marrow cells during acute infection.
In the present study, we investigated the role of TLR2 in bacterial clearance and cytokine response to live S. aureus and HKSA infection in murine peritoneal and bone marrow macrophages. The data presented here indicate that TLR2 blocking before infection restores the release of interleukin (IL)-10 by macrophages, thereby inhibiting excessive proinflammatory cytokine production as well as regulating the production of reactive oxygen species by activating the antioxidant enzymes. It was observed that TLR2-blocked peritoneal macrophages showed impaired release of tumor necrosis factor-alpha (TNF-α), interferon-gamma (IFN-γ) and IL-6 in response to both live and heat-killed S. aureus infection except bone marrow cells. This study supported that S. aureus persists in resident bone marrow cells in a state of quiescence. This work may help us for a better understanding of the host innate immune mechanism against this bacterium and will aid in the development of therapeutics for treating S. aureus.
MATERIALS AND METHODS
Maintenance of Animals and Cells
All experiments involving animals were conducted according to the protocols that had been approved by the Institutional Animal Ethics Committee (IAEC), Department of Physiology, University of Calcutta, under the guidance of the Committee for the Purpose of Control and Supervision of Experiments on Animal (CPCSEA) [Approval Number: 820/04/ac/CPCSEA dated 05 August 2013], Ministry of Environment and Forest, Govt. of India. Wild-type male Swiss albino mice were used throughout the study. To minimize the feeling of hypoxia or discomfort before and during mouse dissection and tissue collection, mice were anaesthetized with inhaling anaesthetics (ether) before terminal surgery. Euthanasia was performed by general anaesthesia followed by vital tissue removal using 2–3 % ether for induction and 1 % for maintenance. Macrophages were prepared from peritoneal fluids of thioglycolate-administered mice. The S. aureus strain AG-789 was obtained from Apollo Gleneagles Hospital, Calcutta, West Bengal, India.
Preparation of Bacteria
S. aureus strain (AG-789) grown overnight in Luria-Bertani broth was diluted with fresh broth and cultured until mid-logarithmic phase of growth. Bacteria were harvested, washed twice with sterile saline and adjusted to the desired inoculum spectrophotometrically before infection (OD620 = 0.2 for 5.0 × 107 cells/ml for S. aureus), and the colony-forming unit (CFU) count of the desired inoculum was confirmed by serial dilution and culture on blood agar [33].
Preparation of Heat-Killed S. aureus
Bacteria were grown in Luria-Bertani broth (LB) overnight under the same conditions. Overnight cultures were washed in 0.9 % NaCl and used for heat-killing for 1 h at 90 °C [34].
Preparation of Resident Fresh Bone Marrow Cells from Mice and Stimulation
The protocols for animal handling were previously approved by our Institutional Animal Ethics Committee (Approval Number: 820/04/ac/CPCSEA dated 05 August 2013). Femurs were obtained from 6 to 12 week old Swiss albino mice. After euthanasia, the mice were sprayed with 70 % ethanol, and the femurs were dissected using scissors, cutting through the tibia below the knee joints as well as through the pelvic bone close to the hip joint. Muscles connected to the bone were removed using clean gauze, and the femurs were placed into a polypropylene tube containing sterile PBS on ice. In a tissue culture hood, the bones were placed in 70 % ethanol for 1 min and washed in sterile RPMI 1640, and then both epiphyses were removed using sterile scissors and forceps. The bones were flushed with a 24Gz syringe filled with RPMI 1640 to extrude bone marrow into a 15 ml sterile polypropylene tube. A 5 ml plastic pipette was used to gently homogenize the bone marrow. The cell suspension generated thereafter is called fresh bone marrow cells. Fresh bone marrow cells (FBMCs) were counted using a hemocytometer, centrifuged for 5 min at 200×g at 4 °C and gently resuspended to obtain a solution containing from 4 to 6 × 106 cells/ml in RPMI 1640 media containing 10 % fetal bovine serum (unless otherwise mentioned) [35]. Murine FBMCs (5 × 106 cells/ml) were infected with S. aureus (5 × 106 CFU/ml) for 30, 60 and 90 min at 37 °C.
Isolation and Stimulation of Peritoneal Macrophages
The mice used, 6–12 weeks of age and fed standard laboratory chow and water, were injected intraperitoneally with 2 ml of 4 % sterile thioglycolate broth, and the resulting peritoneal exudate was harvested by lavage of the peritoneal cavities of mice with endotoxin-free Hanks’ solution 4 to 5 days later. Peritoneal macrophages were suspended in 0.83 % ammonium chloride solution containing 10 % (v/v) Tris buffer (pH 7.65) to lyse erythrocytes. The cells were resuspended in RPMI 1640 medium supplemented with 10 % fetal bovine serum (FBS), 100 IU/ml penicillin and 100 μg/ml streptomycin and then were allowed for plastic adherence. Non-adherent cells were removed by aspiration and by washing with RPMI 1640 medium before the addition of S. aureus. The adherent macrophages, more than 95 % of which appeared to be typical macrophages by light microscopy, were used for each experiment [36]. Murine peritoneal macrophages (5 × 106 cells/ml) were infected with S. aureus (5 × 106 CFU/ml) for 30, 60 and 90 min at 37 °C.
Blocking Antibody Reagents and Culture Conditions
For TLR2 blocking assays, polyclonal antibodies against TLR2 (Cat No: orb11487, Biorbyt Limited, Cambridge, UK) were added at 10 μg/ml, then the peritoneal macrophages and FBMCs were incubated for 30 min at 37 °C in 5 % CO2 with S. aureus or medium alone added, and the cells were incubated for an additional 30, 60 and 90 min at 37 °C in 5 % CO2. Cell-free culture supernatants were collected at 30, 60 and 90 min at 37 °C [37, 38]. This polyclonal antibody is against a human protein (Swiss-Prot: 060603), reacts with mouse, rat, cow, dog and rabbit and was available on PubMed 23800958. Saturation studies were performed beforehand and were found useful at a concentration of 10 μg/ml as suggested earlier [39].
Assays for Colony-Forming Ability of Engulfed Bacteria
Murine peritoneal macrophages and FBMCs (5 × 106 cells/ml) were separately mixed with S. aureus (5 × 106 CFU/ml) in a 1:1 cell/bacterium ratio [40] in RPMI-FBS (5 %) and incubated at 37 °C in a cell culture incubator for different times in the presence and absence of anti-TLR2 antibody (10 μg/ml). After centrifugation, cell culture supernatants were collected and stored for further assay. Phagocytosis was stopped by adding cold (4 °C) RPMI 1640, and extracellular S. aureus were removed by washing the suspension in RPMI (note that we were unable to use antibiotics to kill bacteria present outside of macrophages because the engulfed bacteria died quickly during the period necessary for the action of antibiotics). The pellets were disrupted in sterile water containing 0.01 % bovine serum albumin (BSA) by vigorously vortexing to release intracellular bacteria in the lysate. The lysate-containing bacteria were plated at serial dilutions on mannitol agar plates. The plates were incubated at 37 °C for a day or two and the number of colonies was determined.
Quantification of Hydrogen Peroxide Production
After time-dependent phagocytosis, supernatants were collected and cell lysates were prepared from the pellet. Hydrogen peroxide (H2O2) assay of the supernatant and lysate was performed according to the method as described earlier with slight modification [41]. Briefly, 70 μl of supernatant or lysate recovered from different groups of macrophages, 20 μl horseradish peroxidase (HRP) (500 μg/ml), 70 μl of Phenol red (500 μg/ml) and 40 μl of S. aureus were added to each of the microtiter plate and were allowed to react at 37 °C. The reaction was stopped by adding 25 μl of 2 (N) NaOH, and the absorbance reading was taken at 620 nm using a spectrophotometer (UV-1800 UV–VIS spectrophotometer, Shimadzu, Japan). A standard H2O2 curve was plotted and H2O2 release in supernatants and lysate was evaluated and expressed in micromolars per 106 cells.
Assay for Quantification of Superoxide Anion Release
Superoxide anion release assay measures the change in colour of cytochrome C (cytC), when reduced by O2⋅− released from the stimulated macrophages and FBMCs. Cell-free supernatants recovered from S. aureus-stimulated macrophages and FBMCs in the presence or absence of anti-TLR2 antibody were incubated in the presence of cytochrome C (100 μl at 2 mg/ml in HBSS). The reaction was terminated by placing the tubes on ice for 5 min. The production of superoxide anion was monitored spectrophotometrically (UV-1800 UV–VIS spectrophotometer, Shimadzu, Japan) at 550 nm in reference to the blank. The amount of superoxide production was calculated by the following formula: nanomoles of superoxide anion = (mean absorbance at 550 nm × 15.87) [42].
ROS Determination by Flow Cytometry
Flow cytometric data acquisition and analysis were performed on BD FACSVerse. The flow cytometer was equipped with an iron laser with excitation at 488 nm and 15 mW output power. A single cell suspension was prepared having a density of 105–106 cells/ml and a minimum of 10,000 cells were measured. Nonfluorescent 2′,7′-dichlorofluorescin diacetate (DCFH-DA) upon esterase activity within the cells became DCFH which after oxidation by ROS converted to a fluorescent product (DCF). The amount of DCF was proportional to the amount of ROS present inside the cell. Briefly, 20 mM DCFH-DA stock solution made in DMSO was diluted in the cultured medium from different groups of macrophages, to yield 20 μM working solution. Then, the cells were incubated for 60 min in the dark at 37 °C. Finally, cells were suspended in PBS, and ROS generation was measured by fluorescence intensity at 530 nm [43].
Assay for Quantification of Nitric Oxide Production
Nitric oxide (NO) release was determined by the Griess assay. Thioglycolate-elicited mouse peritoneal macrophage sand FBMCs in serum-free RPMI 1640 medium were mixed with S. aureus, and the mixture was incubated in the presence or absence of anti-TLR2 antibody for different times at 37 °C with 5 % CO2 and centrifuged. Fifty microliters of the supernatant or lysate was incubated separately in 40 μM Tris (pH 7.9) containing 40 μM of the reduced form of β-nicotinamide adenine dinucleotide phosphate, 40 μM flavin adenine dinucleotide and 0.05 U/ml nitrate reductase at 37 °C for 15 min. Reduced samples were incubated with an equal volume of Griess reagent consisting of sulphanilamide (0.25 % (w/v)) and N-1-naphthylethylenediamine (0.025 % (w/v)), the mixture was incubated for 10 min and the absorbance at 550 nm was measured in a spectrophotometer (UV-1800 UV–VIS spectrophotometer, Shimadzu, Japan). The total nitrate/nitrite concentration was determined by comparison to a reduced NaNO3 standard curve [44].
Assay of Catalase Enzyme Activity
Catalase activity in the medium or cell-free lysate was determined spectrophotometrically by measuring the decrease in H2O2 concentration at 240 nm. At time 0, 100 μl of the supernatant or cell-free lysate was added separately to 2.89 ml of potassium phosphate buffer (pH 7.4) taken in a quartz cuvette. Then, 0.1 ml of 300 mM H2O2 was added to it, and the absorbance was taken at 240 nm for 5 min at 1 min intervals. Catalase activity was expressed in terms of millimoles per minute per milligram of protein [45].
Assay of Superoxide Dismutase Enzyme Activity
One hundred microliters of the medium (supernatant) or cell-free lysate was mixed separately with 1.5 ml of a Tris-EDTA-HCl buffer (pH 8.5), then 100 μl of 7.2 mmol/l pyrogallol was added and the reaction mixture was incubated at 25 °C for 10 min. The reaction was terminated by the addition of 50 μl of 1 M HCl and measured at 420 nm. One unit was determined as the amount of enzyme that inhibited the oxidation of pyrogallol by 50 %. The activity was expressed as units per milligram of protein [46].
Tumor Necrosis Factor-α, Interferon-Gamma, Interleukin-6 and Interleukin-10 ELISA Assays
Murine peritoneal macrophages and FBMCs (5 × 106 cells/ml) were mixed separately with S. aureus (5 × 106 CFU/ml) in a 1:1 cell/bacterium ratio [40] in RPMI-FBS (5 %) and incubated at 37 °C in a cell culture incubator for different times in the presence and absence of anti-TLR2 antibody. After incubation, cell culture supernatants were collected and stored at −70 °C prior to analysis. Supernatants from different groups were normalized to the protein content by the Bradford method before the assay, and the levels of TNF-α, IFN-γ, IL-6 and IL-10 production were assayed using ELISA kits according to the instructions provided by the manufacturer (Ray Biotech Inc.) in a Bio-rad ELISA Reader.
Statistical Analysis
Isolated peritoneal macrophages and resident bone marrow cells from mice were pooled together to obtain the requisite amount of macrophages (5 × 106 cells/ml), and the different parameters were measured. This was repeated for three times for each parameter (for e.g. H2O2 production) and then the mean value of these triplicate experiments was taken for calculation. Data was expressed as mean ± SD. Means were compared between groups by using analysis of variance (ANOVA). p < 0.05 was considered significant.
RESULTS
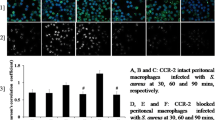
Phagocytic Activity of Murine Resident Fresh Bone Marrow Cells and Peritoneal Macrophage After Neutralisation of Cell Surface TLR2 During S. aureus Infection
We used live S. aureus to investigate whether cell surface TLR2 blocking alters the phagocytic activity of resident bone marrow cells and peritoneal macrophages. As shown in Table 1, a significant difference was found in the degree of phagocytic engulfment of S. aureus between the macrophages of antibody-untreated and TLR2-blocked macrophages at 60 and 90 min post-treatment. Moreover, the number of engulfed bacteria was not the same for both the resident bone marrow cells and peritoneal macrophage groups (Table 1).
Alteration in Hydrogen Peroxide Release by Murine Resident Fresh Bone Marrow Cells and Peritoneal Macrophage Infection with Live S. aureus or with Heat-Killed S. aureus due to TLR2 Blocking
We tested whether hydrogen peroxide produced by resident bone marrow cells and peritoneal macrophages in culture or in cell lysate was TLR2 dependent during short-term S. aureus infection. Both anti-TLR2 antibody-untreated and anti-TLR2 antibody-treated macrophage cultures were exposed to live S. aureus or HKSA and incubated for 30, 60 and 90 min at 37 °C. Anti-TLR2 antibody significantly (p < 0.05) inhibited hydrogen peroxide production in supernatant and lysate by live S. aureus-infected macrophages at 60 min after infection, compared with the antibody-untreated and antibody-infected peritoneal macrophages (Fig. 1a, b). In addition, the amount of H2O2 released in the lysate and supernatant by the anti-TLR2 antibody-neutralized resident bone marrow cells and peritoneal macrophages, followed by heat-killed S. aureus infection, was found to be significantly lower (p < 0.05) when compared with only heat-killed S. aureus-infected macrophages at all the time points studied (Fig. 1c, d).
Alteration in hydrogen peroxide (H2O2) release by murine resident fresh bone marrow cells and peritoneal macrophage infection with live S. aureus or with heat-killed S. aureus (HKSA) due to TLR2 blocking. Peritoneal macrophages and resident bone marrow cells were incubated with or without anti-TLR2 antibodies for 30 min and re-incubated with live and heat-killed S. aureus for the time indicated. Supernatants and lysates were prepared as mentioned earlier. H2O2 content was expressed in terms of micromolars per 106 cells. a H2O2 release in culture supernatant by live S. aureus-infected peritoneal macrophages and resident fresh bone marrow cells, b H2O2 release in lysate by live S. aureus-infected peritoneal macrophages and resident fresh bone marrow cells, c H2O2 release in culture supernatant by heat-killed S. aureus (HKSA)-infected peritoneal macrophages and resident fresh bone marrow cells and d H2O2 release in lysate by heat-killed S. aureus (HKSA)-infected peritoneal macrophages and resident fresh bone marrow cells. Values were expressed as mean ± SD; *p < 0.05 indicates significant difference compared to the uninfected control macrophage group; # p < 0.05 indicates significant difference between control and heat-killed S. aureus-infected macrophage (HKSAM) group; $ p < 0.05 indicates significant difference with respect to only live S. aureus-infected group (LSAM); φ p < 0.05 indicates significant difference with respect to only heat-killed S. aureus-infected group (HKSAM). ATabM anti-TLR2 antibody-treated macrophage, LSAM live S. aureus-infected macrophage, ATabM + LSA anti-TLR2 antibody-treated macrophage + live S. aureus, HKSAM heat-killed S. aureus-infected macrophages, ATabM + HKSA anti-TLR2 antibody-treated macrophage + heat-killed S. aureus.
Alteration in Superoxide Anion Release by Murine Resident Fresh Bone Marrow Cells and Peritoneal Macrophage Infection with Live S. aureus or with Heat-Killed S. aureus due to TLR2 Blocking
Murine resident fresh bone marrow cells and peritoneal macrophages were infected in vitro with live and heat-killed S. aureus, as described in the “MATERIALS AND METHODS,” or left uninfected. Thirty, 60 or 90 min after infection, the amount of superoxide anion produced was evaluated. Both in the culture supernatant (Fig. 2a) and in the lysate (Fig. 2b), live S. aureus-infected macrophages produced significantly (p < 0.05) larger amounts of superoxide than uninfected macrophages after 60 and 90 min of infection, whereas infection of TLR2-neutralized peritoneal macrophages with live S. aureus produced lower levels of superoxide anion compared to macrophages infected with live S. aureus alone at 60 and 90 min in culture as well as in lysate. A similar pattern of results was observed in the culture supernatant in case of resident bone marrow cells, and the lysate showed a significant change in superoxide anion release only at 90 min (Fig. 2a, b). In addition, HKSA that infected both macrophages and resident bone marrow cells only particularly at 90 min showed a significant change in the superoxide anion release in the culture supernatant (Fig. 2c), while peritoneal macrophages and resident fresh bone marrow cells, incubated with heat-killed S. aureus, showed a higher amount of superoxide anion at all three time points and at 30 and 60 min, respectively, compared to that of the control, and at 60 min, TLR2-neutralized infected macrophages showed a marked decrease compared to the infected one. Identical observation was found for resident bone marrow cells at 30 min.
Alteration in superoxide anion release by murine resident fresh bone marrow cells and peritoneal macrophage infection with live S. aureus or with heat-killed S. aureus (HKSA) due to TLR2 blocking. Peritoneal macrophages and resident bone marrow cells were incubated with or without anti-TLR2 antibody for 30 min and re-incubated with live and heat-killed S. aureus for the time indicated. Superoxide anion release assay was determined by measuring the changes in colour of cytochrome C, when reduced by O2⋅− released from the stimulated macrophages. a Superoxide anion release in culture supernatant by live S. aureus-infected peritoneal macrophages and resident fresh bone marrow cells, b superoxide anion release in lysate by live S. aureus-infected peritoneal macrophages and resident fresh bone marrow cells, c superoxide anion release in culture supernatant by heat-killed S. aureus (HKSA)-infected peritoneal macrophages and resident fresh bone marrow cells and d superoxide anion release in lysate by heat-killed S. aureus (HKSA)-infected peritoneal macrophages and resident fresh bone marrow cells. Values were expressed as mean ± SD; *p < 0.05 indicates significant difference compared to the uninfected control macrophage group; # p < 0.05 indicates significant difference between control and heat-killed S. aureus-infected macrophage (HKSAM) group; $ p < 0.05 indicates significant difference with respect to only live S. aureus-infected group (LSAM); φ p < 0.05 indicates significant difference with respect to only heat-killed S. aureus-infected group (HKSAM). ATabM anti-TLR2 antibody-treated macrophage, LSAM live S. aureus-infected macrophage, ATabM + LSA anti-TLR2 antibody-treated macrophage + live S. aureus, HKSAM heat-killed S. aureus-infected macrophages, ATabM + HKSA anti-TLR2 antibody-treated macrophage + heat-killed S. aureus.
Alteration in Cellular ROS Production by Murine Peritoneal Macrophage and Resident Fresh Bone Marrow Cells After Infection with Live S. aureus due to TLR2 Blocking as Determined by Flow Cytometry
Murine resident fresh bone marrow cells and peritoneal macrophages were infected in vitro with live S. aureus, as described in the “MATERIALS AND METHODS,” or left uninfected. As we found that both anti-TLR2 antibody and live S. aureus-infected peritoneal and bone marrow macrophages significantly (p < 0.05) inhibited hydrogen peroxide and superoxide production in the supernatant and lysate at 60 min (Figs. 1 and 2), we determined the ROS production by flow cytometry particularly at 60 min after infection. Following the incubation with DCFH-DA, it has been found that both the macrophages infected with live S. aureus (Figs. 3 and 4) showed a marked increase (p < 0.05) in ROS production compared to that of their respective control groups. In addition, a significant decrease (p < 0.05) in ROS production was observed when anti-TLR2 antibody-treated peritoneal macrophages and resident bone marrow cells were infected with live S. aureus, compared to the macrophages infected with live S. aureus alone.
Alteration in ROS generation by murine peritoneal macrophage infection with live S. aureus due to TLR2 blocking. a Gating of peritoneal macrophages (PMs) infected with or without live S. aureus, into subpopulations of different sizes in a forward (FSC-A)/side scatter (SSC-A) dot plot, in which the abscissa (FSC-A) represents cell size and the ordinate (SSC-A) cell granularity. b Gating of peritoneal macrophages (PMs) pretreated with anti-TLR2 antibody with or without live S. aureus infection, into subpopulations of different sizes in a forward (FSC-A)/side scatter (SSC-A) dot plot. c PMs were pretreated with or without live S. aureus and then incubated with 20 μM DCFH-DA for 60 min. Fluorescence from cells was measured by FACS and indicated as green and red histograms, respectively. d PMs pretreated with anti-TLR2 antibody with or without live S. aureus infection and then incubated with 20 μM DCFH-DA for 60 min. Fluorescence from cells was measured by FACS and indicated as green and red histograms, respectively. e ROS generation of different groups was shown in the fluorescence intensity. Values were expressed as median ± SD. *p < 0.05 indicates significant difference compared to the uninfected control macrophage group; $ p < 0.05 indicates significant difference (p < 0.05) with respect to only live S. aureus-infected group (LSAM).
Alteration in ROS generation by murine resident fresh bone marrow cell infection with live S. aureus due to TLR2 blocking. a Gating of resident bone marrow cells infected with or without live S. aureus, into subpopulations of different sizes in a forward (FSC-A)/side scatter (SSC-A) dot plot, in which the abscissa (FSC-A) represents cell size and the ordinate (SSC-A) cell granularity. b Gating of resident bone marrow cells pretreated with anti-TLR2 antibody with or without live S. aureus infection, into subpopulations of different sizes in a forward (FSC-A)/side scatter (SSC-A) dot plot. c Resident bone marrow cells were pretreated with or without live S. aureus and then incubated with 20 μM DCFH-DA for 60 min. Fluorescence from cells was measured by FACS and indicated as green and red histograms, respectively. d Resident bone marrow cells pretreated with anti-TLR2 antibody with or without live S. aureus infection and then incubated with 20 μM DCFH-DA for 60 min. Fluorescence from cells was measured by FACS and indicated as green and red histograms, respectively. e ROS generation of different groups was shown in the fluorescence intensity. Values were expressed as median ± SD. *p < 0.05 indicates significant difference compared to the uninfected control macrophage group; $ p < 0.05 indicates significant difference with respect to only live S. aureus-infected group (LSAM).
Alteration in Nitric Oxide Production by Murine Resident Fresh Bone Marrow Cells and Peritoneal Macrophage Infection with Live S. aureus or with Heat-Killed S. aureus due to TLR2 Blocking
To investigate the involvement of TLR2 in acute in vitro infection of resident bone marrow cells and peritoneal macrophages with live and heat-killed S. aureus, we determined the nitric oxide release in the supernatant and lysate, respectively, at 30, 60 and 90 min in the presence or absence of anti-TLR2 antibody. A significant decrease in NO production in the lysate and supernatant (p < 0.05) was found due to TLR2 blocking in live and heat-killed S. aureus-infected peritoneal macrophages particularly after 60 and 90 min compared with only live and heat-killed S. aureus-infected macrophage (Fig. 5a, c), whereas anti-TLR2 antibody significantly impaired (p < 0.05) nitric oxide release at 90 min by TLR2-neutralized heat-killed S. aureus-infected resident bone marrow cells compared to anti-TLR2 antibody-untreated HKSA-infected macrophages. The enhancement in nitrite secretion prior to TLR2 blocking was abrogated by N-monomethyl l-arginine, showing that it was being produced through the nitric oxide synthase pathway (data not shown). Hence, the production of superoxide and nitrite and H2O2 by resident bone marrow cells and peritoneal macrophage was identical whether the cells had been stimulated by an extract of S. aureus surface proteins or by the whole bacteria.
Alteration in nitric oxide production by murine resident fresh bone marrow cells and peritoneal macrophage infection with live S. aureus or with heat-killed S. aureus (HKSA) due to TLR2 blocking. Murine peritoneal macrophages (5 × 106 cells/ml) and resident bone marrow cells were allowed to interact with S. aureus (5 × 106 CFU/ml) in a 1:1 cell/bacterium ratio incubated at 37 °C in a cell culture incubator for different times in the presence and absence of anti-TLR2 antibody. Results in this figure represent mean nitric oxide content (μM) in the medium or from cell-free lysate from different groups of triplicate experiments. a Nitric oxide release in culture supernatant by live S. aureus-infected peritoneal macrophages and resident fresh bone marrow cells, b nitric oxide release in lysate by live S. aureus-infected peritoneal macrophages and resident fresh bone marrow cells, c nitric oxide release in culture supernatant by heat-killed S. aureus (HKSA)-infected peritoneal macrophages and resident fresh bone marrow cells and d nitric oxide release in lysate by heat-killed S. aureus (HKSA)-infected peritoneal macrophages and resident fresh bone marrow cells. Values were expressed as mean ± SD; *p < 0.05 indicates significant difference compared to the uninfected control macrophage group; # p < 0.05 indicates significant difference between control and heat-killed S. aureus-infected macrophage (HKSAM) group; $ p < 0.05 indicates significant difference with respect to only live S. aureus-infected group (LSAM); φ p < 0.05 indicates significant difference with respect to only heat-killed S. aureus-infected group (HKSAM). ATabM anti-TLR2 antibody-treated macrophage, LSAM live S. aureus-infected macrophage, ATabM + LSA anti-TLR2 antibody-treated macrophage + live S. aureus, HKSAM heat-killed S. aureus-infected macrophages, ATabM + HKSA anti-TLR2 antibody-treated macrophage + heat-killed S. aureus.
Neutralisation of Cell Surface TLR2 Significantly Alters Cytokine (TNF-α, IFN-γ, IL-6 and IL-10) Production During Infection of Resident Fresh Bone Marrow Cells and Peritoneal Macrophage with Live S. aureus
Short-term infection of starch-elicited peritoneal macrophages or resident bone marrow cells from Swiss albino mice for 60 and 90 min with S. aureus resulted in a significant increase (p < 0.05) in the amount of TNF-α secreted compared to the amount secreted by the control, uninfected cells. In contrast, when peritoneal macrophages or fresh bone marrow cells that have been pretreated with anti-TLR2 antibody to neutralize cell surface TLR2 were used, the TNF-α responses to live S. aureus were significantly (p < 0.05) lower than the corresponding antibody-untreated macrophages (Fig. 6a) at 60 and 90 min. The amount of IFN-γ release was markedly increased (p < 0.05) with respect to the control at three time points for peritoneal cells and at 60 and 90 min for bone marrow cells. But the anti-TLR2 antibody plus bacteria-treated group showed a marked decrease with respect to the only infected group (Fig. 6b) at 60 and 90 min for both the two cell subtypes. Similarly, IL-6 production for both peritoneal as well as bone marrow cells was significantly increased (p < 0.05) in the infected group than that of their respective control at all three time points. In addition, a significant decrease (p < 0.05) was found due to TLR2 blocking in the infected peritoneal macrophages at all time points and in bone marrow cells at 60 and 90 min, when compared to the only S. aureus-infected group (Fig. 6c). In contrast, IL-10 production was significantly lowered (p < 0.05) at 90 min only when both peritoneal and bone marrow cells were infected with S. aureus alone, with respect to their control groups. But anti-TLR2 antibody treatment of peritoneal macrophages as well as of bone marrow cells before S. aureus infection showed a significant rise in IL-10 production at 90 min only than that of their anti-TLR2 antibody-untreated only S. aureus-infected groups (Fig. 6d).
Neutralisation of cell surface TLR2 significantly alters cytokine (TNF-α, IFN-γ, IL-6 and IL-10) production during infection of resident fresh bone marrow cells and peritoneal macrophage by live S. aureus. Peritoneal macrophages and resident bone marrow cells were incubated with or without anti-TLR2 antibody for 30 min and re-incubated with only live S. aureus for the time indicated. Levels of TNF-α (a), IFN-γ (b), IL-6 (c) and IL-10 (d) in the supernatants collected after 30, 60 and 90 min after live S. aureus-infected macrophages in the presence or absence of anti-TLR2 antibody were determined by utilizing ELISA according to the manufacturer’s recommendations and were expressed from triplicate experiments. Values were expressed as mean ± SD. *p < 0.05 indicates significant difference compared to the uninfected control macrophage group; $ p < 0.05 indicates significant difference with respect to only live S. aureus-infected group (LSAM). ATabM anti-TLR2 antibody-treated macrophage, LSAM live S. aureus-infected macrophage, ATabM + LSA anti-TLR2 antibody-treated macrophage + live S. aureus, HKSAM heat-killed S. aureus-infected macrophages, ATabM + HKSA anti-TLR2 antibody-treated macrophage + heat-killed S. aureus.
Neutralisation of Cell Surface TLR2 Significantly Alters Cytokine (TNF-α, IFN-γ, IL-6 and IL-10) Production During Infection of Resident Fresh Bone Marrow Cells and Peritoneal Macrophage with Heat-Killed S. aureus
Acute infection of peritoneal macrophages or resident bone marrow cells for 60 and 90 min with HKSA resulted in a significant rise (p < 0.05) in the amount of TNF-α release compared to the amount secreted by the control. When heat-killed S. aureus infected peritoneal macrophages and resident bone marrow cells, pretreated with anti-TLR2 antibody, a marked decrease (p < 0.05) was shown at 90 min for the first one and at 60 min for the latter, compared to the antibody-untreated only heat-killed S. aureus-infected group (Fig. 7a). Similarly, HKSA-infected bone marrow cells that have been pretreated with anti-TLR2 antibody produced significantly (p < 0.05) a lower amount of IFN-γ at 60 and 90 min, in comparison with their only HKSA-infected group (Fig. 7b). But IFN-γ production was markedly increased (p < 0.05) at three time points in the HKSA-infected bone marrow cells but only at 90 min in the infected peritoneal macrophages, compared to their respective control group. Treatment of resident bone marrow cells with anti-TLR2 antibody before HKSA stimulation showed decreased IL-6 level at 60 min, but it was at 90 min in case of peritoneal macrophages in comparison to the antibody-untreated but HKSA-stimulated cells (Fig. 7c). On the contrary, IL-10 production was significantly (p < 0.05) decreased at 60 min when peritoneal cells were infected with HKSA and at 90 min for HKSA-infected bone marrow cells, with respect to their uninfected control. But anti-TLR2 antibody pretreatment followed by HKSA infection of both peritoneal and bone marrow cells produced a significant (p < 0.05) rise in IL-10 production at 90 min, compared to their antibody-untreated only infected group (Fig. 7d).
Neutralisation of cell surface TLR2 significantly alters cytokine (TNF-α, IFN-γ, IL-6 and IL-10) production during infection of resident fresh bone marrow cells and peritoneal macrophage by heat-killed S. aureus. Peritoneal macrophages and resident bone marrow cells were incubated with or without anti-TLR2 antibody for 30 min and re-incubated with only heat-killed S. aureus for the time indicated. Levels of TNF-α (a), IFN-γ (b), IL-6 (c) and IL-10 (d) in the supernatants collected after 30, 60 and 90 min after heat-killed S. aureus-infected macrophages in the presence or absence of anti-TLR2 antibody were determined by utilizing ELISA according to the manufacturer’s recommendations and were expressed from triplicate experiments. Values were expressed as mean ± SD; # p < 0.05 indicates significant difference compared to the uninfected control macrophage group; φ p < 0.05 indicates significant difference with respect to only heat-killed S. aureus-infected group (HKSAM). ATabM anti-TLR2 antibody-treated macrophage, LSAM live S. aureus-infected macrophage, ATabM + LSA anti-TLR2 antibody-treated macrophage + live S. aureus, HKSAM heat-killed S. aureus-infected macrophages, ATabM + HKSA anti-TLR2 antibody-treated macrophage + heat-killed S. aureus.
Alteration in Antioxidant Enzyme (Catalase) Activity After TLR2 Blocking Followed by Live and Heat-Killed S. aureus Infection in Peritoneal Macrophage and Resident Fresh Bone Marrow Cells
As several lines of report suggested that bacterial catalase played an important role in the intracellular survival of S. aureus in murine peritoneal macrophages and resident bone marrow cells, we became interested to find out whether there are any changes in these antioxidant enzyme activities after TLR2 blocking followed by live and heat-killed S. aureus infection. A marked increase (p < 0.05) in the catalase enzyme activity has been observed in murine peritoneal macrophages (Table 2) and resident bone marrow cells (Table 2), both in the culture supernatant (media) and in the cell-free lysate at 60 and 90 min when macrophages were incubated with anti-TLR2 antibody prior to S. aureus infection than that of macrophages infected with S. aureus alone. A similar observation was found in the cell-free extract when macrophages were infected with heat-killed S. aureus, and though only at 90 min, a significant decrease (p < 0.05) was observed in the cell lysate in antibody-pretreated heat-killed S. aureus-infected macrophage than that of antibody-untreated only HKSA-infected macrophage. As shown in Table 2, a significant difference was found in the catalase activity between the resident bone marrow cells of antibody-untreated and TLR2-blocked bone marrow cells at 60 and 90 min post-treatment.
Alteration in Antioxidant Enzyme (SOD) Activity After TLR2 Blocking Followed by Live and Heat-Killed S. aureus Infection in Peritoneal Macrophage and Resident Fresh Bone Marrow Cells
On the basis of above findings, we further assessed the superoxide dismutase (SOD) activity that has been found to be increased in case of S. aureus-infected macrophages as well as bone marrow cells, compared to their control group. Anti-TLR2 antibody significantly (p < 0.05) inhibited SOD production in the supernatant and lysate by live S. aureus-infected peritoneal and bone marrow cells at 60 min after infection, compared with the antibody-untreated and antibody-infected peritoneal macrophages and bone marrow cells (Table 3). In contrast, the amount of SOD activity in the lysate and supernatant by anti-TLR2 antibody-neutralized resident bone marrow cells, followed by heat-killed S. aureus infection, was found to be significantly lower (p < 0.05) when compared with the only heat-killed S. aureus-infected macrophages particularly at 90 min (Table 3).
DISCUSSION
S. aureus is a formidable, resilient and dangerous opportunistic human pathogen. Recent studies have shown that S. aureus is highly resistant to killing by professional phagocytes and that such cells even provide a favourable environment for intracellular survival of S. aureus [47]. Among the known TLRs, TLR2 is a key sensor for detecting S. aureus invasion. But the function of TLRs, specifically TLR2, during S. aureus infection is still debated. In this study, we investigated the extent to which TLR2 contributes to the host innate response against the bacterial infection using anti-TLR2 antibody in murine peritoneal macrophages and resident fresh bone marrow cells after acute S. aureus infection.
In this context, we analysed the contribution of TLR2 to cytokine and oxidative stress response to S. aureus infection in both peritoneal macrophage and resident fresh bone marrow cell. We choose two different compartments to study the interaction of S. aureus with TLR2 because macrophages are extremely different from one compartment to another and their capacity to respond to bacteria or bacterial products differs from one site to another as shown for intestinal, peritoneal, alveolar, spleen, microglial macrophages and monocytes [48, 49]. We also used live as well as heat-killed preparation of S. aureus for infection. Bacteria are often killed in order to study their induction of in vitro immune responses as this offers the advantage of standardizing conditions and avoiding confounding effects inherent to differences in bacterial proliferation or overt contamination of the culture environment [23]. There are very few data that directly compare the immunological properties of killed bacterial preparations to their live counterpart.
TLR2 has been identified as the receptor responsible for immunorecognition of the Gram-positive bacteria S. aureus as well as the Gram-positive bacterial components, lipoteichoic acid (LTA) and peptidoglycan (PGN) [50–52]. Results of intracellular killing assay in our study demonstrated that higher numbers of S. aureus survived in TLR2-blocked macrophages both in case of peritoneal macrophages and bone marrow cells as evidenced by higher CFU count in TLR2-blocked macrophages. We speculate that due to blocking of TLR2 with anti-TLR2 antibody, there was a lack in TLR2 recognition of one or more staphylococcal PAMPs, resulting in less killing and higher survival of S. aureus inside the macrophages in our study. This may be the plausible explanation for such impaired intracellular killing of bacteria in TLR2-blocked macrophages. In our study, we could not verify whether TLR2 blocking caused any impairment in phagocytosis; however, earlier reports revealed that although the TLR2 on phagocytes does not function as a phagocytic receptor, its absence is reported to cause a decrease in bacterial phagocytosis [53, 54]. It was reported that TLR2 deficiency did not alter phagocytic uptake of S. aureus by macrophages and TLR2 does not contribute to the early clearance of S. aureus. Although the scavenger receptor CD36 has been reported to be involved in the macrophage uptake of S. aureus in cooperation with TLR2 [55], we have not examined whether the absence of TLR2 affects CD36 expression in macrophages in this setup. Moreover, it was suggested that TLR2 and CD36 do not have a direct effect on macrophage phagocytosis of S. aureus. Furthermore, CD36 activation in macrophages is dependent on the presence of the TLR2 molecule [56].
In terms of S. aureus clearance, professional phagocytes such as macrophages and neutrophils constitute the first line of host innate immunity to engulf and kill the bacterium [57]. Cells of the monocytic lineage are essential for innate immunity and also play a critical role in the pathophysiology of bacterial diseases. Among the different cytokines implicated in inflammation and septic shock, TNF-α, IL-1β and IL-6 are proinflammatory cytokines. IL-10 is an anti-inflammatory cytokine that inhibits proinflammatory cytokines such as IL-1β, IL-6, IL-8 and TNF-α. S. aureus is capable of producing systemic cytokine responses, and staphylococcal peptidoglycan has been shown to stimulate the production of proinflammatory cytokines and chemokines (TNF-α, IL-1β, IL-6 and IL-8) in monocytes and macrophages [58–60].
It was earlier reported that S. aureus-induced activation of TLR2 leads to the release of proinflammatory cytokines by macrophages, in which TNF-α is considered to be an early cytokine while IL-6 is considered to be a later cytokine [61]. In our experiments, we also observed that TLR2-blocked peritoneal macrophages showed impaired, but not completely depressed, release of TNF-α, IFN-γ and IL-6 in response to both live and heat-killed S. aureus infection. Results of TNF-α, IFN-γ and IL-6 in our study are also in accordance with an earlier finding [16] which demonstrated that TLR2-deficient peritoneal macrophages had significantly impaired production of both TNF and IL-6 in response to heat-killed S. aureus, compared with macrophages from wild-type mice. However, the anti-inflammatory IL-10 response has also been identified to occur in macrophages/monocytes upon TLR2 signalling initiated by S. aureus [62, 63] as evidenced by increased IL-10 production in TLR2-blocked peritoneal macrophages before S. aureus infection compared to the peritoneal macrophages without TLR2 blocking in this study. Moreover, a similar pattern but to lesser degree of cytokine responses was observed in bone marrow cells. Therefore, it was surprising that the production of proinflammatory cytokine was induced in response to heat-killed S. aureus in TLR2-deficient macrophages, although the level was reduced compared with that prior TLR2 blocking. These results suggested that S. aureus is recognized not only by TLR2 but also by other TLRs. Several studies have also demonstrated that not only TLR2 but also other microbial recognition receptors, such as intracellular nucleotide-binding oligomerization domain-like receptors, are involved in evoking cytokine responses to S. aureus infection [64]. So, we speculate that impaired production of cytokines by macrophages (both peritoneal macrophage and bone marrow cells) treated with anti-TLR2 antibody in this study was associated with impaired intracellular killing, suggesting that TLR2-mediated cytokine production may contribute to the kinetics of bacterial clearance in vitro.
Macrophages are reported to share with other types of cells the ability to produce IFN-γ in response to IL-12 plus IL-18 [65]. Recently, TLRs are believed to play an important role in the balance between the production of IL-12 and its family members (e.g. IL-23 and IL-27) [66]. Although we did not measure IL-12 in this acute infection study, we speculate that decreased IFN-γ in S. aureus-infected macrophages with TLR2 blocking might be IL-12-mediated as reported in our earlier study after long-term infection [46].
The production of IFN-γ by macrophages is likely to amplify their antimicrobial activities and to serve as an auto-regulatory circuit that helps to expand Th1 responses. It was earlier reported that reduced antigen-presenting cell (APC) activation and proinflammatory cytokine secretion in the absence of TLR2 signalling further contribute to the lower IFN-γ production observed with the sera of TLR2−/− mice [67]. Indeed, in in vivo condition, APC-derived IL-12 triggers NK cells to produce IFN-γ, which itself as a feedback loop activates macrophages and recruits neutrophils, which are both important for the clearance of bacteria. Therefore, we hypothesize that a low IFN-γ level in turn prevents effective macrophage activation, thereby reducing the killing of bacteria by macrophages and the generation of protective immunity. Although the production of proinflammatory cytokines is important for mediating the initial host defence against invading pathogens, an excessive inflammatory response can be detrimental to the host. Thus, TLR-mediated inflammation is a double-edged sword that must be precisely regulated [68]. Therefore, it is of paramount importance to identify ways to enhance the innate defences while reducing or avoiding inflammation-induced pathology during infection.
Although traditionally considered an extracellular pathogen, there is increasing evidence that staphylococci are able to invade host cells and that an intracellular lifestyle may facilitate long-term persistence in bone tissue, enabling evasion of antimicrobials and host immune responses. Staphylococcal bone infections are thus likely to be a continuing and probably increasing problem, and understanding of the interaction of these pathogens with bone is central to the development of novel therapeutic strategies required to treat increasingly antibiotic-resistant and persistent infections. The balance of activity between osteoblast and osteoclast is crucial to maintaining the proper homeostasis of bone turnover, and any shift in the relative levels of osteoblast and osteoclast activity can result in bone pathology [69]. Infection with a pathogen such as S. aureus is capable of stimulating such a shift, mediated in part by induction of an inflammatory response. In addition to staphylococcal induction of inflammatory mediators that modulate the actions of osteoblasts and osteoclasts, bacteria of this genus are involved in more direct interactions with resident bone cells. Thus, the suggestion of internalization of S. aureus by resident bone cells in vivo provides a protective niche for the bacterium, where it is shielded from immune effector mechanisms, and antibiotics may help explain persistent cases. However, the true importance of intracellular staphylococci in clinical osteomyelitis has yet to be established [69]. In cell culture systems, S. aureus is able to invade cultured osteoblasts from murine, human and embryonic chick sources [70–74]. However, the internalization of S. aureus and its persistence inside resident bone marrow cells have not been reported. TLR2 blocking prior to infection of bone marrow cells favours increased residence of S. aureus, indicating the beneficial role of TLR2 in the clearing of bacteria from bone cells.
There is strong evidence that production of proinflammatory cytokines is induced by staphylococcal infection of bone and that they directly contribute to bone destruction. In particular, the inflammatory cytokines TNF-α, IL-1 and IL-6 seem to be especially important in bone physiology and pathology [75]. Cell culture models support the view that IL-1 and TNF-α stimulate the proliferation and differentiation of osteoclast progenitors into mature osteoclasts in the presence of osteoblasts [76–78]. Similarly, IL-6 increases bone resorption activity and osteoclast number in cultured mouse calvariae and stimulates osteoclast differentiation in the presence of osteoblasts [79, 80]. Therefore, downregulation of proinflammatory cytokines like TNF-α, IFN-γ and IL-6 and upregulation of anti-inflammatory cytokines like IL-10 due to TLR2 neutralisation of bone marrow cells before S. aureus infection as evident from this study might have a therapeutic approach in regulating bone infection and associated inflammatory diseases like septic arthritis.
In addition, surface-associated material (SAM) from S. aureus stimulates bone resorption and osteoclast formation. Neutralisation of TNF-α and IL-6 fully abolishes SAM-stimulated osteoclastogenesis [81, 82]. Furthermore, live and heat-killed S. aureus-induced TNF-α and IL-6 by resident bone marrow cells in our case is well in line with an earlier report that Staphylococcus epidermidis surface material can also induce bone resorption by a mechanism that is strongly dependent on TNF-α [83]. There is strong evidence that S. aureus infection of bone initiates local and systemic production of TNF-α, IL-1 and IL-6 via host pattern recognition receptors. Elevated levels of these cytokines then shift the homeostatic balance of bone turnover increasing osteoclast differentiation and bone resorption and diminish in osteoblast-mediated bone matrix production and mineralisation, thereby driving bone destruction [84]. Therefore, reduced production of proinflammatory cytokines like TNF-α, IFN-γ and IL-6 and elevated release of anti-inflammatory cytokines like IL-10 due to TLR2 neutralisation of bone marrow cells before HKSA stimulation as evident from this study might have a therapeutic approach in regulating bone resorption and bone destruction as reported earlier from some SAM of S. aureus. The interruption of TLR-mediated recognition by immune cells is a reasonable strategy for immune evasion of bacteria, since the recognition of bacteria and bacterial components via TLRs plays a pivotal role in the initiation of antibacterial innate immunity. There have been only a few reports with respect to interruption of TLR function in primary immune cells by bacteria. Therefore, the modulatory activity of live and killed S. aureus on TLR2 in peritoneal and resident bone marrow cells may follow a different mechanism.
Our study presents support for a mechanism whereby infection with S. aureus persists in bone in a state of quiescence. Once S. aureus invades the osteoblast, its intracellular location is a relative sanctuary against standard chemotherapeutic regimens and the humoral immune response of the host. In addition, these intracellular bacteria may be released by processes which result in the death of host osteoblasts. Bacteria (e.g. S. aureus) could internalize and survive within host cells (e.g. osteoblasts) and, after “hiding” for some time period, which could be up to years [85], these quiescent bacteria could escape from the infected host cells into the surrounding tissue and could be sufficient to initiate a recurrence of symptomatic infection. It is also possible that upon escaping from the host cells, bacteria may internalize into other host cells (e.g. neutrophils, macrophages). All of these may help to understand the pathogenesis of infections like periprosthetic joint infection [86].
ROS generation in response to bacterial lipopolysaccharide and LTA is crucial for pathogen killing by immune cells. However, sustained production of ROS during immune responses and sepsis can cause damage to macromolecules, cell death and tissue injury [87]. Once inside, S. aureus may be exposed to a variety of host killing mechanisms including various ROS, generated by the respiratory burst response of the host [88]. ROS and reactive nitrogen species (RNS) are produced by macrophages as part of their antimicrobial response. The production of ROS is initiated by NADPH oxidase, which catalyzes the reduction of molecular oxygen to superoxide (O2 −). Superoxide can then be converted to H2O2 and hydroxyl radical by SOD [89]. In addition, NO produced by inducible nitric oxide synthase (iNOS) can combine with superoxide to generate additional products with enhanced toxicity, such as peroxynitrite (ONOO-) [90]. In order to survive and proliferate, intracellular pathogens have defence mechanisms to deal with microbial killing of host cells. SOD, catalase and glutathione are the main cellular ROS-degrading enzyme systems; SOD converts superoxide radical (O2 −) into H2O2, which is metabolized by catalase and glutathione peroxidase [91].
We observed increases in superoxide anion and H2O2 when peritoneal and bone marrow cells were stimulated with S. aureus prior to TLR2 blocking. Furthermore, NO synthesis is known to be critical for the control of infection, as the production of this mediator endows macrophages with cytostatic and cytotoxic activities against bacteria [92]. Therefore, it is justifiable to assume that a higher amount of superoxide radical and H2O2 may be released by the macrophages after stimulation with S. aureus, prior to TLR2 blocking, as essential components of the innate immune response against intracellular bacteria. Both were found to be decreased after TLR2 blocking. So, these results lead to a conclusion that intracellular killing of S. aureus was compromised during acute infection due to blocking of cell surface TLR2.
The intracellular environment undergoes a continued change once the bacteria get inside the macrophages. Activities of antioxidant enzymes like catalase and SOD were determined from the supernatant or lysate recovered after time-dependent phagocytosis in the presence or absence of anti-TLR2 antibodies from the peritoneal macrophages or resident fresh bone marrow cells. However, in this study, we also hypothesized that the source of antioxidant enzymes could be S. aureus derived. The increased bacterial SOD content neutralizes the bactericidal activity of O2 − by converting it into H2O2. Catalase, a protein with known free radical scavenging activities, metabolizes H2O2, a toxic oxygen metabolite. In our study, to counteract the increased superoxide radical and H2O2, the bacteria might have released a higher amount of SOD as evidenced by the increased SOD activity prior to TLR2 blocking. The increased bacterial SOD content neutralizes the bactericidal activity of O2 − by converting it into H2O2, another oxygen metabolite having potential antimicrobial activities. Bacterial catalase has received a very prominent role in eliminating the toxic effects of H2O2. But decreased catalase release in our study prior to TLR2 blocking suggests an enhanced phagocytic capacity of the macrophages. When macrophages were treated with anti-TLR2 antibody, we observed decreased SOD activity and increased catalase activity with a concomitant decrease in O2 −, H2O2 and NO levels. These results motivate us to hypothesize that after TLR2 blocking, macrophages have less phagocytic activity as evidenced by increased CFU count after TLR2 blocking in this study. Although we have reported earlier that to understand whether catalase contributing in the intracellular survival was of bacterial origin or not, 3-amino-1,2,4-triazole (ATZ) has to be used to inhibit specifically macrophage-derived catalase. Catalase enzyme activity from the whole staphylococcal cells in the presence of ATZ could suggest that the released catalase was of extracellular origin. From the intracellular survival assay, it could be evident that pretreatment of macrophages with ATZ reduces the bacterial burden in macrophages when infected with the recovered bacteria only from the anti-TLR2 antibody-treated macrophages after phagocytosis [46]. Alteration in antioxidant enzyme activity from the recovered S. aureus after time-dependent phagocytosis in the presence or absence of CXCR1 antibody (IL-8 receptor antibody) has been reported recently from our laboratory [93]. We have also reported that staphylococcal catalase protects intracellularly survived bacteria by destroying H2O2 produced by the murine peritoneal macrophages [28]. Furthermore, contribution of catalase and superoxide dismutase to the intracellular survival of clinical isolates of S. aureus in murine macrophages has also been reported earlier by us [36]. However, we have not tested this during acute S. aureus infection in the presence or absence of anti-TLR2 antibody.
In summary, we showed here that the innate immune receptor TLR2 contributes to the effective control of an early staphylococcal infection in vitro, although compensatory mechanisms may be activated for the subsequent control of infection and bacterial clearance in vivo. Our study also demonstrates that the resident bone marrow cells behave similarly as that of the peritoneal macrophages but the degree of functional response was less in the resident fresh bone marrow cells as compared to the peritoneal macrophages. Furthermore, as opposed to the peritoneal macrophages, the production of cytokines by resident bone marrow cells was not affected when these cells were treated with anti-TLR2 antibody. The only difference was the level of oxidative stress parameters in the culture supernatant and in the lysate of resident bone marrow cells which were lower than those obtained from the peritoneal macrophages.
In the current setup, we found that infection of peritoneal macrophages or resident bone marrow cells with S. aureus resulted in TLR2-mediated cytokine production and increased oxidative killing of internalized bacteria which were abrogated by TLR2 blocking. But we have not evaluated the effect of S. aureus interaction on TLR2 protein expression in the present study using purified resident bone marrow macrophages. So, a future study on the dynamics of TLR2 protein expression after acute infection of peritoneal macrophages or purified resident bone marrow macrophages with S. aureus prior to or after TLR2 blocking is needed. In addition, this approach also may have relevance whether TLR2-mediated cytokine expression has any further impact on TLR2 expression in macrophages infected with S. aureus correlating bone destruction or septic arthritis.
Abbreviations
- CPCSEA:
-
Committee for the Purpose of Control and Supervision of Experiments on Animal
- EDTA:
-
Ethylenediaminetetraacetic acid
- FBMCs:
-
Fresh bone marrow cells
- FBS:
-
Fetal bovine serum
- HBSS:
-
Hank’s balanced salt solution
- iNOS:
-
Inducible nitric oxide synthase
- NaNO3 :
-
Sodium nitrate
- NaOH:
-
Sodium hydroxide
- NaCl:
-
Sodium chloride
- PAMP:
-
Pathogen-associated molecular pattern
- TLR2:
-
Toll-like receptor 2
- TLRs:
-
Toll-like receptors
REFERENCES
Lowy, F.D. 1998. Staphylococcus aureus infections. New England Journal of Medicine 339: 520–532.
Garzoni, C., and W.L. Kelley. 2009. Staphylococcus aureus: new evidence for intracellular persistence. Trends in Microbiology 17: 59–65.
Cohen, J. 2002. The immunopathogenesis of sepsis. Nature 420: 885–891.
Bronner, S., H. Monteil, and G. Prévost. 2004. Regulation of virulence determinants in Staphylococcus aureus: complexity and applications. FEMS Microbiology Reviews 28: 183–200.
Wang, J.E., P.F. Jørgensen, M. Almlöf, C. Thiemermann, S.J. Foster, A.O. Aasen, and R. Solberg. 2000. Peptidoglycan and lipoteichoic acid from Staphylococcus aureus induce tumor necrosis factor alpha, interleukin 6 (IL-6), and IL-10 production in both T cells and monocytes in a human whole blood model. Infection and Immunity 68: 3965–3970.
Müller-Anstett, M.A., P. Müller, T. Albrecht, M. Nega, J. Wagener, Q. Gao, S. Kaesler, M. Schaller, T. Biedermann, and F. Götz. 2010. Staphylococcal peptidoglycan co-localizes with Nod2 and TLR2 and activates innate immune response via both receptors in primary murine keratinocytes. PloS One 5: e13153.
Mattsson, E., R. Heying, J.S. van de Gevel, T. Hartung, and H. Beekhuizen. 2008. Staphylococcal peptidoglycan initiates an inflammatory response and procoagulant activity in human vascular endothelial cells: a comparison with highly purified lipoteichoic acid and TSST-1. FEMS Immunology and Medical Microbiology 52: 110–117.
Gillrie, M.R., L. Zbytnuik, E. McAvoy, R. Kapadia, K. Lee, C.C.M. Waterhouse, S.P. Davis, D.A. Muruve, P. Kubes, and M. Ho. 2010. Divergent roles of Toll-like receptor 2 in response to lipoteichoic acid and Staphylococcus aureus in vivo. European Journal of Immunology 40: 1639–1650.
Fournier, B., and D.J. Philpott. 2005. Recognition of Staphylococcus aureus by the innate immune system. Clinical Microbiology Reviews 18: 521–540.
Kubica, M., K. Guzik, J. Koziel, M. Zarebski, W. Richter, B. Gajkowska, A. Golda, A.M. Gudowska, K. Brix, L. Shaw, T. Foster, and J. Potempa. 2008. A potential new pathway for Staphylococcus aureus dissemination: the silent survival of S. aureus phagocytosed by human monocyte-derived macrophages. PloS One 3: e1409.
Von Köckritz-Blickwede, M., M. Rohde, S. Oehmcke, L.S. Miller, A.L. Cheung, H. Herwald, S. Foster, and E. Medina. 2008. Immunological mechanisms underlying the genetic predisposition to severe Staphylococcus aureus infection in the mouse model. American Journal of Pathology 173: 1657–1668.
Akira, S., S. Uematsu, and O. Takeuchi. 2006. Pathogen recognition and innate immunity. Cell 124: 783–801.
Hoebe, K., P. Georgel, S. Rutschmann, X. Du, S. Mudd, K. Crozat, S. Sovath, L. Shamel, T. Hartung, U. Zähringer, and B. Beutler. 2005. CD36 is a sensor of diacylglycerides. Nature 433: 523–527.
Silva, M.T. 2011. Macrophage phagocytosis of neutrophils at inflammatory/infectious foci: a cooperative mechanism in the control of infection and infectious inflammation. Journal of Leukocyte Biology 89: 675–683.
Takeuchi, O., and S. Akira. 2010. Pattern recognition receptors and inflammation. Cell 140: 805–820.
Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, T. Ogawa, H. Takada, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of Gram-negative and Gram-positive bacterial cell wall components. Immunity 11: 443–451.
Knuefermann, P., Y. Sakata, J.S. Baker, C.H. Huang, K. Sekiguchi, H.S. Hardarson, O. Takeuchi, S. Akira, and J.G. Vallejo. 2004. Toll-like receptor 2 mediates Staphylococcus aureus-induced myocardial dysfunction and cytokine production in the heart. Circulation 110: 3693–3698.
Spiller, S., G. Elson, R. Ferstl, S. Dreher, T. Mueller, M. Freudenberg, B. Daubeuf, H. Wagner, and C.J. Kirschning. 2008. TLR-4 induced IFN-γ production increases TLR-2 sensitivity and drives Gram negative sepsis in mice. Journal of Experimental Medicine 205: 1747–1754.
Netea, M.G., J.W.M. Van der Meer, and B.J. Kullberg. 2004. Toll-like receptors as an escape mechanism from the host defense. Trends in Microbiology 12: 484–488.
Arko-Mensah, J., E. Julian, M. Singh, and C. Fernández. 2007. TLR2 but not TLR4 signalling is critically involved in the inhibition of IFN-γ induced killing of Mycobacteria by murine macrophages. Scandinavian Journal of Immunology 65: 148–157.
Watanabe, I., M. Ichiki, A. Shiratsuchi, and Y. Nakanishi. 2007. TLR2-mediated survival of Staphylococcus aureus in macrophages: a novel bacterial strategy against host innate immunity. Journal of Immunology 178: 4917–4925.
Singh, A., D. Rost, N. Tvardovskaia, A. Roggenkamp, A. Wiedemann, C.J. Kirschning, M. Aepfelbacher, and J. Heesemann. 2002. Yersinia V-antigen exploits toll-like receptor 2 and CD14 for interleukin 10 mediated immunosuppression. Journal of Experimental Medicine 196: 1017–1024.
Strunk, T., P. Richmond, A. Prosser, K. Simmer, O. Levy, D. Burgner, and A. Currie. 2011. Method of bacterial killing differentially affects the human innate immune response to Staphylococcus epidermidis. Innate Immunity 17: 508–516.
Dong, C., H. Sexton, A. Gertrudes, T. Akama, S. Martin, C. Virtucio, C.W. Chen, X. Fan, A. Wu, W. Bu, L. Liu, L. Feng, K. Jarnagin, and Y.R. Freund. 2013. Inhibition of Toll-like receptor-mediated inflammation in vitro and in vivo by a novel benzoxaborole. Journal of Pharmacology and Experimental Therapeutics 344: 436–446.
Kondo, T., T. Kawai, and S. Akira. 2012. Dissecting negative regulation of Toll-like receptor signaling. Trends in Microbiology 33: 450–460.
Takeuchi, O., K. Hoshino, and S. Akira. 2000. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. Journal of Immunology 165: 5392–5396.
Shin, J.E., Y.S. Kim, J.E. Oh, B.M. Min, and Y. Choi. 2010. Treponema denticola suppresses expression of human beta defensin-3 in gingival epithelial cells through inhibition of the toll-like receptor 2 axis. Infection and Immunity 78: 672–679.
Das, D., and B. Bishayi. 2009. Staphylococcal catalase protects intracellularly survived bacteria by destroying H2O2 produced by the murine peritoneal macrophages. Microbial Pathogenesis 47: 57–67.
Paul-Clark, M.J., S.K. McMaster, and R. Sorrentino. 2009. Toll-like receptor 2 is essential for sensing of oxidants during inflammation. American Journal of Respiratory and Critical Care Medicine 179: 299–306.
Sorci, G., and B. Faivre. 2009. Inflammation and oxidative stress in vertebrate host-parasite system. Philosophical Transactions of the Royal Society B 364: 71–83.
West, A.P., I.E. Brodsky, C. Rahner, D.K. Woo, H. Erdjument-Bromage, P. Tempst, M.C. Walsh, Y. Choi, S. Gerald, G.S. Shadel, and S. Ghosh. 2011. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature 272: 476–482.
Martínez-Pulgarín, S., G. Dominguez-Bernal, J.A. Orden, and R. de la Fuente. 2009. Simultaneous lack of catalase and beta-toxin in Staphylococcus aureus leads to increased intracellular survival in macrophages and epithelial cells and to attenuated virulence in murine and ovine models. Microbiology 155: 1505–1515.
Mal, P., S. Dutta, D. Bandyopadhyay, K. Dutta, A. Basu, and B. Bishayi. 2012. Gentamicin in combination with ascorbic acid regulates the severity of Staphylococcus aureus infection-induced septic arthritis in mice. Scandinavian Journal of Immunology 76: 528–540.
Mathias, S., J.J. Naja, F. Ferracin, and R. Landmann. 2011. T and B cells are not required for clearing Staphylococcus aureus in systemic infection despite a strong TLR2–MyD88-dependent T cell activation. Journal of Immunology 186: 443–452.
Marim, F.M., T.N. Silveira, D.S. Lima Jr., and D.S. Zamboni. 2010. A method for generation of bone marrow-derived macrophages from cryopreserved mouse bone marrow cells. PloS One 5: e15263.
Das, D., and B. Bishayi. 2010. Contribution of catalase and superoxide dismutase to the intracellular survival of clinical isolates of Staphylococcus aureus in murine macrophages. Indian Journal of Microbiology 50: 375–384.
Lancioni, C.L., Q. Li, J.J. Thomas, X.D. Ding, B. Thiel, M.G. Drage, N.D. Pecora, A.G. Ziady, S. Shank, C.V. Harding, W.H. Boom, and R.E. Rojas. 2011. Mycobacterium tuberculosis lipoproteins directly regulate human memory CD4+ T cell activation via Toll-like receptors 1 and 2. Infection and Immunity 79: 663–673.
Swisher, J.F.A., N. Burton, S.M. Bacot, S.N. Vogel, and G.M. Feldman. 2010. Annexin A2 tetramer activates human and murine macrophages through TLR4. Blood 115: 549–558.
Gebbia, J.A., J.L. Coleman, and J.L. Benach. 2004. Selective induction of matrix metalloproteinases by Borrelia burgdorferi via Toll-like receptors 2 in monocyte. Journal of Infectious Diseases 189: 113–119.
Sen, R., D. Das, and B. Bishayi. 2010. Staphylococcal catalase regulates its virulence and induces arthritis in catalase deficient mice. Indian Journal of Physiology and Pharmacology 53: 307–317.
Leigh, P.C.J., R. van Furth, and T.L. van Zwet. 1986. In vitro determination of phagocytosis and intracellular killing by polymorphonuclear and mononuclear phagocytes. In Handbook of experimental Immunology, ed. D.M. Weir, 46.1–46.26. Oxford: Blackwell Scientific.
Absolom, D.R. 1986. Basic methods for the study of phagocytosis. Methods Enzymology 132: 95–180.
Bae, Yun Soo, Jee Hyun Lee, Soo Ho. Choi, Sunah Kim, Felicidad Almazan, Joseph L. Witztum, and Yury I. Miller. 2009. Macrophages generate reactive oxygen species in response to minimally oxidized low density lipoprotein: Toll like receptor 4 and spleen tyrosine kinase dependent activation of NADPH oxidase-2. Circulation Research 104: 210–218.
Nandi, D., M.K. Mishra, A. Basu, and B. Bishayi. 2010. Protective effects of interleukin-6 in lipopolysaccharide (LPS)-induced experimental endotoxemia is linked to alteration in hepatic anti-oxidant enzymes and endogenous cytokines. Immunobiology 215: 443–451.
Mal, P., D. Ghosh, D. Bandyopadhyay, K. Dutta, and B. Bishayi. 2012. Ampicillin alone and in combination with riboflavin modulates Staphylococcus aureus infection induced septic arthritis in mice. Indian Journal of Experimental Biology 50: 677–689.
Bishayi, B., D. Bandyopadhyay, A. Majhi, and R. Adhikary. 2014. Possible role of Toll like receptor-2 (TLR-2) in the intracellular survival of Staphylococcus aureus in murine peritoneal macrophages: involvement of cytokines and anti-oxidant enzymes. Scandinavian Journal of Immunology 80: 127–143.
Miller, R.A., and B.E. Britigan. 1997. Role of oxidants in microbial pathophysiology. Clinical Microbiology Reviews 10: 1–18.
Philippart, F., C. Fitting, and J.M. Cavaillon. 2012. Lung microenvironment contributes to the resistance of alveolar macrophages to develop tolerance to endotoxin. Critical Care Medicine 40: 2987–2996.
Gautier, E.L., T. Shay, J. Miller, M. Greter, C. Jakubzick, S. Ivanov, J. Helft, A. Chow, K.G. Elpek, S. Gordonov, A.R. Mazloom, A. Ma’ayan, W.J. Chua, T.H. Hansen, S.J. Turley, M. Merad, and G.J. Randolph. 2012. Gene expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nature Immunology 13: 1118–1128.
Michelsen, K.S., A. Aicher, M. Mohaupt, T. Hartung, S. Dimmeler, C.J. Kirschning, and R.R. Schumann. 2001. The role of Toll-like receptors (TLRs) in bacteria-induced maturation of murine dendritic cells (DCS): peptidoglycan and lipoteichoic acid are inducers of DC maturation and require TLR2. Journal of Biological Chemistry 276: 25680–25686.
Schwandner, R., R. Dziarski, H. Wesche, M. Rothe, and C.J. Kirschning. 1999. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by Toll like receptor 2. Journal of Biological Chemistry 274: 17406–17409.
Dziarski, R., and D. Gupta. 2005. Staphylococcus aureus peptidoglycan is a Toll-like receptor 2 activator: a reevaluation. Infection and Immunity 73: 5212–5216.
Mae, M., M. Iyori, M. Yasuda, H.M. Shamsul, H. Kataoka, K. Kiura, A. Hasebe, Y. Totsuka, and K. Shibata. 2007. The diacylated lipopeptide FSL-1 enhances phagocytosis of bacteria by macrophages through a Toll-like receptor 2-mediated signaling pathway. FEMS Immunology and Medical Microbiology 49: 398–409.
Jann, N.J., M. Schmaler, F. Ferracin, and R. Landmann. 2011. TLR2 enhances NADPH oxidase activity and killing of Staphylococcus aureus by PMN. Immunology Letters 135: 17–23.
Stuart, L.M., J. Deng, J.M. Silver, K. Takahashi, A.A. Tseng, E.J. Hennessy, R.A.B.. Ezekowitz, and K.J. Moore. 2005. Response to Staphylococcus aureus requires CD36-mediated phagocytosis triggered by the COOH-terminal cytoplasmic domain. Journal of Cell Biology 170: 477–485.
Yimin, M. Kohanawa, Z. Songji, M. Ozaki, S. Haga, G. Nan, Y. Kuge, and N. Tamaki. 2013. Contribution of Toll-like receptor 2 to the innate response against Staphylococcus aureus infection in mice. PloS One 9: e74287.
Rigby, K.M., and F.R. DeLeo. 2012. Neutrophils in innate host defense against Staphylococcus aureus infections. Seminars in Immunopathology 34: 237–259.
Heumann, D., C. Barras, A. Severin, M.P. Glauser, and A. Tomasz. 1994. Gram-positive cell walls stimulate synthesis of tumor necrosis factor alpha and interleukin-6 by human monocytes. Infection and Immunity 62: 2715–2721.
Timmerman, C.P., E. Mattsson, L. Martinez-Martinez, L. De Graaf, J.A. Van Strijp, H.A. Verbrugh, J. Verhoef, and A. Fleer. 1993. Induction of release of tumor necrosis factor from human monocytes by staphylococci and staphylococcal peptidoglycans. Infection and Immunity 61: 4167–4172.
Wakabayashi, G., J.A. Gelfand, W.K. Jung, R.J. Connolly, J.F. Burke, and C.A. Dinarello. 1991. Staphylococcus epidermidis induces complement activation, tumor necrosis factor and interleukin-1, a shock-like state and tissue injury in rabbits without endotoxemia. Comparison to Escherichia coli. Journal of Clinical Investigation 87: 1925–1935.
Jiang, W., B. Li, X. Zheng, X. Liu, Y. Cen, J. Li, X. Pan, H. Cao, J. Zheng, and H. Zhou. 2011. Artesunate in combination with oxacillin protect sepsis model mice challenged with lethal live methicillin-resistant Staphylococcus aureus (MRSA) via its inhibition on proinflammatory cytokines release and enhancement on antibacterial activity of oxacillin. International Immunopharmacology 11: 1065–1073.
Frodermann, V., T.A. Chau, S. Sayedyahossein, J.M. Toth, D.E. Heinrichs, and J. Madrenas. 2011. A modulatory interleukin-10 response to staphylococcal peptidoglycan prevents Th1/Th17 adaptive immunity to Staphylococcus aureus. Journal of Infectious Diseases 204: 253–262.
Manicassamy, S., and B. Pulendran. 2009. Modulation of adaptive immunity with Toll-like receptors. Seminars in Immunology 21: 185–193.
Deshmukh, H.S., J.B. Hamburger, S.H. Ahn, D.G. McCafferty, S.R. Yang, G. Vance, and Fowler Jr. 2009. Critical role of NOD2 in regulating the immune response to Staphylococcus aureus. Infection and Immunity 77: 1376–1382.
Schindler, H., M.B. Lutz, M. Röllinghoff, and C. Bogdan. 2001. The production of IFN-γ by IL-12/IL-18-activated macrophages requires STAT4 signaling and is inhibited by IL-4. Journal of Immunology 166: 3075–3082.
Presky, D.H., H. Yang, L.J. Minetti, A.O. Chua, N. Nabavi, C.Y. Wu, M.K. Gately, and U. Gubler. 1996. A functional interleukin 12 receptor complex is composed of two beta-type cytokine receptor subunits. Proceedings of the National Academy of Sciences of the United States of America 93: 14002–14007.
Torres, D., M. Barrier, F. Bihl, V.J.F. Quesniaux, I. Maillet, S. Akira, B. Ryffel, and F. Erard. 2004. Toll-like receptor 2 is required for optimal control of Listeria monocytogenes infection. Infection and Immunity 72: 2131–2139.
Conroy, H., N.A. Marshall, and K.H. Mills. 2008. TLR ligand suppression or enhancement of Treg cells? A double-edged sword in immunity to tumors. Oncogene 27: 168–180.
Henderson, B., and S.P. Nair. 2003. Hard labour: bacterial infection of the skeleton. Trends in Microbiology 11: 570–577.
Ellington, J.K., S.S. Reilly, W.K. Ramp, M.S. Smeltzer, J.F. Kellam, and M.C. Hudson. 1999. Mechanisms of Staphylococcus aureus invasion of cultured osteoblasts. Microbial Pathogenesis 26: 317–323.
Hudson, M.C., W.K. Ramp, N.C. Nicholson, A.S. Williams, and M.T. Nousiainen. 1995. Internalization of Staphylococcus aureus by cultured osteoblasts. Microbial Pathogenesis 19: 409–419.
Jevon, M., C. Guo, B. Ma, N. Mordan, S.P. Nair, M. Harris, B. Henderson, G. Bentley, and S. Meghji. 1999. Mechanisms of internalization of Staphylococcus aureus by cultured human osteoblasts. Infection and Immunity 67: 2677–2681.
Khalil, H., R.J. Williams, G. Stenbeck, B. Henderson, S. Meghji, and S.P. Nair. 2007. Invasion of bone cells by Staphylococcus epidermidis. Microbes and Infection 9: 460–465.
Reilly, S.S., M.C. Hudson, J.F. Kellam, and W.K. Ramp. 2000. In vivo internalization of Staphylococcus aureus by embryonic chick osteoblasts. Bone 26: 63–70.
Kwan, T.S., M. Padrines, S. Theoleyre, D. Heymann, and Y. Fortun. 2004. IL-6, RANKL, TNF-alpha/IL-1: interrelations in bone resorption pathophysiology. Cytokine Growth Factor Reviews 15: 49–60.
Mundy, G.R. 1991. Inflammatory mediators and the destruction of bone. Journal of Periodontal Research 26: 213–217.
Pfeilschifter, J., C. Chenu, A. Bird, G.R. Mundy, and G.D. Roodman. 1989. Interleukin-1 and tumor necrosis factor stimulate the formation of human osteoclast-like cells in vitro. Journal of Bone and Mineral Research 4: 113–118.
Tokukoda, Y., S. Takata, H. Kaji, R. Kitazawa, T. Sugimoto, and K. Chihara. 2001. Interleukin-1beta stimulates transendothelial mobilization of human peripheral blood mononuclear cells with a potential to differentiate into osteoclasts in the presence of osteoblasts. Journal of Endocrinology 48: 443–452.
Ishimi, Y., C. Miyaura, C.H. Jin, T. Akatsu, E. Abe, Y. Nakamura, A. Yamaguchi, S. Yoshiki, T. Matsuda, and T. Hirano. 1990. IL-6 is produced by osteoblasts and induces bone resorption. Journal of Immunology 145: 3297–3303.
Kotake, S., K. Sato, K.J. Kim, N. Takahashi, N. Udagawa, I. Nakamura, A. Yamaguchi, T. Kishimoto, T. Suda, and S. Kashiwazaki. 1996. Interleukin-6 and soluble interleukin-6 receptors in the synovial fluids from rheumatoid arthritis patients are responsible for osteoclast-like cell formation. Journal of Bone and Mineral Research 11: 88–95.
Meghji, S., S.J. Crean, P.A. Hill, M. Sheikh, S.P. Nair, K. Heron, B. Henderson, E.B. Mawer, and M. Harris. 1998. Surface-associated protein from Staphylococcus aureus stimulates osteoclastogenesis: possible role in S. aureus-induced bone pathology. British Journal of Rheumatology 37: 1095–1101.
Nair, S., Y. Song, S. Meghji, K. Reddi, M. Harris, A. Ross, S. Poole, M. Wilson, and B. Henderson. 1995. Surface-associated proteins from Staphylococcus aureus demonstrate potent bone resorbing activity. Journal of Bone and Mineral Research 10: 726–734.
Yokoyama, R., S. Itoh, G. Kamoshida, T. Takii, S. Fujii, T. Tsuji, and K. Onozakia. 2012. Staphylococcal superantigen-like protein 3 binds to the Toll-like receptor 2 extracellular domain and inhibits cytokine production induced by Staphylococcus aureus, cell wall component, or lipopeptides in murine macrophages. Infection and Immunity 80: 2816–2825.
Wright, J.A., and S.P. Nair. 2010. Interaction of staphylococci with bone. International Journal of Medical Microbiology 300: 193–204.
Bosse, M.J., H.E. Gruber, and W.K. Ramp. 2005. Internalization of bacteria by osteoblasts in a patient with recurrent, long-term osteomyelitis: a case report. Journal of Bone and Joint Surgery 87: 1343–1347.
Hamza, T., M. Dietz, D. Pham, N. Clovis, S. Danley, and B. Li. 2013. Intracellular Staphylococcus aureus alone causes infection in vivo. European Cells and Materials 25: 341–350.
Facecchia, K., L.A. Fochesato, S.D. Ray, S.J. Stohs, and S. Pandey. 2011. Oxidative toxicity in neurodegenerative diseases: role of mitochondrial dysfunction and therapeutic strategies. Journal of Toxicology :Article ID 683728. doi:10.1155/2011/683728
Hampton, M.B., A.J. Kettle, and C.C. Winterbourn. 1998. Inside the neutrophil phagosome: oxidants, myeloperoxidase and bacterial killing. Blood 92: 3007–3017.
Stor, Z.G., L.A. Tartaglia, S.B. Farr, and B.N. Ames. 1990. Bacterial defense against oxidative stress. Trends in Genetics 6: 363–368.
Bogdan, C., M. Rollinghoff, and A. Diefenach. 2000. Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Current Opinion Immunology 12: 64–76.
Komuro, I., N. Keicho, A. Iwamoto, and K.S. Akagawa. 2001. Human alveolar macrophages and granulocyte macrophages colony stimulating factor-induced monocyte derived macrophages are resistant to H2O2 via their high basal and inducible levels of catalase activity. Journal of Biological Chemistry 276: 24360–24364.
Tripp, C.S., S.F. Wolf, and E.R. Unanue. 1993. Interleukin 12 and tumor necrosis factor alpha are costimulators of interferon gamma production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proceedings of the National Academy of Sciences of the United States of America 90: 3725–3729.
Bishayi, B., D. Bandyopadhyay, A. Majhi, and R. Adhikary. 2014. Expression of CXCR1 (interleukin-8 receptor) in murine macrophages after Staphylococcus aureus infection and its possible implication on intracellular survival correlating with cytokines and bacterial anti-oxidant enzymes. Inflammation. doi:10.1007/s10753-014-9991-1.
ACKNOWLEDGMENTS
This work was supported/funded by the Department of Science and Technology (DST), Science and Engineering Research Board (SERB), Ministry of Science and Technology, Government of India, New Delhi, India [Grant Number: SR/SO/HS/0013/2012, dated 21 May 2013 to Biswadev Bishayi]. The author (BB) is indebted to the Department of Science and Technology, Government of India for providing the instruments procured under the DST-PURSE programme to the Department of Physiology, University of Calcutta. The Department of Science and Technology, Government of India is also thanked for providing the DST-INSPIRE fellowship to Mrs. Ajeya Nandi [Grant Number: DST INSPIRE FELLOWSHIP/2013/1118, dated 23 June 2014]. The authors remained thankful to Dr Debajit Bhowmick, Ph.D., CU BD COE Manager, of the Centre for Research in Nanoscience and Nanotechnology, Acharya Prafulla Chandra Roy Siksha Prangan affiliated to the University of Calcutta, JD-2, Sector III, Salt Lake, Kolkata 700098, West Bengal, India for performing the flow cytometry.
Conflict of Interest
All authors declared that they have no conflict of interest. They also state that they do not have a direct financial relation with the commercial identities mentioned in this manuscript that might lead to a conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nandi, A., Dey, S., Biswas, J. et al. Differential Induction of Inflammatory Cytokines and Reactive Oxygen Species in Murine Peritoneal Macrophages and Resident Fresh Bone Marrow Cells by Acute Staphylococcus aureus Infection: Contribution of Toll-Like Receptor 2 (TLR2). Inflammation 38, 224–244 (2015). https://doi.org/10.1007/s10753-014-0026-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-014-0026-8