Abstract
The increasing prevalence of both asthma and obesity are major health problems. Recent studies established a possible link between obesity and asthma; however, the underlying mechanism is not clear. The aim of the study was to analyze the prevalence of metabolic syndrome in postmenopausal subjects with asthma and search the interactions between adipokines, metabolic syndrome, and asthma. A total of 45 female patients (57.5 ± 13.9 years) with asthma and 30 healthy subjects (59.6 ± 12.8 years) in postmenopausal status were enrolled in this study. For the diagnosis of metabolic syndrome, modified World Health Organization diagnostic criteria were used. Blood levels of glucose, lipid profile, HbA1c, insulin, CRP, leptin, adiponectin, tumor necrosis factor-alpha, interleukin (IL)-6, IL-8 and plasminogen activator inhibitor-1 (PAI-1) were measured. The mean body mass index was 29.6 ± 5.4 for asthma patients and 28.2 ± 5.3 for the control group. The incidence of metabolic syndrome was found as 26 % for both groups. Insulin resistance as calculated by homeostasis model assessment (HOMA-IR) and fasting insulin levels were significantly higher in asthma patients (p < 0.001 for both parameters). Leptin levels were significantly higher (p = 0.001) and adiponectin levels were lower (p = 0.029) in asthma patients compared to controls. We concluded that although incidence of obesity and metabolic syndrome was not higher in postmenopausal asthma patients than controls, there was an impairment of glucose metabolism and altered adipokine levels in asthma patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Metabolic syndrome (MetS) which was firstly described by Reaven as syndrome X, is a major public health challenge worldwide [1]. The main components of the syndrome are obesity, insulin resistance (IR), hypertension, and dyslipidemia [2]. There is agreement that MetS causes pro-inflammatory and thrombogenic state, leading to late-onset diabetes mellitus and cardiovascular diseases with increased mortality and morbidity. So a clinical diagnosis of the MetS is useful because it affects therapeutic strategy in patients at higher risk.
Asthma is a common chronic inflammatory disease of the airways with reversible airflow obstruction. The prevalence of asthma has been steadily increasing over the past two decades, especially in developed countries. Obesity is defined as an increase in body weight resulting from excessive body fat and a rise in the prevalence of obesity seems to have occurred over the same time period with asthma. Several studies have found an interaction between obesity and asthma [3–6]. Inflammation is corner stone in the pathogenesis of asthma. In obese subjects there is a low grade systemic inflammation that originates from adipocytes and a tendency to Th2 inflammation and atopy [7]. Adipocytes work as an endocrine organ and secret a variety of adipokines including adiponectin, leptin, plasminogen activator inhibitor-1 (PAI-1) and cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin (IL)-6, and IL-8 [6]. Adiponectin, which has anti-inflammatory properties, are actually lower among obese subjects and level of leptin which is a pro-inflammatory hormone, is also lower among lean subjects. Leptin can upregulate systemic inflammation and may lead impairment in lung function [8]. Increased expression and secretion of pro-inflammatory cytokines such as TNF- α, IL-6, and IL-12 were detected when exposed to leptin [9]. Also, this systemic inflammation may help to drive IR, endothelial dysfunction and high blood pressure conditions. Adipokines secreted by adipose tissue seems to have direct effect on asthma pathophysiology especially in animal models; however, human studies are currently indecisive. Association between obesity and asthma has often been reported to be stronger in women than in men [4, 5]. High-serum leptin and low-serum adiponectin concentrations helped to predict asthma in selected premenopausal women, independent of obesity [10]. Women patients have 40 % higher asthma prevalence and 50 % more asthma exacervations than men [11, 12]. The aim of our study was to find out whether MetS was an additional risk factor for asthma in postmenopausal women and to search the interactions between adipokines, metabolic syndrome, and asthma.
METHODS
Subjects
A total of 45 adult female patients with asthma, defined according to the criteria of the Global Initiative for Asthma (GINA), and 30 female healthy controls all in postmenopause status were enrolled in this study from our University Hospital Pulmonology outpatient clinic. Respiratory symptoms and medications were assessed in detail and pulmonary function tests were performed in a standard fashion using electronic spirometer (MIR), for every subject. The patient had to complete at least three forced vital capacity (FVC) maneuvers, and the best one was chosen according to the recommendation of the European Respiratory Society. Exclusion criteria included the following: current smokers or ex-smokers >10 pack-years, co-morbidity that could potentially increase systemic inflammatory markers like rheumatoid arthritis, any participants taking any medication (including recent oral steroids) which may alter systemic inflammation such as statins or hormonal replacement therapy, the presence of any respiratory symptoms; atopy or airway hyper-responsiveness in the non-asthmatic subjects.
This study has been approved by Ethics Committee and complied with the Declaration of Helsinki (date of issue, December 19, 2006; registration number, 10).
Body Mass Index
All the subjects’ height and weight were measured by the same person using the same equipment. Weight (in kilograms) was measured by using a calibrated hospital scale with subjects dressed in normal indoor clothing without shoes. Height (in centimeters) was measured against a wall using a fixed tape measure. Body mass index was calculated by dividing body weight to height square (in kilogram per square meter). Patients were evaluated in two groups according to their BMI; BMI ≤ 30 (group 1, non-obese) and BMI > 30 (group 2, obese).
Diagnosis of Metabolic Syndrome
For the diagnosis of metabolic syndrome, modified WHO diagnostic criteria were used. The WHO definition for MetS [13] required impaired glucose tolerance plus two of the following three disorders: obesity (BMI > 30 kg/m2 and/or waist-to-hip ratio >0.9 in men or >0.85 in women), dyslipidemia (triglyceride level 150 mg/dL and/or HDL cholesterol level <35 mg/dL in men or <39 mg/dL in women), and high blood pressure (systolic blood pressure 140 mmHg and/or diastolic blood pressure 90 mmHg and/or pharmacological treatment). Insulin resistance was defined as one of the following: type 2 diabetes, impaired fasting glucose values >110 mg/dL or for those with normal fasting glucose values (<110 mg/dL), a glucose uptake below the lowest quartile for background population under hyperinsulinemic, euglycemic conditions with the homeostasis model assessment insulin resistance index (HOMA-IR). IR was calculated as the product of the fasting plasma insulin level (in microunits per milliliter) and fasting plasma glucose level (in milligrams per deciliter) divided by 22.5 [14].
Biochemical Markers
Blood specimens were obtained in the morning after a 10- to 12-h fasting. Serum glucose and lipids [total cholesterol, triglycerides, low-density lipoprotein cholesterol (LDL) and high-density lipoprotein cholesterol (HDL)] were measured with commercially available kits (Roche Cobas Integra 800, Roche Diagnostics GmbH; Mannheim, Germany). HbA1c levels were detected by HPLC (Chromsystems, Germany). C-reactive protein (CRP) level was measured by an automatic nephelometer (Immage, Beckman-Coulter Inc., Fullerton, CA, USA). Insulin and IL-6 were measured by a DPC Immulite One autoanalyzer (Diagnostic Products Corporation, Los Angeles, CA, USA). The serum samples were stored at −80 °C until further analysis. Adiponectin concentrations were determined by AviBion Human Adiponectin enzyme-linked immunosorbent assay (ELISA) kit (Orgenium, Finland). Leptin was measured by human Leptin ELISA kit (DRG Instruments GmbH, Germany). IL-8 (Orgenium, Finland), PAI-1 (American Diagnostica GmbH, USA), and TNF-α (Biosource, USA) were measured in serum and plasma by commercial ELISA assays according to manufacturer’s instructions.
Statistics
Variables are presented as percentage, mean ± standard deviation (SD), or median (interquartile range, IQR) as required depending on their distribution. Significance of difference between groups for continuous variables was assessed by Kruskal–Wallis and one-way ANOVA (with Tukey post hoc test) where appropriate. Unpaired Student’s t test or Mann–Whitney tests were used for two-group comparisons. The chi-square test or Fischer’s exact test was used for testing prevalence between groups. Because of skewed distributions, log-transformed values for HDL cholesterol, LDL cholesterol, insulin, HOMA-IR, and TNF-α were used in analyses and back-transformed for data presentation when necessary. The Spearman rank-order correlation coefficient was calculated for correlation analysis. R indicates the correlation coefficient. The statistical analysis was performed using SPSS-13 programme (SPSS Inc., Chicago, IL., USA) and p values ≤ 0.05 are considered significant.
RESULTS
A total of 75 subjects were included in our study. Women in both groups were similar with respect to baseline characteristics of age, weight, body mass index, waist circumferences, and years since menopause. Their demographic and anthropometric data are shown in Table 1. The average duration of asthma was 8.9 ± 7.5 years. 22 (48.9 %) patients in asthma group and 10 (33.3 %) patients in control group had BMI higher than 30 (p = 0.182). Twenty patients with asthma have also allergic rhinitis. Twenty-three (51.1 %) patients in asthma group and 18 (60 %) patients in control group had hypertension. There were no differences between groups for lipid panels including total cholesterol, triglycerides, LDL cholesterol, and HDL cholesterol. Obvious diabetes mellitus was found in 11 (24.4 %) asthma patients and in 6 (20 %) control subjects. IR as calculated by HOMA-IR and fasting insulin levels was significantly higher in asthma patients (p < 0.001 for both parameters; Table 2). No significant relationship between inhaled steroids usage and insulin levels was found. Incidence of MetS was 26.7 % for both groups.
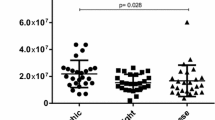
Although, we failed to identify a significant obesity asthma interaction, in regard to both body mass index and waist circumferences between groups, we determined that leptin levels were significantly higher (Z = 3.244, p = 0.001) and adiponectin levels were lower (Z = 2.185, p = 0.029) in asthma patients than controls (Table 3). Additionally, serum log (TNF-α) levels were significantly higher in asthma group (t = 2.026, p = 0.046). Serum IL-8 levels showed a borderline significant increase in asthma patients. PAI-1 and IL-6 levels showed no significant difference between asthma and control groups in asthma group, metabolic syndrome was positively correlated with IL-6 and leptin (r = 0.313, p = 0.037; r = 0.379, p = 0.010, respectively).
We could not find any association between other adipokines, asthma duration, asthma severity, forced expiratory volume in second (FEV1) and presence of MetS in asthma group in general. FEV1 was negatively correlated with IL-6, leptin, and IL-8 levels (r = 0.364, p = 0.016; r = 0.463, p = 0.001; r = 0.370, p = 0.014, respectively) in asthma group. Asthma severity has no association with adipokines except a weak positive correlation with leptin in asthma group (r = 0.297, p = 0.048).
Asthma group with metabolic syndrome has higher leptin and IL-6 levels than asthma group without MetS (Z = 2.515, p = 0.011; Z = 2.074, p = 0.038, respectively; Table 4). Other inflammatory markers and adipokines showed no difference between these two groups. With regard to asthma severity, leptin and log (TNF-α) levels were significantly different in three asthma groups (mild, moderate, and severe asthma groups) (χ2 = 7.059, p = 0.029; F = 5.424, p = 0.008, respectively; Table 5). These differences were derived from differences between mild and moderate asthma severity groups. Asthma severity has strong positive correlation with log (TNF-α; r = 0.720, p = 0.008) in asthma group with metabolic syndrome.
DISCUSSION
In this study, we determined that in postmenopausal period incidence of MetS was similar between asthma and control groups. Although we failed to identify a significant obesity asthma interaction, in regard to both body mass index and waist circumferences, IR was significantly higher in asthma patients. Also, we determined that leptin levels were significantly higher and adiponectin levels were lower in asthma patients than controls.
Results from several large population-based studies have shown that the presence of metabolic syndrome using different definitions is associated with a significantly increased risk of total mortality, cardiovascular morbidity, and mortality [15]. Larsson showed that among different definitions, WHO criteria had the highest predictive power to identify risk factors associated with mortality [16]. Hence, we preferred to use WHO criteria in our study. The overall prevalence of MetS in adults over the age of 20 in the US is estimated at 36.3 % [17]. Menopausal status is an independent risk factor for the metabolic syndrome and the risk for the metabolic syndrome increased up to 14 years since menopause [18]. The association between obesity and asthma appears to be stronger in women and may be affected by hormones such as estrogen levels. Barr [19] found that exogenous hormone replacement therapy in postmenopausal women was associated with increased risk of asthma. In our study to minimize any potential confounders, we only included postmenopausal women. Age is also an important factor for the relationship between obesity and asthma. An association between obesity and asthma was found in young children (6–7 years old) but not in adolescents (13–14 years old) [20]. According to our data, no difference between asthma and control groups in postmenopause stage was observed with regard to obesity and MetS incidence. We explained our findings like that by aging in postmenopausal term, influence of hormones besides obesity on asthma seemed to get lost.
Obesity affects mechanics of diaphragm, chest, and abdominal wall and causes a decrease in tidal volume, expiratory reserve volume and an increase in breathing frequency [21]. Also, obesity is associated with increased upper airway collapsibility with greater symptoms for a given degree of lung function impairment. Besides that co-morbidities of obesity, such as gastroesophageal reflux, sleep-disordered breathing, or hypertension may provoke or worsen asthma [22]. Sin [23] and Schacter [24] showed that while obese subjects reported more wheeze and dyspnea with increased self-reported asthma, they are at a lower risk for objective airflow obstruction and their levels of atopy - bronchial hyper-responsiveness did not support the suggestion of a higher prevalence of asthma in this group. These data suggest that misdiagnosis of asthma was more prevalent in obese population. One of the strength of our study was that, all our patients had doctor diagnosed definite asthma. Their lung function tests with reversibility were consentient with asthma.
Hyperglycemic tendencies and IR may play a role in the causation of asthma and allergy. Sood [25] showed significant higher IR in 246 current asthma patients than controls. Thuesen [26] found increased risk of developing asthma-like symptoms in patients with IR and effect of IR was stronger than that of obesity. It is hypothesized that IR and asthma may share some common inflammatory pathways or mediators that secreted from adipocytes. Husemoen [27] found that in Danish 3609 adults, IR was asssociated with aeroallergen sensitization and allergic asthma, but not nonallergic asthma. Similarly in allergic asthmatics children, higher IR with higher serum IL- 6 and TNF-α levels were revealed compared to healthy children with similar BMI [28]. Hormones such as leptin and adiponectin have a role in energy regulation. In our study, altered adiponectin and leptin levels with impaired glucose metabolism was found in asthmatics. Higher leptin, IL-8, TNF-α, and lower adiponectin levels in asthma group might led a dysregulation in adipose tissue in asthma patients. Leptin regulates normal body weight and energy consumption [29, 30]. Adiponectin has anti-inflammatory effects and it has been shown to improve glucose metabolism and stimulate fatty acid oxidation and nitric oxide synthesis. In the third national health and nutrition examination, survey’s results supported that leptin levels were highly associated with asthma especially in premenopausal women independent of BMI [8]. Guler [31] also suggested that serum leptin concentrations were a predictive factor for asthma in boys, even after adjusting for obesity. Shore [32] has previously noted leptin-mediated increased bronchial hyperactivity in obese mice models. Animal studies reported the protective role of adiponectin from allergic airways disease. In mice, allergen inhalation lowers serum adiponectin, and adiponectin infusion attenuates allergic airway responses, suggesting that low levels of adiponectin associated with obesity might contribute to asthma [33, 34]. On the other hand, there are studies in which levels of leptin and adiponectin showed no association with asthma. In a Scandinavian prospective cohort study of children and adults, incident asthma was associated with obesity, but there was no association between leptin, adiponectin, C-reactive protein, or insulin with asthma in multivariate analysis [35]. Different study populations and experimental designs might be the reason of divergence of results but contradictory results should be further analyzed to clearly answer if possible cross-talk between adipocyte tissue and lungs might exist through adipokines. IL- 6 was considered as negative predictor of FEV1 in men [36]. In our study, forced expiratory volume in second (FEV1) was negatively associated with IL- 6, IL-8, and leptin. TNF-α levels and insulin resistance was found higher in asthma patients. When asthma severity increased from mild to moderate, a relatively strong positive correlation between asthma severity and TNF-α was observed. There seems to be an association between circulating inflammatory markers and asthma severity but its extent could not be explained in this study. This could be the result of small study group size which might be a limitation of our study. In order to strength our study we prefer to study a special subgroup (postmenopausal asthmatics women), to eliminate other confounding factors such as age, menopausal status, and hormones. Finally, we thought that insulin resistance could be the possible link between asthma and inflammatory mechanisms. Detection and adequate treatment of metabolic risk factors such as insulin resistance may prevent the onset and progression to more severe disease. Further studies are needed to elucidate the interaction between insulin resistance and asthma severity from different age groups with different body mass index.
REFERENCES
Reaven, G.M. 1988. Banting lecture. Role of insulin resistance in human disease. Diabetes 37(12): 1595–1607.
Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. 2001. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). Journal of the American Medical Association 285(19): 2486–2497.
Visness, C.M., S.J. London, J.L. Daniels, J.S. Kaufman, K.B. Yeatts, A.M. Siega-Riz, A. Calatroni, and D.C. Zeldin. 2010. Association of childhood obesity with atopic and nonatopic asthma: results from the National Health and Nutrition Examination Survey 1999–2006. The Journal of Asthma 47: 822–829.
Chen, Y., D. Rennie, Y. Cormier, and J. Dosman. 2005. Sex specificity of asthma associated with objectively measured body mass index and waist circumference: the Humboldt study. Chest 128: 3048–3054.
Brumpton, B., A. Langhammer, P. Romundstad, et al. 2013. General and abdominal obesity and incident asthma in adults: the HUNT study. European Respiratory Journal 41: 323–329.
Tkacova, R. 2010. Systemic inflammation in chronic obstructive pulmonary disease: may adipose tissue play a role? Review of the literature and future perspectives. Mediators of Inflammation 2010: 585989.
Poulain, M., M. Doucet, G.C. Major, V. Drapeau, F. Sériès, L.P. Boulet, A. Tremblay, and F. Maltais. 2006. The effect of obesity on chronic respiratory diseases: pathophysiology and therapeutic strategies. CMAJ 174: 1293–1299.
Sood, A., E.S. Ford, and C.A. Camargo Jr. 2006. Association between leptin and asthma in adults. Thorax 61: 300–305.
Sarraf, P., R.C. Frederich, E.M. Turner, G. Ma, N.T. Jaskowiak, D.J. Rivet 3rd, J.S. Flier, B.B. Lowell, D.L. Fraker, and H.R. Alexander. 1997. Multiple cytokines and acute inflammation raise mouse leptin levels: potential role in inflammatory anorexia. The Journal of Experimental Medicine 185: 171–175.
Sood, A. 2010. Obesity, adipokines, and lung disease. Journal of Applied Physiology 108(3): 744–753.
Camargo Jr., C.A., Weiss S.T., Zhang S., Willett W.C., and Speizer F.E.. 1999. Prospective study of body mass index, weight change, and risk of adult-onset asthma in women. Archives of Internal Medicine 159(22): 2582–2588.
National Center for Health Statistics. Asthma Prevalence, Health Care Use and Mortality: United States, 2003–05. http://www.cdc.gov/nchs/data/hestat/asthma03-05/asthma03-05.htm.
WHO Consultation. 1999. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Geneva: World Health Organization. Report No 99.2.
Matthews, D.R., J.P. Hosker, A.S. Rudenski, B.A. Naylor, D.F. Treacher, and R.C. Turner. 1985. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419.
Wells, J.C. 2012. Ethnic variability in adiposity, thrifty phenotypes and cardiometabolic risk: addressing the full range of ethnicity, including those of mixed ethnicity. Obesity Reviews 2: 14–29.
Larsson, I., A. Lindroos, T.C. Lystig, I. Näslund, and L. Sjöström. 2005. Three definitions of the metabolic syndrome: relations to mortality and atherosclerotic morbidity. Metabolic Syndrome and Related Disorders 3: 102–112.
Ford, E.S., C. Li, and G. Zhao. 2010. Prevalence and correlates of metabolic syndrome based on a harmonious definition among adults in the US. Journal of Diabetes 2(3): 180–193.
Cho, G.J., J.H. Lee, H.T. Park, J.H. Shin, S.C. Hong, T. Kim, J.Y. Hur, K.W. Lee, Y.K. Park, and S.H. Kim. 2008. Postmenopausal status according to years since menopause as an independent risk factor for the metabolic syndrome. Menopause 15: 524–529.
Barr, R.G., C.C. Wentowski, F. Grodstein, S.C. Somers, M.J. Stampfer, J. Schwartz, F.E. Speizer, and C.A. Camargo Jr. 2004. Prospective study of postmenopausal hormone use and newly diagnosed asthma and chronic obstructive pulmonary disease. Archives of Internal Medicine 164: 379–386.
Gonzalez-Barcala, F.J., et al. 2012. Obesity and asthma: an association modified by age. Allergol Immunopathol (Madr). doi:10.1016/j.aller.2012.05.011.
Chlif, M., D. Keochkerian, C. Mourlhon, D. Choquet, and S. Ahmaidi. 2005. Noninvasive assessment of the tension-time index of inspiratory muscles at rest in obese male subjects. International Journal of Obesity 29: 1478–1483.
Sin, D.D., and E.R. Sutherland. 2008. Obesity and the lung: 4. Obesity and asthma. Thorax 63: 1018–1023.
Sin, D.D., R.L. Jones, and S.F. Man. 2002. Obesity is a risk factor for dyspnea but not for airflow obstruction. Archives of Internal Medicine 162: 1477–1481.
Schachter, L.M., C.M. Salome, J.K. Peat, and A.J. Woolcock. 2001. Obesity is a risk for asthma and wheeze but not airway hyperresponsiveness. Thorax 56: 4–8.
Sood, A., X. Cui, C. Qualls, W.S. Beckett, M.D. Gross, M.W. Steffes, L.J. Smith, and D.R. Jacobs Jr. 2008. Association between asthma and serum adiponectin concentration in women. Thorax 63: 877–882.
Thuesen, B.H., L.L. Husemoen, L.G. Hersoug, C. Pisinger, and A. Linneberg. 2009. Insulin resistance as a predictor of incident asthma-like symptoms in adults. Clinical and Experimental Allergy 39(5): 700–707.
Husemoen, L.L., C. Glümer, C. Lau, C. Pisinger, L.S. Mørch, and A. Linneberg. 2008. Association of obesity and insulin resistance with asthma and aeroallergen sensitization. Allergy 63(5): 575–582.
Segal, K.R., M. Landt, and S. Klein. 1996. Relationship between insulin sensitivity and plasma leptin concentration in lean and obese men. Diabetes 45: 988–991.
Dyck, D.J. 2009. Adipokines as regulators of muscle metabolism and insulin sensitivity. Applied Physiology, Nutrition and Metabolism 34: 396–402.
Baratta, R., S. Amato, C. Degano, M.G. Farina, G. Patanè, R. Vigneri, and L. Frittitta. 2004. Adiponectin relationship with lipid metabolism is independent of body fat mass: evidence from both cross-sectional and intervention studies. Journal of Clinical Endocrinology and Metabolism 89: 2665–2671.
Guler, N., E. Kirerleri, U. Ones, Z. Tamay, N. Salmayenli, and F. Darendeliler. 2004. Leptin: does it have any role in childhood asthma? The Journal of Allergy and Clinical Immunology 14: 254–259.
Shore, S.A. 2007. Obesity and asthma: lessons from animal models. Journal of Applied Physiology 102: 516–528.
Shore, S.A., R.D. Terry, L. Flynt, A. Xu, and C. Hug. 2006. Adiponectin attenuates allergen-induced airway inflammation and hyperresponsiveness in mice. The Journal of Allergy and Clinical Immunology 118: 389–395.
Kilic, H., I.K. Oguzulgen, F. Bakir, and H. Turktas. 2011. Asthma in obese women: outcomes and factors involved. Journal of Investigational Allergology and Clinical Immunology 21: 290–296.
Jartti, T., L. Saarikoski, L. Jartti, I. Lisinen, A. Jula, R. Huupponen, J. Viikari, and O.T. Raitakari. 2009. Obesity, adipokines and asthma. Allergy 64: 770–777.
Wood, L.G., J. Attia, P. McElduff, M. McEvoy, and P.G. Gibson. 2010. Assessment of dietary fat intake and innate immune activation as risk factors for impaired lung function. European Journal of Clinical Nutrition 64: 818–825.
ACKNOWLEDGMENTS
The authors wish to express their sincere appreciation to the study participants and to Fatih University. This work was financially supported by the Scientific Research Fund of Fatih University under the project number P53010703.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aydin, M., Koca, C., Ozol, D. et al. Interaction of Metabolic Syndrome with Asthma in Postmenopausal Women: Role of Adipokines. Inflammation 36, 1232–1238 (2013). https://doi.org/10.1007/s10753-013-9660-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-013-9660-9