Abstract
Berberine (Ber), the major constituent of Coptidis Rhizoma, possesses anti-inflammatory properties. In this study, we investigated the effects of Ber on cigarette smoke (CS)-mediated acute lung inflammation. C57BL/6 mice (6–8 weeks) were exposed to CS to induce acute lung injury. Ber was used to pretreat CS-exposed mice (50 mg/kg, intragastrically). Lung tissues were collected for histological examination, myeloperoxidase (MPO) activity assay, Western blot analysis, and electrophoretic mobility shift assay. Bronchoalveolar lavage fluid (BALF) was measured for cell counts and cytokine analysis. Histological examination showed that CS exposure caused infiltration of inflammatory cells into alveolar spaces and interstitial edema. Pretreatment with Ber significantly attenuated CS-induced lung inflammation. The numbers of total cells, macrophages, and neutrophils in BALF were decreased by 43, 40, and 53 %, respectively, by Ber pretreatment in CS-exposed mice, accompanied by decreased MPO activity, a marker of neutrophil accumulation. Ber pretreatment also profoundly diminished CS-induced secretions of macrophage inflammatory protein 2, tumor necrosis factor alpha, interleukin-6, and monocyte chemotactic protein-1 in BALF, along with less nuclear translocation of the pro-inflammatory transcription factor nuclear factor-kappa B (NF-κB) p65 subunit and lower NF-κB DNA-binding activity (P < 0.01). Thus, our results indicated that Ber ameliorates CS-induced acute lung injury through its anti-inflammatory activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is one of the leading causes of morbidity and mortality worldwide [1]. Airflow obstruction in COPD is associated with an abnormal inflammatory response to the lung noxious particles or gases [2]. Cigarette smoke (CS) is the most commonly encountered risk factor for the pathogenesis of COPD [2]. CS contains nearly 5,000 chemicals, a variety of which are known as carcinogens [3], induces lung inflammation through initiating the infiltration of innate and adaptive inflammatory cells into the airways and the lung parenchyma and further destroys the alveolar structure [4].
Inflammation is the normal physiological response to tissue damage, which appears to be amplified by the stimulations of chronic irritants, such as CS, to the respiratory tract of patients with COPD [2]. Inflammation is typified by increased leukocyte, particularly neutrophil and macrophage, infiltration [5]. Cellular inflammation is associated with enhanced cytokine and chemokine secretion [6]. In patients with COPD, neutrophils, macrophages, and other inflammatory cells accumulate around airways [2]. In mouse models, CS exposure enhances inflammatory cell infiltration into the bronchoalveolar space and promotes production of several pro-inflammatory cytokines and chemokines, such as tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), macrophage inflammatory protein 2 (MIP-2), and keratinocyte-derived chemokine [5, 6]. While it is well known that CS has deleterious effects on respiratory tract and is the major cause of COPD [2], the molecular and cellular basis of the CS-induced inflammation in lung injury is still unclear. The nuclear factor kappa B (NF-κB) is a complex signaling pathway and plays an important role in the induction of pro-inflammatory gene expression, leading to the production of cytokines, adhesion molecules, chemokines, growth factors, and enzymes [7]. Recent evidence implicates the involvement of NF-κB pathway in CS-mediated pulmonary inflammation [7, 8]. NF-κB has been shown to be activated in alveolar macrophages and peripheral lungs of smokers and patients with COPD [7]. Moreover, selective NF-κB inhibition is an emerging therapeutic strategy to suppress CS-induced pulmonary pro-inflammatory response [8].
Berberine (Ber) is the major constituent of Coptidis Rhizoma with multiple pharmacological activities, including antitumor [9], anti-inflammation [10], and antibiotic activity [11]. Recent studies documented that Ber has therapeutic effects on lung injury [12, 13]. Liu et al. showed that Ber has protective effects on radiation-induced lung injury via soluble intercellular adhesion molecular-1 (sICAM-1) and transforming growth factor-beta-1 (TGF-β1) in patients with lung cancer [12]. Zhang et al. documented that Ber protects against lipopolysaccharide (LPS)-induced lung injury and inhibits cytosolic phospholipase A2 [13]. In the present study, we investigated the effects of Ber on CS-induced acute lung injury in mouse model. We found that Ber attenuated CS-mediated pulmonary inflammation and suppressed CS-induced NF-κB activation.
MATERIAL AND METHODS
Mice
Specific pathogen-free male C57BL/6 mice (6–8 weeks) were obtained from Laboratory Animal Center of Third Military Medical University (Chongqing, China). Mice were housed at 20 °C on a 12-h day/night cycle in sterile microisolators and fed ad libitum with sterile chow and water. All mouse procedures in this study were conducted according to international and institutional guidelines for animal care, and approved by the Animal Experimentation Ethics Committee of the Second Affiliated Hospital of the Third Military Medical University (Chongqing, China).
Thirty-six mice (12 mice for each group) were randomly divided into three groups: normal air-exposed control group (C); cigarette smoke-exposed group (CS); and Ber pretreatment group (Ber) in which mice were pretreated with Ber before CS exposure.
Drug Treatment
Ber (Sigma-Aldrich Co., Louis, MO, USA) was freshly dissolved in dimethyl sulfoxide (DMSO), which was then diluted by phosphate-buffered saline (PBS; pH 7.4). The final DMSO concentration in the PBS applied to animals was 0.1 %. Mice were pretreated with Ber (50 mg/kg, intragastrically) (13) 1 h before every CS exposure.
Cigarette Smoke Exposure
For cigarette smoke exposure experiment, a commercial filter cigarette was employed (White Shark brand, Tobacco Company, Hunan, China). Each cigarette contained 1 mg of nicotine and 13 mg of tar according to the manufacturer’s specification. CS exposure was performed according to previously described methods [14]. Briefly, the mice (12 per group) were placed in a closed plastic box connected to a smoke generator (CH Technologies, Westwood, NJ, USA) and exposed to cigarette smoke generated from nine cigarettes per day for four consecutive days. On the fifth day, mice were killed by an intraperitoneal overdose of anesthetic (pentobarbital sodium, 300 mg/kg ip).
The left lung from four mice of each group was employed for histopathological examination, and the right lung for analysis of myeloperoxidase (MPO) activity. The lung from another four mice of each group was used to bronchoalveolar lavage, and the right lung was excised for Western blot analysis. The lung of both sides from the remaining four mice of each group was subjected to electrophoretic mobility shift assay (EMSA)
Bronchoalveolar Lavage Fluid Analysis
The right lung was lavaged with three aliquots of .0.5 ml of PBS (pH 7.4) containing 0.6 mM EDTA. Bronchoalveolar lavage fluid (BALF) was centrifuged at 800×g for 10 min. The supernatants were collected and stored at −80 °C for cytokine and chemokine analysis. The cell pellet was resuspended in 200 μl of PBS with 0.6 mM EDTA, and cell count was performed by the Easy Count System (Immunicon, Huntingdon Valley, PA). Differential cell count was determined by cytocentrifugation and Wright-Giemsa staining.
MPO Ability Assay
The lung tissues were homogenized in ten volumes of 100 mM phosphate buffer (pH 7.4) containing protease inhibitor. The activity of pulmonary MPO, an indicator of neutrophil accumulation, was analyzed in the homogenized lung tissues as described before [15]. Briefly, the assay mixture composed of 400 μl of phosphate buffer (100 mM, pH 7.4) with 0.5 % hexadecyltrimethylammonium bromide (Sigma-Aldrich, St. Louis, MO), 40 μl of tetramethylbenzidine (20 mM in dimethyll sulfoxide, prepared fresh and protected from light), and H2O (23 μl). Twenty microliters of enzyme extract was added to this assay mixture. The mixture was incubated at 37 °C for 3 min, and 17 μl of H2O2 (0.03 %) was added. The mixture was further incubated for 3 min. Two milliliters of acetate buffer (0.2 M, pH 3.2) was added to stop the reaction. The mixture was kept on ice, and the absorbance was read at 655 nm. MPO activity was expressed as units per milligram of protein.
Enzyme-linked Immunosorbent Assay
The concentrations of MIP-2, TNF-α, IL-6, and monocyte chemotactic protein-1 (MCP-1) in BALF were analyzed by enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN) according to the protocol provided by the manufacturer.
Histological Analysis
Mice lung was fixed with 4 % paraformaldehyde, embedded in paraffin, and sliced at 4 μm. Paraffin sections were stained with hematoxylin and eosin (H&E) for histopathological analysis.
Preparation of Nuclear Extracts
Pulmonary nuclear extracts were prepared by hypotonic lysis and subsequent high salt extraction as described before [16]. Briefly, 100 mg of lung tissue was homogenized in 500 μl of ice-cold buffer I, consisted of 10 mM HEPES (pH 7.9), 10 mM KCl, 2 mM MgCl2, 0.1 mM EDTA, 1.0 mM dithiothreitol (DTT), and 0.5 mM phenylmethylsulfonylfluoride (PMSF; Sigma-Aldrich). After incubation on ice for 15 min, the lung homogenate was supplemented with 50 μl of 10 % Nonidet P-40 solution (Sigma-Aldrich). The mixture was vortexed for 30 s and centrifuged at 6,000×g, 4 °C for 1 min. The crude nuclear pellet was resuspended in 200 μl of buffer II (20 mM HEPES (pH 7.9), 420 mM NaCl, 1.5 mM MgCl2, 0.1 mM EDTA, 1 mM DTT, 0.5 mM PMSF, and 25 % (v/v) glycerol) and incubated on ice for 30 min with intermittent mixing. The suspension was centrifuged at 12,000×g, 4 °C for 15 min. The supernatant containing the nuclear proteins was removed to a new tube and kept at −80 °C.
Western Blot Analysis
Equal amounts (20 μg) of protein samples prepared as above were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes. The membrane was blocked in 5 % milk in Tris-buffered saline and 0.1 % Tween 20 at room temperature for 1 h and incubated with primary antibodies against p65 NF-κB (1:1,000, Cell Signaling Technology, Inc., Danvers, MA, USA) or histone (1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight at 4 °C, and washed for three times with TBS. Then, the membrane was incubated with horseradish peroxidase-conjugated secondary antibody (1:10,000; Santa Cruz Biotechnology), washed for three times with TBS, and developed by an enhanced chemiluminescent detection system (Amersham, Piscataway, NJ, USA). Signal intensities were quantitated by the Gel Image Analysis System (Tanon Science & Technology Co., Ltd., Shanghai, China).
EMSA
NF-κB DNA binding activity was determined by the EMSA assay in the nuclear proteins of mouse lungs. To detect whether the NF-κB subunit p65 was in the NF-κB DNA complex, 2 μl of p65 antibodies (Cell Signaling Technology) was incubated with the nuclear proteins prior to addition of radiolabeled probe to visualize any supershift-retarded bands. A double-stranded oligonucleotide containing NF-κB binding site with the sequence 5′-AGT TGA GGG GAC TTT CCC AGG C-3′ (Santa Cruz Biotechnology) was end-labeled with γ-32P ATP via T4 polynucleotide kinase. To identify nonspecific binding of nuclear protein, competition reactions were conducted by additionally adding either 100-fold excess of non-labeled competitor (NF-κB consensus oligonucleotide) or 100-fold excess of non-radiolabeled mutated NF-κB oligonucleotide (5′-AGT TGA GGC GAC TTT CCC AGG C-3′). After the binding reactions, the DNA protein complexes were separated on 4 % nondenaturing polyacrylamide gels in 0.5× Tris borate-EDTA buffer. Gels were vacuum-dried and exposed to x-ray film at −80 °C by an intensifying screen. Specific band intensities were quantified by an image scanning densitometer.
Statistical Analysis
Data are expressed at mean ± SD. All statistical analyses were done with SPSS.11 software for windows (SPSS Inc., Chicago, IL, USA). Differences between group means were evaluated by one-way analysis of variance followed by a Tukey post hoc test. P values less than 0.05 were considered as significant.
RESULTS
Ber Attenuated CS-mediated Pulmonary Histological Changes
H&E staining in control mice showed normal lung morphology (Fig. 1). CS exposure caused lung injury including infiltration of inflammatory cells into alveolar spaces and interstitial edema (Fig. 1). Ber pretreatment markedly reduced CS-induced alveolar injury (Fig. 1). Ber alone and vehicle control had no effect on pulmonary histological changes (data not shown).
The effects of Ber on CS-induced histological changes. Mice (n = 4) were exposed to normal air or CS with or without Ber pretreatment. Lungs were fixed with 4 % paraformaldehyde, embedded in paraffin, and cut into 4-μm slices. After H&E staining, histological examination was performed by light microscopy. a Control group, b cigarette smoke group, c CS-treated mice with berberine pretreatment (×200 magnifications).
Ber Reduced CS-induced BALF Cellularity and MPO Activity
There was a significant increase in the numbers of total cells, neutrophils, and macrophages in the BALF of CS-exposed mice (P < 0.01; Fig. 2a–c). Ber pretreatment remarkably reduced the numbers of total cells, macrophages, and neutrophils by 43, 40, and 53 %, respectively (Fig. 2a–c; P < 0.01). However, Ber alone and vehicle control had no effect on the baseline numbers of total cells, macrophages, and neutrophils compared with control group (P > 0.01; data not shown).
The effects of Ber on BALF cellularity in CS-exposed mice. Differential cell count in BALF was determined by cytocentrifugation and Wright-Giemsa staining. The bar graph shows the numbers of total cells (a), macrophages (b), and neutrophils (c) in BALF. C control group, CS cigarette smoke group, Ber CS-treated mice with berberine pretreatment. Data are presented as mean ± SD (n = 4). *P < 0.01 vs. normal air-exposed control group; # P < 0.01 vs. CS-exposed group.
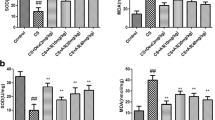
Pulmonary neutrophil accumulation was quantitated by analyzing MPO activity in lung homogenates. CS exposure induced a more than fourfold increase in MPO activity (Fig. 3). Ber administration reduced CS-promoted MPO activity by 58 % (Fig. 3).
The effects of Ber on pulmonary MPO activity in CS-treated mice. Lung sections from different groups were homogenized, and the MPO activity was determined spectrophotometrically. C control group, CS cigarette smoke group, Ber CS-treated mice with berberine pretreatment. Data are presented as mean ± SD (n = 4). *P < 0.01 vs. normal air-exposed control group; # P < 0.01 vs. CS-exposed group.
Berberine Decreased CS-induced Pro-inflammatory Cytokine and Chemokine Production in BALF
The production of pro-inflammatory cytokines and chemokines in BALF was determined by ELISA assay. CS significantly enhanced the production of MIP-2 (sevenfold), TNF-α (tenfold), IL-6 (ninefold), and MCP-1 (twofold) in BALF compared with those in control mice (P < 0.01; Fig. 4). Ber pretreatment dampened the secretion of MIP-2, TNF-α, IL-6, and MCP-1 by 35, 46, 44, and 27 %, respectively, in CS-treated mice (Fig. 4).
The effects of Ber on the production of BALF cytokines and chemokines in CS-exposed mouse lung. BALF was collected from different groups of mouse lung. The concentrations of MIP-2 (a), TNF-α (b), IL-6 (c), and MCP-1 (d) in BALF were analyzed by ELISA. C control group, CS cigarette smoke group, Ber CS-treated mice with berberine pretreatment. Data are presented as mean ± SD (n = 4). *P < 0.01 vs. normal air-exposed control group; # P < 0.01 vs. CS-exposed group.
Ber Diminished CS-induced p65 NF-κB Nuclear Translocation and NF-κB DNA Binding Activity in Mouse Lung
CS exposure markedly enhanced nuclear translocation of p65 NF-κB (Fig. 5a; P < 0.01) and promoted NF-κB DNA binding activity (Fig. 5b). Ber administration significantly decreased p65 NF-κB nuclear translocation (Fig. 5a; P < 0.01) and NF-κB DNA binding activity (Fig. 5b).
The effects of Ber on nuclear translocation and DNA binding activity of NF-κB in CS-treated mice. a Nuclear translocation of p65 NF-κB was measured by Western blot analysis. Histone protein was used as an internal control to monitor equal protein loading (top). The bar graph shows the relative levels of nuclear p65 NF-κB (bottom). b Nuclear NF-κB DNA binding activity in the lungs was analyzed by EMSA using radiolabeled NF-κB oligonucleotide. p65 antibodies were incubated with the nuclear extracts prior to addition of radiolabeled probe to visualize supershift-retarded bands in the NF-κB complex. The specificity of NF-κB DNA binding was identified by adding excess of nonlabeled NF-κB oligonucleotides or nonlabeled mutated NF-κB oligonucleotides to mouse lung nuclear extracts. C control group, CS cigarette smoke group, Ber CS-treated mice with berberine pretreatment group. 1 control group; 2 cigarette smoke group; 3 CS-treated mice with berberine pretreatment group; 4 lung nuclear extracts of CS group incubated with radiolabeled probe together with nonlabeled NF-κB oligonucleotides; 5 lung nuclear extracts of CS group incubated with radiolabeled probe together with non-radiolabeled mutated NF-κB oligonucleotide. 6 lung nuclear extracts of CS group incubated with p65 antibodies prior to addition of radiolabeled probe. Data are presented as mean ± SD (n = 4). *P < 0.01 vs. normal air-exposed control group; # P < 0.01 vs. CS-exposed group.
DISCUSSION
In the present study, we found that pretreatment with Ber attenuated CS-induced acute lung injury through suppressing infiltration of inflammatory cells into alveolar spaces and inhibiting pulmonary edema. Ber administration resulted in a significant decrease in the numbers of total cells, macrophages, and neutrophils in the BALF of CS-treated mice (P < 0.01), along with lowered lung MPO activity and less production of MIP-2, TNF-α, IL-6, and MCP-1 in BALF. Furthermore, Ber also exhibited inhibition of p65 NF-κB nuclear translocation and decreased NF-κB DNA binding activity. Our results indicated that Ber has therapeutic potential on CS-induced acute lung injury via its anti-inflammatory properties.
Accumulating evidence suggests that Ber has anti-inflammatory effects on lung injury [12, 13, 17]. Ber administration reduces LPS-induced pulmonary edema, neutrophil infiltration, and histopathological changes [13, 17]. Ber also attenuates radiation-induced lung injury through suppressing plasma TGF-β1 and sICAM-1 in patients with lung cancer [12]. In the present study, we found that Ber inhibits CS-induced acute lung injury through decreasing pulmonary edema, reducing neutrophil and macrophage infiltration, lowering lung MPO activity, lessening the secretion of pro-inflammatory cytokines and chemokines (MIP-2, TNF-α, IL-6, and MCP-1) in BALF, and attenuating the translocation of p65 NF-κB into nucleus and NF-κB DNA binding activity. Collectively, these results suggest that Ber has protective effects on lung injury induced by different irritants and might provide a new approach for the treatment of lung injury.
NF-κB complexes are bound to nuclear factor of kappa light polypeptide gene enhancer in B cells inhibitor (IκB) protein and kept inactive in the cytoplasm [8]. IκB kinase2 (IKK2) induces the degradation of IκBα. Upon the degradation of IκB protein, NF-κB is released and translocated into nucleus and binds to the consensus sequences on DNA [8]. Rajendrasozhan et al. showed that oral administration of IKKβ/IKK2 inhibitor PHA-408 inhibits CS-mediated neutrophil influx in BALF, decreases the production of BALF cytokine-induced neutrophil chemoattractant (CINC-1) and pulmonary IL-6, TNF-α, interleukin-1 beta, and granulocyte macrophage-colony stimulating factor, and suppresses nuclear translocation and DNA binding activity of NF-κB in CS-exposed lungs in rats [8]. These results indicated that the activation of NF-κB plays an important role in CS-induced lung inflammation. Consistent with these observations, we also found that CS exposure significantly enhanced nuclear accumulation of p65 NF-κB and promoted NF-κB DNA binding activity, suggesting that CS exposure induces NF-κB activation in mouse lung. Anto et al. showed that cigarette smoke condensate (CSC)-induced NF-κB activation is comparable with that of TNF, and is not cell type-specific because CSC can promote the activation of NF-κB in human histiocytic lymphoma U937 cells, Jurkat T cells, lung cells (H1299), and head and neck squamous cell lines (1483 and 14B) [18]. Moreover, the activation of NF-κB is also enhanced in alveolar macrophages and peripheral lungs of smoker and patients with COPD [7]. All of these results suggest that NF-κB activation is involved in CS-mediated lung inflammation. Inhibition of NF-κB activation by administration of IKKβ/IKK2 inhibitor PHA-408 profoundly attenuates CS-induced acute lung injury [8]. Similar observation was obtained in our study, in which Ber protects against CS-induced lung pro-inflammatory response via suppressing the activation of NF-κB, evidenced by suppressing of pulmonary edema, less inflammatory cell influx into BALF, lower secretion of BALF pro-inflammatory cytokines and chemokines, in terms of MIP-2, TNF-α, IL-6, and MCP-1. Moreover, in human lung epithelial cells, curcumin downregulates CS-induced NF-κB activation, which is correlated with suppression of cyclooxygenase-2, matrix metalloproteinase (MMP-9), and cyclin D1 [19]. These observations suggest that suppression of NF-κB could serve as a new therapeutic approach to treat CS-induced lung inflammation.
CS has adverse effects on general lung health as it causes inflammatory lung injury and alveolar epithelial damage supported by animal models [20, 21]. Although clinical features of chronic exposure to CS-mediated lung injury are not as acute and profound as these in animal studies [22], animal models of CS-induced acute lung injury may offer a histological or biochemical base upon which additional insults could bring out clinical manifestations more quickly. In line with this hypothesis that smokers are prone to lung inflammation and pulmonary edema, an association between cigarette smoking and risk of acute lung injury/acute respiratory distress syndrome has been found in a 15-year cohort study [23]. Thus, in the present study, the protective effects of Ber on CS-mediated acute lung injury provide a therapeutic possibility in pulmonary inflammation in CS-induced chronic airway disease, such as COPD, an inflammatory disease mainly associated with exposure to CS [2]. Although the mechanism of how CS leads to COPD is still unclear, it is well established that NF-κB activation is implicated in the production of many inflammatory mediators present in the COPD lung [24]. Therefore, the NF-κB signaling pathway may represent a therapeutic target to suppress the inflammation associated with COPD. Our results indicated that Ber has inhibitory effects on NF-κB activation in CS-mediated acute lung injury. We propose that Ber may have potential in the treatment of CS-induced inflammation (e.g., COPD), via attenuating the inflammatory cascade closely associated with NF-κB activation. Nevertheless, further studies are required to determine the potential clinical usefulness of Ber in the anti-inflammatory therapy for chronic CS exposure-induced lung diseases. The underlying mechanism of how Ber regulates NF-κB signaling pathway deserves further investigation, which was not touched upon in the present study. Taken together, our results indicated that Ber ameliorates CS-induced inflammation and NF-κB activation in acute lung injury.
References
Rabe, K.F., B. Beghé, F. Luppi, and L.M. Fabbri. 2007. Update in chronic obstructive pulmonary disease 2006. American Journal of Respiratory and Critical Care Medicine 175: 1222–1232.
Rabe, K.F., S. Hurd, A. Anzueto, P.J. Barnes, S.A. Buist, P. Calverley, Y. Fukuchi, C. Jenkins, R. Rodriguez-Roisin, C. van Weel, J. Zielinski, and Global Initiative for Chronic Obstructive Lung Disease. 2007. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. American Journal of Respiratory and Critical Care Medicine 176: 532–555.
Stampfli, M.R., and G.P. Anderson. 2009. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nature Reviews Immunology 9: 377–384.
Hogg, J.C., F. Chu, S. Utokaparch, R. Woods, W.M. Elliott, L. Buzatu, R.M. Cherniack, R.M. Rogers, F.C. Sciurba, H.O. Coxson, and P.D. Paré. 2004. The nature of small-airway obstruction in chronic obstructive pulmonary disease. The New England Journal of Medicine 350: 2645–2653.
Profita, M., A. Sala, A. Bonanno, L. Riccobono, M. Ferraro, S. La Grutta, G.D. Albano, A.M. Montalbano, and M. Gjomarkaj. 2010. Chronic obstructive pulmonary disease and neutrophil infiltration: role of cigarette smoke and cyclooxygenase products. American Journal of Physiology. Lung Cellular and Molecular Physiology 298: L261–L269.
Thatcher, T.H., S.B. Maggirwar, C.J. Baglole, H.F. Lakatos, T.A. Gasiewicz, R.P. Phipps, and P.J. Sime. 2007. Aryl hydrocarbon receptor-deficient mice develop heightened inflammatory responses to cigarette smoke and endotoxin associated with rapid loss of the nuclear factor-kappaB component RelB. American Journal of Pathology 170: 855–864.
Wright, J.G., and J.W. Christman. 2003. The role of nuclear factor kappa B in the pathogenesis of pulmonary diseases: implications for therapy. American Journal of Respiratory Medicine 2: 211–219.
Rajendrasozhan, S., J.W. Hwang, H. Yao, N. Kishore, and I. Rahman. 2010. Anti-inflammatory effect of a selective IkappaB kinase-beta inhibitor in rat lung in response to LPS and cigarette smoke. Pulmonary Pharmacology & Therapeutics 23: 172–181.
Park, J.J., S.M. Seo, E.J. Kim, Y.J. Lee, Y.G. Ko, J. Ha, and M. Lee. 2012. Berberine inhibits human colon cancer cell migration via AMP-activated protein kinase-mediated downregulation of integrin β1 signaling. Biochemical and Biophysical Research Communications 426: 461–467.
Iizuka, N., K. Miyamoto, K. Okita, A. Tangoku, H. Hayashi, S. Yosino, T. Abe, T. Morioka, S. Hazama, and M. Oka. 2000. Inhibitory effect of Coptidis Rhizoma and berberine on the proliferation of human esophageal cancer cell lines. Cancer Letters 148: 19–25.
Marinova, E.K., D.B. Nikolova, D.N. Popova, G.B. Gallacher, and N.D. Ivanovska. 2000. Suppression of experimental autoimmune tubulointerstitial nephritis in BALB/c mice by berberine. Immunopharmacology 48: 9–16.
Liu, Y., H. Yu, C. Zhang, Y. Cheng, L. Hu, X. Meng, and Y. Zhao. 2008. Protective effects of berberine on radiation-induced lung injury via intercellular adhesion molecular-1 and transforming growth factor-beta-1 in patients with lung cancer. European Journal of Cancer 44: 2425–2432.
Zhang, H.Q., H.D. Wang, D.X. Lu, R.B. Qi, Y.P. Wang, Y.X. Yan, and Y.M. Fu. 2008. Berberine inhibits cytosolic phospholipase A2 and protects against LPS-induced lung injury and lethality independent of the alpha2-adrenergic receptor in mice. Shock 29: 617–622.
Vlahos, R., S. Bozinovski, J.E. Jones, J. Powell, J. Gras, A. Lilja, M.J. Hansen, R.C. Gualano, L. Irving, and G.P. Anderson. 2006. Differential protease, innate immunity, and NF-kappaB induction profiles during lung inflammation induced by subchronic cigarette smoke exposure in mice. American Journal of Physiology. Lung Cellular and Molecular Physiology 290: L931–L945.
Yao, H., I. Edirisinghe, S.R. Yang, S. Rajendrasozhan, A. Kode, S. Caito, D. Adenuga, and I. Rahman. 2008. Genetic ablation of NADPH oxidase enhances susceptibility to cigarette smoke-induced lung inflammation and emphysema in mice. American Journal of Pathology 172: 1222–1237.
Tian, J., X. Lin, R. Guan, and J.G. Xu. 2004. The effects of hydroxyethyl starch on lung capillary permeability in endotoxic rats and possible mechanisms. Anesthesia and Analgesia 98: 768–774.
Li, F., H.D. Wang, D.X. Lu, Y.P. Wang, R.B. Qi, Y.M. Fu, and C.J. Li. 2006. Neutral sulfate berberine modulates cytokine secretion and increases survival in endotoxemic mice. Acta Pharmacologica Sinica 27: 1199–1205.
Anto, R.J., A. Mukhopadhyay, S. Shishodia, C.G. Gairola, and B.B. Aggarwal. 2002. Cigarette smoke condensate activates nuclear transcription factor-kappaB through phosphorylation and degradation of IkappaB(alpha): correlation with induction of cyclooxygenase-2. Carcinogenesis 23: 1511–1518.
Shishodia, S., P. Potdar, C.G. Gairola, and B.B. Aggarwal. 2003. Curcumin (diferuloylmethane) down-regulates cigarette smoke-induced NF-kappaB activation through inhibition of IkappaBalpha kinase in human lung epithelial cells: correlation with suppression of COX-2, MMP-9 and cyclin D1. Carcinogenesis 24: 1269–1279.
Milner, D. 2004. The physiological effects of smoking on the respiratory system. Nursing Times 100: 56–59.
Blake, D.J., A. Singh, P. Kombairaju, D. Malhotra, T.J. Mariani, R.M. Tuder, E. Gabrielson, and S. Biswal. 2010. Deletion of Keap1 in the lung attenuates acute cigarette smoke-induced oxidative stress and inflammation. American Journal of Respiratory Cell and Molecular Biology 42: 524–536.
Podowski, M., C. Calvi, S. Metzger, K. Misono, H. Poonyagariyagorn, A. Lopez-Mercado, T. Ku, T. Lauer, S. McGrath-Morrow, A. Berger, C. Cheadle, R. Tuder, H.C. Dietz, W. Mitzner, R. Wise, and E. Neptune. 2012. Angiotensin receptor blockade attenuates cigarette smoke-induced lung injury and rescues lung architecture in mice. The Journal of Clinical Investigation 122: 229–240.
Iribarren, C., D.R. Jacobs Jr., S. Sidney, M.D. Gross, and M.D. Eisner. 2000. Cigarette smoking, alcohol consumption, and risk of ARDS: a 15-year cohort study in a managed care setting. Chest 117: 163–68.
Rajendrasozhan, S., S.R. Yang, I. Edirisinghe, H. Yao, D. Adenuga, and I. Rahman. 2008. Deacetylases and NF-kappaB in redox regulation of cigarette smoke-induced lung inflammation: epigenetics in pathogenesis of COPD. Antioxidants & Redox Signaling 10: 799–811.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lin, K., Liu, S., Shen, Y. et al. Berberine Attenuates Cigarette Smoke-Induced Acute Lung Inflammation. Inflammation 36, 1079–1086 (2013). https://doi.org/10.1007/s10753-013-9640-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-013-9640-0