Abstract
Exposure to nickel (Ni2+) can trigger allergic reactions in susceptible individuals, which is widely accepted as the major cause of allergic contact hypersensitivity (CHS) worldwide. Although Ni2+-induced proinflammatory responses clearly play a pivotal role in CHS, the underlying molecular mechanism has not been fully defined. Here we report that Ni2+ activates the NLRP3–ASC–caspase-1 immune signaling pathway in antigen-presenting cells, leading to the proteolytic processing and secretion of a proinflammatory cytokine, interleukin-1β (IL-1β). The activation of this signaling axis is independent of phagolysosome–cathepsin B pathway. Instead, Ni2+ induces mitochondrial reactive oxygen species accumulation and cation fluxes, both of which are required for activating the NLRP3–ASC–caspase-1 pathway. Together, these results identified a novel innate immune signaling pathway (NLRP3–ASC–caspase-1–IL-1β) activated by Ni2+ and provided a mechanistic basis for optimizing the therapeutic intervention against Ni2+-induced allergy in patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Allergic contact dermatitis (ACD) has been continuously regarded as one of the major immunologic problems worldwide. Allergies to nickel (Ni2+) is one of the well-known major causes of ACD, and it is estimated that about 18 % of women and 3.4 % of men are allergic to the Ni2+ released from jewelry (i.e., necklaces and rings), buttons of clothing, and even coins [1, 2]. ACD is initiated based on the exposure to an allergic substance (termed “contact hypersensitivity” (CHS)) that requires antigen presentation by dendritic cells and subsequent generation of allergen-specific T cell responses. After the first allergen encounter (the “sensitization” step), if the susceptible individuals are re-exposed to the contact allergy, clinical skin manifestations, such as rash, itching, skin thickening, and lesion, will appear [1, 3, 4]. Besides the crucial role of T cells in CHS, it has been shown that the innate proinflammatory signals are also essential for the efficient sensitization because these innate immune signals are responsible for inducing cytokines/chemokines secretion from dendritic cells/phagocytes, upregulating co-stimulatory molecules and facilitating maturation of these innate immune cells [3, 5, 6].
Gene profiling studies have shown that Ni2+ treatment can elicit upregulation of a pool of innate immune genes, predominantly with proinflammatory functions. In line with this, recent studies have demonstrated that Ni2+ can activate nuclear factor-κB (NF-κB) and the mitogen-activated protein (MAP) kinase p38, presumably via direct interaction and activation of human Toll-like receptor 4 (TLR4) [6]. However, little is known regarding whether other innate immune sensors, such as nucleotide-binding oligomerization domain-like receptors (NLRs), are also involved in recognition of Ni2+. As studies in the past decades have indicated the crucial roles of Nod-like receptors in sensing both the exogenous pathogens/irritants and endogenous danger signals [7–9], we hypothesized that certain Nod-like receptor may also serve as an innate immune sensor for Ni2+, and the recognition and subsequent activation of such innate immune signaling pathway may contribute to the Ni2+-associated contact dermatitis.
In this study, we report that Ni2+ activates the NLRP3–ASC–caspase-1 inflammasome in antigen-presenting cells, resulting in the release of bioactive interleukin-1β (IL-1β). Moreover, we further demonstrate that this innate immune recognition process involves lysosome rupture, mitochondrial reactive oxygen species (ROS) generation, and cation fluxes. Our study provides a previously unknown innate immune sensing mechanism for Ni2+, which may thus benefit future therapies against Ni2+-associated allergic contact dermatitis.
MATERIALS AND METHODS
Reagents
NiCl2 · 6H2O (Ni2+), phorbol 12-myristate 13-acetate (PMA), cytochalasin D, ATP, uricase, Ca-074-Me, and BAPTA-AM were purchased from Sigma-Aldrich. Imject Alum and streptavidin–HRP were purchased from Pierce. Z-YVAD-fmk was purchased from Alexis Biochemicals; monosodium urate crystals and Mito-TEMPO were purchased from Enzo Life Science. Ultrapure lipopolysaccharide (LPS) and poly(dA/dT) were from InvivoGen. Lipofectamine 2000 and MitoSOX were purchased from Invitrogen. Antibodies used for immunoblotting were listed as the following: anti-human cleaved caspase-1 (p20) (catalog no. 4199), anti-human β-actin (catalog no. 4967), and anti-rabbit IgG HRP-conjugated antibody (catalog no. 7074). All the above three antibodies were from Cell Signaling. Anti-human cathepsin B (catalog no. sc-13985) was from Santa Cruz Biotechnology.
Cell Culture and Stimulation
Bone marrow-derived macrophages (BMDMs) and bone marrow dendritic cells (BMDCs) were generated from murine bone marrow cells as previously described [10, 11]. After pretreatment with ultrapure LPS (100 ng/ml) overnight, the cells were then treated with stimuli. THP1 cells were purchased from ATCC and cultured in RPMI1640 medium, supplemented with 10 % fetal bovine serum (FBS) and 1 % penicillin–streptomycin. THP1 cell differentiation towards a macrophage-like phenotype was achieved by stimulation with PMA (100 nM) for 3 h. The differentiated THP1 cells were then washed, re-suspended and rested overnight. Various stimuli as described in individual experiments were added the next day, and supernatants were collected to detect IL-1β by ELISA. The immortalized murine macrophages from Nlrp3 −/−, Asc −/−, Capase-1 −/−, Nlrc4 −/−, Cathepsin B−/−, Aim2 −/− mice, and their corresponding wild-type control cells were from Dr. Katherine Fitzgerald and as previously described [10]. For experiments in which poly(dA/dT) were used, they were transfected using Lipofectamine 2000 (3 μg/ml) according to the manufacturer's instructions. For the experiments using various inhibitors, they were added 30 to 60 min before NLRP3 inflammasome stimuli. Potassium efflux was blocked by 150 mM of KCl (the same concentration of NaCl were used as controls) as previously described [12]. After inflammasome agonists’ stimulation (Ni2+ and alum for 18 h, poly(dA/dT) for 6 h, and ATP for 1 h), the culture supernatants and/or lysates were collected and subjected to ELISA and/or immunoblot analysis as described in individual experiments.
Enzyme-Linked Immunosorbent Assay
The corresponding “capture” and “detection” antibodies and standard recombinant proteins for human or mouse IL-1β were purchased from eBioscience. They were used at concentrations as recommended by the manufacturer.
Immunoblot Analysis
The immunoblot analysis was performed as previously described [13–16]. Briefly, the cellular protein extracts were prepared by lysing the cells in 300 μl of lysis buffer (1 % NP-40, 200 μM PMSF, 0.1 % SDS, 0.5 % sodium deoxycholate, 0.2 mM sodium vanadate, 50 mM sodium fluoride, protease inhibitor, and phosphatase inhibitor cocktails in PBS). After harvesting the cells, they were subjected to sonication followed by centrifugation at 15,000 rpm for 8 min. The supernatants were then collected and transferred into fresh tubes. A standard protein concentration determination protocol (Bradford assay kit from Bio-Rad) was used to determine the total concentrations of cellular protein. For immunoblotting of secreted proteins, the preparations of protein extracts from culture supernatants were performed as previously described [10]. The total proteins obtained were then separated by SDS-PAGE, and after the nitrocellulose membrane transfer, they were immunoblotted using corresponding antibodies.
Detection of Cathepsin B Release from Lysosome
The cytosolic release of cathepsin B was measured in human THP1 cells. Briefly, the THP1 cells (grown in 6-well plate at 3 × 106 cells per well using plain DMEM medium) were first differentiated into macrophages by stimulation of 100 nM PMA for 3 h. Then the differentiated THP1 cells were washed with PBS and rested overnight in plain DMEM medium without addition of FBS or antibiotics. The next morning, the cells were washed four times with PBS before stimulation with alum or Ni2+ (for 18 h). Then, the cells were collected and subjected to permeabilization with 50 μg/ml digitonin and 1 mM phenylmethylsulfonyl fluoride in PBS (pH 7.4) for 10 min on ice. This procedure has been shown to successfully lyse the plasma membrane while still keeping the integrity of membranes of intracellular organelles [17]. The cellular extract was then subjected to immunoblot analysis for the detection of human cathepsin B.
Mitochondrial ROS Detection
The level of mitochondrial ROS was measured by flow cytometry as previously described [18, 19]. MitoSOX, a dye that stains superoxide specifically produced from mitochondria, was used to quantify the relative amounts of mitochondrial ROS after stimulations. Briefly, cells (PMA-primed THP1) were first stimulated with Ni2+ for 18 h, and then loaded with 2.5 μM of MitoSOX for 20 min. The cells were then washed four times with phosphate buffered saline (PBS) and the levels of mitochondrial ROS were measured via flow cytometry. The obtained data were further analyzed using FlowJo software (Treestar).
Statistics
All the presented data are shown as mean ± SD. Statistical analysis between groups was performed using a two-tailed Student’s t test in all studies. P values less than 0.05 were considered statistically significant.
RESULTS
Ni2+ Induces Caspase-1 Activation and Mature IL-1β Secretion from Antigen-Presenting Cells
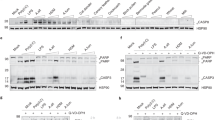
We first tested whether Ni2+ could induce IL-1β release from antigen-presenting cells (APCs). As shown in Fig. 1, Ni2+ triggered IL-1β secretion from human THP1 cells (Fig. 1a), human periphery blood mononuclear cells (PBMCs) (Fig. 1b), murine bone BMDMs (Fig. 1c), or BMDCs (Fig. 1d) after PMA or LPS priming step which induces pro-IL-1β generation as previously suggested [7–9]. In consistence with Ni2+-induced IL-1β secretion, procaspase-1 was auto-cleaved into mature caspase-1 because the active p20 subunit of caspase-1 was detected after Ni2+ stimulation (Fig. 1e). Similar to other inflammasome agonists [12, 20–23], active caspase-1 was required for Ni2+-induced IL-1β secretion, because the blockade of caspase-1 activity by Z-YVAD-fmk [12, 20, 24] abolished IL-1β secretion (Fig. 1f). Together, these results demonstrate that Ni2+ can induce caspase-1 activation and IL-1β secretion in antigen-presenting cells.
Ni2+ induces caspase-1 activation and IL-1β secretion from antigen-presenting cells. The levels of IL-1β from supernatants of PMA-primed THP1 cells (a), LPS-primed human PBMCs (b), mouse BMDMs (c), or BMDCs (d) after stimulation with PBS or various concentration of Ni2+ as indicated in (a–d). *p < 0.05; **p < 0.01; ***p < 0.001 versus controls. Immunoblot analysis for caspase-1 p20 (cleaved caspase-1) and β-actin in cell lysates or cleaved IL-1β (p17) in culture supernatants from PMA-primed THP1 cells after stimulation with PBS or Ni2+ (1.5 mM) (e). IL-1β from supernatants of PMA-primed THP1 cells that were treated with Z-YVAD-fmk and then followed by addition of Ni2+ (1.5 mM) or alum (250 μg/ml) (f). ns not significant. **p < 0.01; ***p < 0.001. Data in (a–d) and (f) are shown as mean ± SD, and all data are representative of three to five independent experiments.
The NLRP3–ASC–Caspase-1 Inflammasome Directs Ni2+-Induced IL-1β Secretion
The NLRs are known to direct caspase-1 activation in response to various stimuli, including pathogenic bacteria/viruses, environmental irritants, and endogenous danger molecules [7–10, 12, 16, 25]. Therefore, we next tested which NLR(s) mediate the Ni2+-induced IL-1β secretion. As the NLRP3 has been previously shown to sense foreign pathogens or irritants [7–9], we first determined if NLRP3 was involved in Ni2+-induced IL-1β secretion. Indeed, Nlrp3 −/− BMDMs were defective of secreting IL-1β after Ni2+ stimulation (Fig. 2a). In line with this, Asc −/− or caspase-1 −/− BMDMs also had impaired IL-1β secretion (Fig. 2a). However, BMDMs that are deficient in NLRC4, a sensor protein of another inflammasome that recognizes bacterial components, such as the flagellin [26], released comparable levels of IL-1β as wild-type cells after Ni2+ stimulation (Fig. 2a). Similarly, we found that deficiency in AIM2 (absence in melanoma 2) inflammasome also had no effect on Ni2+-induced IL-1β secretion (Fig. 2b, c). These data together imply that Ni2+-induced IL-1β secretion is exclusively mediated by the NLRP3 inflammasome. To rule out the possibility that Ni2+-induced IL-1β secretion may be mediated via other NLRP3 inflammasome endogenous activators, such as ATP or uric acid crystals [21, 23], we next investigated whether blocking ATP/uric acid crystals triggered inflammasome activation may affect Ni2+-induced IL-1β secretion. As shown in Fig. 3a, addition of apyrase (an ATP-hydrolyzing enzyme) [27, 28] did not alter Ni2+-induced IL-1β secretion. Similarly, Ni2+-induced IL-1β release was also independent of uric acid crystals because addition of uricase, which degrades uric acid [12, 27], did not block IL-1β secretion by Ni2+ (Fig. 3b). Together, these data demonstrate that the NLRP3 serves as an innate immune sensor for Ni2+.
The NLRP3–ASC–caspase-1 inflammasome mediates Ni2+-induced IL-1β secretion. a The secretion of IL-1β was measured after stimulation of Ni2+ (1.5 mM) or alum (200 μg/ml) in LPS-primed immortalized mouse macrophages that are derived from wild type, Nlrp3 −/−, Asc −/−, caspase-1 −/−, or Nlrc4 −/− mice. b Levels of IL-1β were shown in LPS-primed Aim2-sufficient (Aim2 +/+) or Aim2-deficient (Aim2 −/−) BMDMs after stimulation of Ni2+ or poly(dA/dT) (2 μg/ml). c Levels of IL-1β were shown in WT, Asc −/−, and Casp1 −/− BMDMs after stimulation with Ni2+ or poly(dA/dT). ns not significant. *p < 0.05; **p < 0.01. All data are shown as mean ± SD and are representative of at least three independent experiments.
ATP and uric acid are not intermediates for Ni2+-induced IL-1β secretion. a The secretion of IL-1β from LPS-primed mouse BMDMs that were treated with apyrase (10 U/ml) before the addition of Ni2+ (1.5 mM) or ATP (1 mM). b The level of IL-1β from supernatants of LPS-primed mouse BMDMs that were treated with uricase (8 U/ml) before the addition of Ni2+ (1.5 mM) or monosodium urate crystals (MSU, 300 μg/ml). Data are shown as mean ± SD and are representative of three independent experiments. **p < 0.01.
Ni2+ Activates NLRP3 Inflammasome Activation Independently of the Phagolysosome Pathway
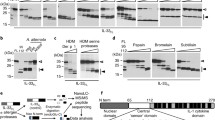
Antigen-presenting cells, such as macrophages and dendritic cells, are important players for initiation and progression of Ni2+-associated CHS [1, 2, 5], we therefore investigated the molecular mechanism underlying the Ni2+-induced NLRP3 inflammasome activation in these cells. The actin-dependent endocytic pathway has been previously shown as a prerequisite for NLRP3 inflammasome activation by many NLRP3 agonists, such as alum and silica crystals [10, 12, 29]. To test the role of actin-dependent phagocytosis in Ni2+-induced NLRP3 inflammasome activation, we utilized an actin polymerization inhibitor, cytochalasin D [30], to block actin-dependent endocytic pathways. Consistent with previous reports10, cytochalasin D greatly reduced alum-induced IL-1β secretion (Fig. 4a), whereas the ATP-induced NLRP3 inflammasome activation was largely unaffected. In contrast to alum, Ni2+-induced NLRP3 inflammasome activation appears to be independent of phagocytosis because cytochalasin D pretreatment had minimal effect on Ni2+-induced IL-1β secretion (Fig. 4a). These data suggest that actin-dependent phagocytic pathway is not required step for Ni2+-induced NLRP3 inflammasome activation and IL-1β secretion.
The phagolysosome pathway is dispensable for Ni2+-induced NLRP3 inflammasome activation. a The secretion of IL-1β in PMA-primed THP1 cells that were treated with cytochalasin D before the addition of Ni2+, alum, or ATP. b IL-1β levels in supernatants from PMA-primed THP1 cells after pretreatment of bafilomycin A1 followed by stimulation with Ni2+, alum, or ATP. c Immunoblot analysis for detecting the release of cathepsin B into the cytosol of THP1 cells after stimulation with Ni2+ or alum. d The secretion of IL-1β from LPS-primed mouse BMDMs that were treated with Ca-074-Me (10 μM) after stimulation with Ni2+ or alum. Data in (a, b, d) are shown as mean ± SD, and all data are representative of three to five independent experiments. *p < 0.05 and **p < 0.01 versus controls.
Additionally, to further verify our results, we tested whether cathepsin B, a lysosomal cysteine protease known to mediate NLRP3 inflammasome activation by many NLRP3 agonists [10, 31] was required for Ni2+-induced NLRP3 inflammasome activation. To this end, we first determined whether blocking lysosomal acidification, a crucial step that facilitates cathepsin B release from lysosome, would affect Ni2+-induced NLRP3 inflammasome activation. In support to previous studies, the addition of bafilomycin A1, which blocks the lysosomal acidification, significantly attenuated alum-induced IL-1β secretion. However, the Ni2+-induced IL-1β release was not significantly affected (Fig. 4b). Furthermore, we were only able to detect cathepsin B in the cytosol after treatment of alum, but not Ni2+ (Fig. 4c). Consistently, we found that inhibition of cathepsin B activity by CA-074-Me only impaired IL-1β release after stimulation with alum, but not Ni2+ (Fig. 4d). Together, these data demonstrate that the “lysosome–cathepsin B” pathway is dispensable for Ni2+-induced NLRP3 inflammasome activation.
Ni2+ Activates NLRP3 Inflammasome via Mitochondrial Reactive Oxygen Species
Previous studies have shown that most, if not all, NLRP3 agonists treatment leads to mitochondrial stress and/or damage, resulting in mitochondrial ROS leaking from the oxidative respiration chain to activate the NLRP3 inflammasome [18, 19]. Therefore, to test whether ROS is also required for Ni2+-induced NLRP3 inflammasome activation, we first sought to determine whether Ni2+ could increase mitochondrial ROS production. Indeed, Ni2+ stimulation significantly elevated mitochondrial ROS production (Fig. 5a) in antigen-presenting cells. As Mito-TEMPO has been previously shown to specifically block mitochondrial ROS generation [18], we next tested if Mito-TEMPO could block Ni2+-induced mitochondrial ROS production and subsequent IL-1β secretion. As expected, similar to ATP, the Ni2+-induced mitochondrial ROS and IL-1β secretion were dramatically diminished (Fig. 5b, c). In contrast, the double-stranded DNA (polydA/dT)-induced IL-1β release, which is independent of ROS and NLRP3 inflammasome [32, 33], was largely unaffected by Mito-TEMPO (Fig. 5c). Taken together, these data suggest that mitochondrial ROS production is a critical step during Ni2+-induced NLRP3 inflammasome activation.
Ni2+ activates the NLRP3 inflammasome via inducing mitochondrial ROS. a Flow cytometric analysis for mitochondrial ROS (labeled by MitoSOX) was shown in PMA-primed THP1 cells after stimulation with PBS or Ni2+. b The levels of mitochondrial ROS, measured by MitoSOX flow cytometry, were normalized to untreated controls (n = 3) in PMA-primed THP1 cells that were treated with Mito-TEMPO before stimulation of Ni2+ or ATP. c The secretion of IL-1β in PMA-primed THP1 cells that were treated with Mito-TEMPO before the addition of Ni2+, ATP, or poly(dA/dT). Data in (b) and (c) are shown as mean ± SD, and all data are representative of three independent experiments. *p < 0.05 and **p < 0.01 versus controls.
Potassium and Calcium Mobilizations Orchestrate Ni2+-Induced NLRP3 Inflammasome Activation
The mobilizations of two cations (potassium and calcium) have been recently linked to inflammation in myeloid cells [34–36]. Cytosolic depletion of potassium and elevation of intracellular free calcium have been shown to be prerequisites for the assembly of NLRP3 inflammasome [34, 36]. To determine whether cation fluxes are required for Ni2+ to activate the NLRP3 inflammasome, we first tested whether the inhibition of potassium efflux would affect the IL-1β release. When the cells were treated with Ni2+ in the presence of high concentration of extracellular KCl, but not NaCl, the activation of NLRP3 inflammasome was greatly diminished (Fig. 6a). Moreover, intracellular calcium elevation is also essential for NLRP3 inflammasome activation because the addition of intracellular calcium chelator BAPTA-AM prior to Ni2+ stimulation eradicated the IL-1β release, whereas the poly(dA/dT)-induced IL-1β secretion was unaffected (Fig. 6b). Collectively, these data indicate that potassium depletion and calcium elevation in the cytosol are prerequisites for the NLRP3 inflammasome activation in response to Ni2+.
Potassium and calcium fluxes orchestrate the Ni2+-induced NLRP3 inflammasome activation. a The secretion of IL-1β in PMA-primed THP1 cells that were placed in 150 mM of KCl or NaCl and then stimulated with Ni2+, alum, or poly(dA/dT). b The levels of released IL-1β from LPS-primed mouse BMDMs that were treated with BAPTA-AM (20 μM) before the stimulation with Ni2+, alum, or poly(dA/dT). Data are shown as mean ± SD, and all data are representative of three independent experiments. *p < 0.05 and **p < 0.01 versus controls.
DISCUSSION
Metal-induced allergic contact dermatitis has been one of the major clinical manifestations of contact hypersensitivity [3–5]. Ni2+ is widely accepted to be the most frequently encountered contact allergen that is commonly found in jewelry, buttons of clothing, and coins [3–5]. Although the precise mechanism of Ni2+-induced allergic responses has not been completely defined, both the innate and adaptive immunities are thought to cooperatively drive allergic immune responses induced by Ni2+ exposure [3–5]. Typically, the onset of contact dermatitis requires two steps: the “sensitization” step and the “challenge” step. During the first sensitization step, which resembles the “priming” step of pathogen encounter, the allergen is thought to be initially detected by the innate immune system, presumably via engaging pattern recognition receptors (PRRs). This recognition results in the activation of downstream immune signaling pathways to elevate the expression of multiple proinflammatory genes and immune adhesion molecules. These events would ultimately contribute to the efficient allergen uptake/presentation by antigen-presenting cells and amplification of local inflammation. The allergen-loaded APCs will then migrate to secondary lymphoid organs to present allergen-associated epitopes to T cells, which can then differentiate, clonally divide to large colonies, and are ready to rapidly respond to the allergy “re-exposure” in the challenge step. During the second challenge step, the encounter of the same allergen in susceptible individuals will lead to more rapid allergen-specific immune responses, presumably driven by T cells. This rapid secondary allergen recognition can cause massive undesirable inflammatory responses, characterized by local skin rash, itchiness, redness, swelling, and lesion [2–5].
Although Ni2+ has been recently shown to bind to Toll-like receptor 4 at the unconventional histidine residues [6], whether other PRRs can also sense the presence of Ni2+ remains largely unknown. Given that multiple innate sensing pathways exist for most pathogens and endogenous danger signals, a comprehensive appreciation of the innate immune recognition mechanisms for Ni2+ is of fundamental importance for optimizing the therapies against Ni2+-induced CHS. In this study, we identified the NLRP3–ASC–caspase-1 inflammasome as a novel innate immune sensor for Ni2+ and further revealed that Ni2+ can induce NLRP3 inflammasome-dependent secretion of bioactive IL-1β, a powerful proinflammatory cytokine.
Furthermore, we also delineated the mechanism involved in this innate immune recognition process. By utilizing the combination of genetic and pharmacologic approaches, we first found that Ni2+-triggered NLRP3 inflammasome activation was independent of actin-mediated phagocytosis. This finding suggests that unlike NLRP3 insoluble agonists, such as alum and silica crystals, the soluble NiCl2 · 6H2O (Ni2+)-induced inflammasome activation occurs independent of actin-mediated phagocytosis, which is not unexpected. In line with this, we confirmed that the lysosome–cathepsin B pathway was not required, because the blockade of lysosomal acidification and cathepsin B activity had no effect on NLRP3 inflammasome activation by Ni2+. These results together demonstrate that unlike the insoluble crystalline substances, Ni2+ activates the NLRP3 inflammasome in a manner that is independent of phagolysosome pathway.
In support of previous findings, we found that mitochondrial ROS, which have been shown to regulate NLRP3 inflammasome activation by almost all activators [37–41], also played a role in Ni2+-induced NLRP3 inflammasome activation. Macrophages stimulated with Ni2+ accumulated excess mitochondrial ROS, the inhibition of which abolished Ni2+-induced NLRP3 inflammasome activation. ROS are notorious at causing cellular anomalies via affecting the normal functions of proteins, lipids, and DNA [39, 40, 42], all of which might either independently or cooperatively contribute to NLRP3 inflammasome activation. Moreover, our results also showed that the blockade of potassium and calcium fluxes abolished Ni2+-induced NLRP3 inflammasome activation. These data suggest that similar to other NLRP3 stimuli [7–9, 34, 36], the unique ionic environment also drives NLRP3 inflammasome activation by Ni2+. Interestingly, recent studies have also demonstrated that ER calcium release may directly facilitate pumping calcium into the mitochondria to induce more ROS generation [19, 34]. Therefore, it appears that ROS and cation mobilization are interconnected in regulating the activation of NLRP3 inflammasome, although the precise mechanism is still largely unknown.
In summary, our study reveals that Ni2+ can activate the NLRP3–ASC–caspase-1 inflammasome via inducing mitochondrial stress and cation fluxes, which eventually lead to the secretion of proinflammatory cytokine IL-1β. Therefore, these results have expanded the current understanding of innate immune activating features of Ni2+ and provided a novel mechanistic basis for targeting Ni2+-induced innate immune pathways to counteract Ni2+-associated ACD.
References
Freudenberg, M.A., P.R. Esser, T. Jakob, C. Galanos, and S.F. Martin. 2009. Innate and adaptive immune responses in contact dermatitis: Analogy with infections. Giornale Italiano di Dermatologia e Venereologia: Organo Ufficiale, Societa Italiana di Dermatologia e Sifilografia 144: 173–185.
Grabbe, S., and T. Schwarz. 1998. Immunoregulatory mechanisms involved in elicitation of allergic contact hypersensitivity. Immunology Today 19: 37–44.
Spiewak, R., J. Pietowska, and K. Curzytek. 2007. Nickel: A unique allergen - from molecular structure to European legislation. Expert Review of Clinical Immunology 3: 851–859.
Liden, C., L. Skare, and M. Vahter. 2008. Release of nickel from coins and deposition onto skin from coin handling–comparing euro coins and SEK. Contact Dermatitis 59: 31–37.
Martin, S.F., and T. Jakob. 2008. From innate to adaptive immune responses in contact hypersensitivity. Current Opinion in Allergy and Clinical Immunology 8: 289–293.
Schmidt, M., et al. 2010. Crucial role for human Toll-like receptor 4 in the development of contact allergy to nickel. Nature Immunology 11: 814–819.
Gross, O., C.J. Thomas, G. Guarda, and J. Tschopp. 2011. The inflammasome: An integrated view. Immunological Reviews 243: 136–151.
Ogura, Y., F.S. Sutterwala, and R.A. Flavell. 2006. The inflammasome: First line of the immune response to cell stress. Cell 126: 659–662.
Schroder, K., and J. Tschopp. 2010. The inflammasomes. Cell 140: 821–832.
Hornung, V., et al. 2008. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nature Immunology 9: 847–856.
Lutz, M.B., et al. 1999. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. Journal of Immunological Methods 223: 77–92.
Eisenbarth, S.C., O.R. Colegio, W. O’Connor, F.S. Sutterwala, and R.A. Flavell. 2008. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature 453: 1122–1126.
Li, X., Z. Zhong, S. Liang, X. Wang, and F. Zhong. 2009. Effect of cryopreservation on IL-4, IFNγ and IL-6 production of porcine peripheral blood lymphocytes. Cryobiology 59: 322–326.
Zhong, F., Z.Y. Zhong, S. Liang, and X.J. Li. 2006. High expression level of soluble SARS spike protein mediated by adenovirus in HEK293 cells. World Journal of Gastroenterology: WJG 12: 1452–1457.
Wen, J., et al. 2013. Soluble form of canine transferrin receptor inhibits canine parvovirus Infection in vitro and in vivo. BioMed Research International 2013: 172479.
Zhang, K., et al. 2013. Porcine reproductive and respiratory syndrome virus activates inflammasomes of porcine alveolar macrophages via its small envelope protein E. Virology 442: 156–162.
McGuire, K.A., A.U. Barlan, T.M. Griffin, and C.M. Wiethoff. 2011. Adenovirus type 5 rupture of lysosomes leads to cathepsin B-dependent mitochondrial stress and production of reactive oxygen species. Journal of Virology 85: 10806–10813.
Nakahira, K., et al. 2011. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nature Immunology 12: 222–230.
Zhou, R., A.S. Yazdi, P. Menu, and J. Tschopp. 2011. A role for mitochondria in NLRP3 inflammasome activation. Nature 469: 221–225.
Wen, H., et al. 2011. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nature Immunology 12: 408–415.
Martinon, F., V. Petrilli, A. Mayor, A. Tardivel, and J. Tschopp. 2006. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440: 237–241.
Dostert, C., et al. 2008. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 320: 674–677.
Mariathasan, S., et al. 2006. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440: 228–232.
Winter, R.N., J.G. Rhee, and N. Kyprianou. 2004. Caspase-1 enhances the apoptotic response of prostate cancer cells to ionizing radiation. Anticancer Research 24: 1377–1386.
Ichinohe, T., H.K. Lee, Y. Ogura, R. Flavell, and A. Iwasaki. 2009. Inflammasome recognition of influenza virus is essential for adaptive immune responses. Journal of Experimental Medicine 206: 79–87.
Miao, E.A., et al. 2006. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nature Immunology 7: 569–575.
Li, H., A. Ambade, and F. Re. 2009. Cutting edge: Necrosis activates the NLRP3 inflammasome. Journal of Immunology 183: 1528–1532.
Lee, B.H., et al. 2012. Activation of P2X(7) receptor by ATP plays an important role in regulating inflammatory responses during acute viral infection. PLoS One 7: e35812.
Sharp, F.A., et al. 2009. Uptake of particulate vaccine adjuvants by dendritic cells activates the NALP3 inflammasome. Proceedings of the National Academy of Sciences of the United States of America 106: 870–875.
Casella, J.F., M.D. Flanagan, and S. Lin. 1981. Cytochalasin D inhibits actin polymerization and induces depolymerization of actin filaments formed during platelet shape change. Nature 293: 302–305.
Halle, A., et al. 2008. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nature Immunology 9: 857–865.
Fernandes-Alnemri, T., J.W. Yu, P. Datta, J. Wu, and E.S. Alnemri. 2009. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 458: 509–513.
Hornung, V., et al. 2009. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458: 514–518.
Murakami, T., et al. 2012. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proceedings of the National Academy of Sciences of the United States of America 109: 11282–11287.
Zhong, Z., et al. 2013. TRPM2 links oxidative stress to NLRP3 inflammasome activation. Nature Communications 4: 1611.
Petrilli, V., et al. 2007. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death and Differentiation 14: 1583–1589.
Zhong, Z., Y. Zhai, and L. Qiao. 2013. Transient receptor potential melastatin 2: A novel target for treatment of gout. Expert Opinion on Therapeutic Targets. doi:10.1517/14728222.2013.835399.
Bolisetty, S., and E.A. Jaimes. 2013. Mitochondria and reactive oxygen species: Physiology and pathophysiology. International Journal of Molecular Sciences 14: 6306–6344.
West, A.P., G.S. Shadel, and S. Ghosh. 2011. Mitochondria in innate immune responses. Nature Reviews Immunology 11: 389–402.
Tschopp, J. 2011. Mitochondria: Sovereign of inflammation? European Journal of Immunology 41: 1196–1202.
Kepp, O., L. Galluzzi, and G. Kroemer. 2011. Mitochondrial control of the NLRP3 inflammasome. Nature Immunology 12: 199–200.
Iyer, S.S., et al. 2013. Mitochondrial cardiolipin is required for nlrp3 inflammasome activation. Immunity 39: 311–323.
Acknowledgments
We thank Dr. Katherine Fitzgerald (from University of Massachusetts Medical School, USA) for providing immortalized inflammasome-deficient macrophages. This work is supported by the National Natural Science Foundation of China (31140093) and Natural Science Foundation of Hebei Province (C2013204130).
Conflict of interest
The authors declared no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, X., Zhong, F. Nickel Induces Interleukin-1β Secretion via the NLRP3–ASC–Caspase-1 Pathway. Inflammation 37, 457–466 (2014). https://doi.org/10.1007/s10753-013-9759-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-013-9759-z