Abstract
The immunological properties of rat S100A8 (r-S100A8) and S100A9 (r-S100A9) in immune cells are poorly understood. Enzyme-linked immunosorbent assay (ELISA) for r-S100A9 enabled us to discuss the differential functional roles of the two proteins, and their localization in the cells was observed microscopically. Recombinant human S100A8 (rh-S100A8) or S100A9 (rh-S100A9) were intravenously administrated into rats with LPS-induced liver damage. ELISA was used to measure the serum concentration of S100A9 in the rats. Western blotting and a preparative ELISA were used to prove specificity and avidity of monoclonal antibodies for r-S100A8 and r-S100A9. Immunohistochemical staining was carried out to visualize intracellular localization of the two proteins in the immune cells using the antibodies. When rh-S100A8 was intravenously injected in the rats (B group), the serum concentration of r-S100A9 apparently decreased as compared with that of the positive control rats (A group). The activities of AST, ALT, and LD in the rat sera (B group) also significantly went down in comparison with those of the rats (A group). Although both the S100A8 and S100A9 were abundantly expressed in activated immune cells, quite difference of not only their intracellular localization but also distribution of the cells expressing the two proteins was microscopically observed. In the rats (B group), less number of the immune cells or less amount of r-S100A8 and r-S100A9 in the cells than those of the rats (A group) was also seen. The r-S100A8 could serve as a regulator of acute inflammatory reaction in the rats with LPS-induced damage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
S100A8 and S100A9, which are calcium-binding proteins, belong to the S100 family, and are abundantly expressed in immune cells of myeloid origin, such as neutrophiles and macrophages. S100A8 and S100A9 (S100 proteins) were first found in the synovial fluid of patients with rheumatoid arthritis [1], and were subjected to subsequent investigation to elucidate their immunological functions [2–7]; however, comprehensive elucidation of the immunological function of S100 proteins has not been achieved. Thus, despite in-depth and continuous study for three decades, the immunological potential of the two proteins is still unclear. S100 proteins presumably play important roles intra- and extracellularly because the mass of the S100 family including S100A8/A9 amounted to more than 40% of the intracellular protein concentration in each cell [8]. Interestingly, it was recently reported that S100 proteins could serve as anti-inflammatory proteins [9, 10]. We also reported the possibility that human S100A8/A9 could function as an anti-inflammatory factor in acute inflammation associated with rejection after liver transplantation [11, 12]. In contrast, S100 proteins were reportedly inducible for acute inflammation [13, 14]. This discrepancy suggests the existence of S100 proteins with a variety of immunological properties in immune cells in vivo, which is worthy of note. Localization of S100A8 and/or S100A9 in the cells and their distribution in rat liver tissue with LPS-induced damage was seen microscopically, and further marked fluctuation in the serum level of r-S100A9 was also demonstrated discriminatively. To achieve our aim, fluorescent immunohistochemical staining was performed using monoclonal antibodies specific for rat S100A8 (r-S100A8) and S100A9 (r-S100A9), so that many images could be observed microscopically.
In the present study, we focused on observing the effect of the administration of recombinant human S100A8 (rh-S100A8) or S100A9 (rh-S100A9) in rats with damage on suppression or induction of inflammatory reactions in vivo, and the dynamic mobility of immune cells expressing r-S100A8 and r-S100A9, leading to elucidation of their functional roles. Another immunological property of the two proteins, characterized by their differential localization in immune cells, was also discussed.
MATERIALS AND METHODS
Reagents
Streptavidin (STA)-TexasRed and STA-fluorescein 5-isothiocyanate (FITC), STA-horseradish peroxidase (HRP), anti-rabbit IgG (goat) IgG-HRP, anti-mouse IgG (horse) IgG-HRP and -FITC conjugates, and biotin (Long arm)-NHS were obtained from Vector Co. (Burlingame, CA); anti-rat CD68 monoclonal antibody was purchased from Santa Cruz Biotechnology Co. (Santa Cruz, CA, USA); anti-myeloperoxidase (MPO) (rabbit) antibody was obtained from Thermo Fisher Scientific (Fremont, CA, USA); 4′, 6-diamino-2-phenylindole (DAPI) (KPL Co., Gaithersburg, MD, USA); lipopolysaccharide (LPS) from Salmonella typhimurium was from Sigma Chemicals, Co. (St. Louis, MO, USA); all others were obtained from Nacalai Tesque or Wakenyaku Co. (Kyoto, Japan).
Expression of cDNA for Human and Rat S100A8 or S100A9
cDNA with histidine tag sequences for human S100A8 and S100A9 was synthesized as described previously [15–17]. Escherichia coli cells expressing rh-S100A8 and rh-S100A9 were harvested, washed, and finally kept frozen at −80°C until use. Also, E. coli cells expressing recombinant r-S100A8 (rr-S100A8) and r-S100A9 (rr-S100A9) were obtained according to the same procedures as described above.
Purification of Recombinant Human or Rat S100A8 and S100A9
Purification of rh-S100A8 and rh-S100A9 was performed as described previously [16]. The concentrations of the products were determined by the method of Lowry et al. using bovine serum albumin as a standard [18]. A single band of rh-S100A8 or rh-S100A9 on the gel was confirmed with SDS-PAGE [19]. Similarly, rr-S100A8 and rr-S100A9 were prepared and then purified.
Preparation of Monoclonal Antibodies and their Specificity
We prepared monoclonal antibodies directed to r-S100A8 and r-S100A9 as described previously [11]. The specificity of monoclonal antibodies for r-S100A8 and r-S100A9 was confirmed by Western blotting, and the sensitivity was examined using a polycarbonate plate, preliminarily coated with a mixture of r-S100A8 and r-S100A9 (approximately 0.5 mg/l in 50 mmol/l phosphate buffer, pH 7.2, each), for an enzyme-linked immunosorbent assay (ELISA).
Development of an Enzyme-Linked Immunosorbent Assay
We developed a new ELISA system for the protein using two monoclonal antibodies specific for r-S100A9 (mAb1D11 and mAb10D11) as described previously [11]. In this ELISA, mAb1D11 and F(ab′)2-HRP derived from mAb10D11 were used as the first and second antibodies, respectively, and rr-S100A9 was used as the standard.
Protocols for Animal Experiments
Animal experimental protocols were approved by the Institutional Animal Care and Use Committee, Kyoto University. Twenty-four Japanese male Wistar rats (250–280 g) were intraperitoneally injected with 4 mg LPS/rat and then divided into four groups (A–D; n = 6/group, rh-S100A8 (approximately 1 mg/0.1 ml/rat) and rh-S100A9 (approximately 1.4 mg/0.1 ml/rat) were injected into the tail vein of the rats in B and C groups, respectively, soon after the injection of LPS. Meanwhile, an equivalent volume of saline was intravenously administrated into each rat in the A and D groups. Eight hours after LPS injection, whole blood (approximately 8 ml) was drawn from the heart of each rat in the A, B, and C groups under anesthesia condition with sodium pentobarbital (10 mg, intraperitoneally). The serum was separated by centrifugation and kept frozen at −80°C. In addition, the liver tissues were quickly removed from each rat and placed in fixative solution, 10% formalin/distilled water, to immobilize the tissues, followed by replacing 1.5 L of the fresh fixative solution at least two times. For biochemical analysis, the residual liver tissue was homogenized in an equal weight of 50 mmol/l PBS 0.9% NaCl as soon as possible. Finally, the whole blood and liver tissues of the rats in D group were obtained from each rat 24 h after LPS injection. These samples were similarly treated as described above, and then kept frozen at −80°C until use.
For biochemical analysis of superoxide anions, all Japanese male Wistar rats (n = 8, 250–280 g) were injected intraperitoneally with 4 mg LPS/rat and then divided into two groups (E and F, n = 4/group). One hour after the injection of LPS, rh-S100A8 (approximately 1 mg/0.1 ml/rat) and rh-S100A9 (approximately 1.4 mg/0.1 ml/rat) were injected into the tail vein of each rat in the two groups (E and F), respectively. Liver tissues were quickly removed from the body and immersed into cold 10 mmol/l phosphate buffer (pH 7.4) 24 h after the injection of LPS. The tissues were embedded in optimal cutting temperature compound (Tissue-Tek®) and used for dihydroethidium (DHE) staining.
Measurement of r-S100A9 and Other Laboratory Markers
The concentrations of r-S100A9 in the serum and liver extract of all rats were measured by ELISA [11]. The samples were usually diluted to 10- to 400-fold with standard matrix solution. For comparison, other laboratory markers, such as AST, ALT, and LDH, were also assayed using an automatic chemical analyzer (TBA200FR-Neo; TOSHIBA Co., Tokyo).
Immunohistochemistry
To reveal the distribution of immune cells expressing r-S100A8 and/or r-S100A9 in the liver tissue of rats with LPS-induced damage, immunohistochemistry was performed using mAb2H6 and mAb15E9 as described previously [12]. Meanwhile, fluorescent immunohistochemical staining was also carried out to verify the localization of r-S100A8 and r-S100A9 in the immune cells in damaged liver. A liver section was stained on the slide with mAb15E9 (approximately 5 μg/ml) at 4°C overnight. After washing five times with 10 mmol/l phosphate buffer, pH 7.2 (buffer A), the liver section was stained with anti-mouse IgG (horse) IgG-FITC conjugate (approximately 5 μg/ml) for 1 h at room temperature in the dark. After washing thoroughly, the section was stained with mAb2H6-biotin (approximately 5 μg/ml) at 4°C overnight in the dark. After washing as above, the section was further stained with STA-Texas Red conjugate (approximately 5 μg/ml) for 2 h at room temperature in the dark. Nuclei of liver and immune cells were counterstained with 4′, 6-diamino-2-phenylindole (DAPI). The stained section was mounted with an adequate volume of non-fluorescent glycerol (90%)/buffer A. Finally, the fluorescence intensity of Texas Red and FITC was observed microscopically.
Superoxide Anions in the Liver Tissue of Rats with LPS-Induced Damage
Superoxide anions were detected in frozen 10-μm-thick liver sections with 10 μmol/l DHE as described previously [20]. The fluorescent intensity of DHE was observed using confocal microscopy.
Statistical Analysis
Statistical analysis was performed by the parametric test for pair-wise comparisons with controls. Significant differences between groups were identified using Student's t test (t test of the difference between the two means). Values of p < 0.05 were considered to be significant.
RESULTS
Expression and Purification of Recombinant Human or Rat S100S8 and S100A9
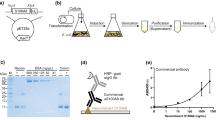
rh-S100A8 and rh-S100A9 were successfully purified as described previously [16]. rr-S100A8 and rr-S100A9 were successfully expressed in E.coli cells and purified using a Ni-agarose affinity column by the method of Namura et al. [16]. As estimated from protein bands in SDS-PAGE, the purity of the two proteins together was ~95% visually, indicating that they were applicable for subsequent experiments in this study (Fig. 1, lane P).
Specificity of monoclonal antibodies for rat S100 proteins and development of an enzyme-linked immunosorbent assay. In a, lane M shows molecular mass markers. Lane P shows protein bands of the mixture of rr-S100A8 and rr-S100A9. Lanes 1 to 5 contains the same proteins as lane P (100 μg/lane). Anti-r-S100A8 antibody (mAb2H6) was used as the first antibody (lane 1). Anti-r-S100A9 antibodies (mAb1d11, mAb10D11, and mAb15E9) were used as the first antibody (lanes 2–4). Anti-mouse IgG (horse) IgG-HRP conjugate was used as the second antibody (lanes 1–4). In b, a polycarbonate ELISA plate preliminarily coated with rat S100 proteins partially purified from rat leukocytes was used. In contrast, an ELISA plate with no antigen was used as a negative control. Each monoclonal antibody was detected with anti-mouse IgG (horse) IgG-HRP conjugate. In c and d, an ELISA plate preliminarily coated with mAb1D11 (5 μg/ml) was used. In d, two pooled sera with different concentrations (low and high) were used to confirm the linearity of the ELISA.
Properties of Monoclonal Antibodies for Rat S100A8 and S100A9 (Specificity and Sensitivity)
As determined by Western blot analysis, mAb2H6 strongly reacted with rr-S100A8, but not with rr-S100A9, indicating its specificity for rr-S100A8 (Fig. 1a, lane 1). In addition, mAb10D11, mAb1D11, and mAb15E9 specifically reacted with rr-S100A9, but hardly with rr-S100A8, indicating that these antibodies are also specific for rr-S100A9 (Fig. 1a, lanes 2–4). One of these, mAb1D11 may be more specific for rr-S100A9 monomer than the other two antibodies because it hardly reacted with rr-S100A9 homodimer (Fig. 1a, lane 3).
The three antibodies, mAb10D11, mAb1D11, and mAb15E9, strongly reacted with r-S100A9, and mAb2H6 also with r-S100A8 (Fig. 1b). Thus, the monoclonal antibodies for r-S100A8 and r-S100A9 were specific for the two proteins, respectively.
Development of an Enzyme-Linked Immunosorbent Assay for r-S100A9 and its Precision
In this ELISA, a novel standard curve was obtained using the rr-S100A9 standard (2600 ng/ml; Fig. 1c). Under standard conditions, the ELISA reaction was linear for r-S100A9 from 0 to 500 ng/ml (Fig. 1d). In addition, we assessed the reproducibility of the method using purified rr-S100A9 and pooled sera from rats with liver damage. Within- and between-day CVs were 2.2–3.7% and 4.7–10.1%, respectively. The recovery of endogenous plus exogenous r-S100A9 was 118% ± 3% (mean ± 1SD).
Changes in the Levels of r-S100A9 and other Laboratory Markers
The concentrations of r-S100A9 in the serum and liver tissue of the positive control rats (A group) markedly increased 125 and 290 times, respectively, 8 h after injection of LPS, as compared with other rats (NC group; Table 1), but both levels subsequently decreased 10 to 30 times 24 h after the injection (data not shown). In contrast, in B group rats, the r-S100A9 levels in both samples were less than half the amount of those in A group rats. In C group rats, the serum level of r-S100A9 slightly decreased, whereas the level in the liver tissue was about half that of A group rats. Meanwhile, when rh-S100A8 or rh-S100A9 alone, together with contaminated ET, was intravenously injected into rats of G (n = 5) and H (n = 5) groups, respectively, the increased levels of r-S100A9 in the serum of rats in both groups ranged from 10.3- to 15.4-fold that of negative control rats. In the contrast, the r-S100A9 level reached about 66-fold that of the liver tissues of G group rats (Table 1). On the other hand, the activities of AST, ALT, and LDH in the serum of rats (A, B, and C groups) were apparently higher than in other rats (NC group); however, in B group rats, the activities of these enzymes were significantly lower than in other rats (A and C groups). Surprisingly, LDH activity increased markedly in the serum of rats (C group), although the reason is unknown.
Effects of rh-S100A8 or rh-S100A9 on Dynamic Mobility of Immune Cells
A large number of immune cells expressing much endogenous S100A8 and/or S100A9 infiltrated and accumulated in inflamed liver tissues of the rats (A group) 8 h after injection of LPS (Fig. 2, panels A2 and A3). Microscopic images, in which a large number of immune cells had accumulated in liver tissues, were seen more distinctly 24 h after the injection (Fig. 2, panels D2, D3, D5, and D6); however, intravenous injection of rh-S100A8 (approximately 1 mg/rat) into the rats (A group) resulted in fewer immune cells expressing endogenous S100A8 and/or S100A9 (Fig. 2, panels B2 and B3). In contrast, when rh-S100A9 (approximately 1.4 mg/rat) was intravenously injected into the rats (C group), very few significant changes in the microscopic images of the liver of rats between A and C groups were seen (Fig. 2, panels C2 and C3).
Effect of rh-S100A8 or rh-S100A9 intravenously administrated on regulation of condition in acute inflammation. r-S100A8 and r-S100A9 were immunohistochemically stained using mAb2H6 and mAb15E9 as the first antibody, respectively. Groups are as follows: A panels A1*, A2, and A3; B panels B1*, B2, and B3; C panels C1*, C2 and C3; D panels D1*, D2*, D3*, D4, D5, and D6; Negative control, panels N1, N2, and N3. Panels with and without asterisk (*) were scanned with low (×100) and high (×400) power fields, respectively. In panel D1*, horizontal arrows indicate focal necrosis.
Intracellular Localization of Endogenous S100A8 and S100A9 in Immune Cells
To visualize the localization of r-S100A8 and r-S100A9 in immune cells in the acute inflammatory phase, fluorescent immunohistochemical staining was carried out. r-S100A8 and r-S100A9 in immune cells were positively stained with mAb2H6 and mAb15E9, respectively, in all groups, but there was almost no difference among A to C groups in the expression of S100 proteins in the cells. In the liver tissue in A to C groups (Fig. 3, panels A1–A4, B1–B4, C1–C4), the different localization of r-S100A8 and/or r-S100A9 in immune cells was clearly demonstrated, in which r-S100A8 existed dominantly in the cytoplasm of the cells, but r-S100A9 was localized near the cell membrane of immune cells (Fig. 3, panels A3, A4, B3, B4, C3, and C4). In addition, notable staining patterns of the two proteins in the cells was observed 24 h after the injection, with a large number of the immune cells expressing r-S100A8 and/or r-S100A9, which were seen over inflamed liver tissues (Fig. 3, panels D1 and D2). It was also noted that r-S100A8 and r-S100A9 tended to visually localize in immune cells in the center and around inflamed areas of the liver tissues, respectively (Fig. 3, panels D1 and D2). Interestingly, microscopic images suggesting cell-to-cell connection among immune cells associated with the secretion of r-S100A9 outside the cells were observed (Fig. 3, panel D4). For comparison, the nucleus of immune cells was counterstained with DAPI. As assessed microscopically, a little endogenous S100A8 and/or S100A9 was likely to exist inside the nucleus of immune cells in the liver (Fig. 3, panels D5, D6, and D7).
Intracellular localizations of endogenous S100A8 and/or S100A9 in the immune cells in the rats with LPS-induced damage. A gr: panels A1–A4, B gr: panels B1–B4, C gr: C1–C4, D gr: panels D1–D7. Panels D5–D7: nuclei were stained with DAPI. In panel D1, two groups of immune cells in the center and around inflamed areas of the liver tissues are indicated by two white circles and horizontal arrows, respectively. Color of microscopic images: red, r-S100A8; green, r-S100A9; yellow, merge; blue, nuclei. A1, B1, C1, and D1: low power field (×100). A2, B2, C2, and D2: high power field (×600). A3, A4, B3, B4, C3, C4, and D3 to D7: super high power field (×4,000).
Identification of Immune Cells all over and Around Inflamed Areas of Liver Tissues
When stained with anti-rat CD68 monoclonal antibody, the immune cells showed a positive reaction localized all over inflamed areas of the liver tissues, indicating macrophages (Fig. 4, panels B1 and B2). Meanwhile, a few immune cells around inflamed areas of the liver tissues were stained with anti-myeloperoxidase antibody positively, indicating neutrophils (Fig. 4, panels A2 and B2, indicated by horizontal arrows).
Identification of immune cells all over and around inflamed areas of liver tissues. Neutrophils were stained with anti-myeloperoxidase (rabbit) antibody and anti-rabbit IgG (goat) IgG-TexasRed conjugate (indicated by horizontal arrows). Macrophages were stained with anti-rat CD68 monoclonal antibody and anti-mouse IgG (horse) IgG-FITC conjugate. Panels A1 and B1: low-power field (×100). Panels A2 and B2: high-power field (×600). Microscopic images: red, neutrophils; green, macrophages.
Detection of Superoxide Anions
To detect superoxide anions in the rat liver tissues in E and F groups, DHE staining was carried out. Many superoxide anions were produced in the rat tissues in F group (Fig. 5, panel F), but such internal oxidants were hardly detected in E group (Fig. 5, panel E), strongly suggesting that rh-100A8 effectively suppresses their production in liver tissues. When rh-S100A9 was intravenously injected into the rats (F group), very little difference in the production of superoxide anions between F and positive control groups rats was observed microscopically (data not shown). This strongly suggests no effect of rh-S100A9 on the suppression of such internal oxidants (Fig. 5, panel F).
DISCUSSION
The biochemical properties of S100 proteins that have been reported by many investigators have varied [21–24]. In this paper, we described the influence of the treatment of rh-S100A8 or rh-S100A9 on the expression of r-S100A8 and/or r-S100A9 in LPS-treated rats, in which we showed the intracellular localization of r-S100A8 and/or r-S100A9 in immunological cells and their distribution in the inflamed liver tissue of rats and presented data supporting their potential as immunological regulators. The value of the two proteins as regulators of immune reactions is based on their origin and clinical data previously reported [11]. In addition, the reliability of our data also depends on the properties of monoclonal antibodies directed to r-S100A8 and r-S100A9. Meanwhile, when recombinant proteins are purified from E. coli cells, endotoxin (ET) should contaminate the purified materials. When the ET, which had an equal amount of ET in the preparation of rh-S100A8 or rh-S100A9, was intravenously injected into rats, the serum level of r-S100A9 slightly increased in (G group). This increase was probably due to contaminated ET in rh-S100A8, because if rh-S100A8 is an inducer of acute inflammation, the r-S100A9 level in B group rats should reach a higher level than that in A group rats, while the serum level of r-S100A9 in G group rats was about one eighth of that in A group rats; therefore, our data suggest that there was no significant influence of the ET on the activation of immune cells, such as neutrophils and macrophages.
In general, most immune cells, such as macrophages and activated neutrophils, in the acute inflammatory phase rapidly move to inflamed areas of liver tissues with inflammatory changes and play functional roles there in self-defense. If rh-S100A8 induced inflammatory reactions in the liver, the condition of liver tissues in B group rats might be more serious, so that many immune cells should accumulate in inflamed areas of the liver; however, as shown in Fig. 2 (panels B2 and B3), not only the number of immune cells expressing r-S100A8 and/or r-S100A9 but also the expression of both proteins in immune cells in the liver tissues was apparently suppressed, suggesting that administrated rh-S100A8 could attenuate LPS-induced r-S100A9 expression, although the mechanism for understanding such regulation is still unclear. Toll-like receptor 4 (TLR4) is recognized as a receptor for LPS. Intravenously injected rh-S100A8 might influence the interactions between LPS and TLR4 on immune cells in vivo, which could result in a significant decrease in the serum level in B group rats. We could not measure r-S100A8 in the serum in the present study; however, it was recently found by flow cytometry that r-S100A8 as much as r-S100A9 was induced on macrophages by stimulating with rh-S100A8 alone (data not shown), in which both proteins may be consumed to form r-S100A8/A9 complex on the cells in vivo, so that less r-S100A9 may be released in the serum.
In this study, the levels of r-S100A9 were very high in the liver tissues treated with LPS plus rh-S100A8 or rh-S100A9; however, whether these marked increases are secreted from hepatocytes and/or immune cells is not clear. It is therefore important to confirm the origin of r-S100A9 in liver tissues with damage. In the near future, we intend to isolate hepatocytes from rat liver tissue to verify the origin of r-S100A9 in liver tissues.
In B group rats, the serum levels of not only r-S100A9 but also hepatic markers, such as AST, ALT, and LDH, fell significantly in comparison with those in the positive control group rats (A group rats), suggesting that rh-S100A8 suppressed the release or secretion of r-S100A9 and hepatic markers outside immune cells in vivo (Table 1). However, S100 proteins are reportedly recognized as inducers of acute inflammation [13, 14]. This discrepancy may be due to differences in the origin of rh-S100A8 or rh-S100A9 intravenously injected, or the experimental conditions between in vivo and in vitro because immunological reactions in vivo are generally very complicated. In contrast, the serum activities of hepatic enzymes rather tended to increase, particularly LDH, although the levels of r-S100A9 in the sera of C group rats slightly fell compared with those of A group rats. These results suggest that the functional role of rh-S100A8 is essentially different from that of r-S100A9.
Endogenous S100A8 and S100A9 are secreted from immune cells, such as neutrophils and macrophages, of myeloid origin. We hypothesized that both proteins could share their functional roles by exchanging signals among these cells in acute inflammatory phase. Not only the expression of both r-S100A8 and r-S100A9 in immune cells but also a number of such immune cells in the liver was significantly suppressed in B group rats (Fig. 2b, panels B1–B3), but these obvious changes were not found in C group rats (Fig. 2, panels C1–C3). These data support our hypothesis concerning the differential role between r-S100A8 and r-S100A9. Furthermore, microscopic images of immune cells expressing r-S100A8 did not necessarily correspond to those expressing r-S100A9, suggesting the possibility of the existence of immune cells sharing immunological functions between the two proteins in vivo (Fig. 2, panels D4–D6; Fig. 3, panels D1–D4). On the other hand, as shown in Fig. 5, the production of superoxide anions in the liver tissue with LPS-induced damage was clearly inhibited when rh-S100A8 was intravenously injected into B group rats. This may indicate the effect of rh-S100A8 on suppression of the production of such internal oxidants, although the mechanism is unclear. In addition, it was recently reported that s-nitrosylated S100A8 might regulate leukocyte-endothelial cell interactions in the microcirculation, and the suppression of mast cell-mediated inflammation [10]. This report may also support our concept concerning another immunological property of r-S100A8 and r-S100A9 in acute inflammation in vivo.
It is generally well known that a large number of immune cells, such as macrophages, neutrophiles, and lymphocytes, have a complex involvement in the regulation of immunological reactions in vivo in their network system, or are functionally regulated according to one or pleural mediators in an unknown manner except for the immune cells. The differential expression and localization of r-S100A8 and r-S100A9 in the immune cells are very interesting to understand the variety of their immunological mobility in acute inflammation. Further distinct observation of morphological images of immune cells expressing r-S100A8 and r-S100A9 may be needed to elucidate their potential, as shown in Fig. 3 (panels D2–D4).
In conclusion, the various intracellular localization of r-S100A8 and/or r-S100A9 in immune cells, neutrophils and macrophages, may provide valuable information for understanding their functional roles in and outside the cells. Our current investigation focused on elucidating the mechanism of intracellular functional roles and dynamic mobility of the two proteins using an immunosuppressant drugs (FK506) and IL-10 KO mice.
Abbreviations
- Rat S100A8:
-
r-S100A8
- Rat S100A9:
-
r-S100A9
- Recombinant human S100A8:
-
rh-S100A8
- Recombinant human S100A9:
-
rh-S100A9
- LPS:
-
Lipopolysaccharide
- STA:
-
Streptavidin
- HRP:
-
Horseradish peroxidase
- FITC:
-
Fluorescein 5-isothiocyanate
- SDS-PAGE:
-
Polyacrylamide gel electrophoresis in the presence of SDS
REFERENCES
Odink, K., N. Cerletti, J. Brüggen, R.G. Clerc, L. Tarcsay, G. Zwadlo, G. Gerhards, R. Schlegel, and C. Sorg. 1987. Two calcium-binding proteins in infiltrate macrophages of rheumatoid arthritis. Nature 330: 380–382.
Rammes, A., J. Roth, M. Goebeler, M. Klempt, M. Hartmann, and C. Sorg. 1997. Myeloid-related protein (MRP) 8 and MRP14, calcium-binding proteins of the S100 family, are secreted by activated monocytes via novel, tubulin-dependent pathway. The Journal of Biological Chemistry 272: 9496–9502.
Guignard, F., J. Mauel, and M. Markert. 1995. Identification and characterization of a novel human neutrophil protein related to the S100 family. The Biochemical Journal 309: 395–401.
Kerkhoff, C., M. Klempt, and C. Sorg. 1998. Novel insights into structure and function of MRP8 (S100A8) and MRP14 (S100A9). Biochimica et Biophysica Acta 1448: 200–211.
Vogl, T., C. Pröpper, M. Hartmann, A. Strey, K. Strupat, C. van den Bos, C. Sorg, and J. Roth. 1999. S100A12 is expressed exclusively by granulocytes and acts independently from S100A8 and S100A9. The Journal of Biological Chemistry 274: 25291–25296.
Nacken, W., C. Sopalla, C. Pröpper, C. Sorg, and C. Kerkhoff. 2000. Biochemical characterization of the murine S100A9 (MRP14) protein suggests that it is functionally equivalent to its human counterpart despite its low degree of sequence homology. European Journal of Biochemistry 267: 560–565.
Nacken, W., J. Roth, C. Sorg, and C. Kerkhoff. 2003. S100A9/S100A8: myeloid representatives of the S100 protein family as prominent players in innate immunity. Microscopy Research and Technique 60: 569–580.
Edgeworth, J., M. Gorman, R. Bennett, P. Freemont, and N. Hogg. 1991. Identification of p8, 14 as a highly abundant heterodimeric calcium binding protein complex of myeloid cells. The Journal of Biological Chemistry 266: 7706–7713.
Lim, S.Y., M.J. Raftery, J. Goyette, K. Hsu, and C.L. Geczy. 2009. Oxidative modifications of S100 proteins: functional regulation by redox. Journal of Leukocyte Biology 86: 577–587.
Lim, S.Y., M.J. Raftery, H. Cai, K. Hsu, W.X. Yan, H.L. Hseih, R.N. Watts, D. Richardson, S. Thomas, M. Perry, and C.L. Geczy. 2008. S-Nitrosylated S100A8: Novel Anti-Inflammatory Properties. Journal of Immunology 181: 5627–5636.
Ikemoto, M., T. Tanaka, Y. Takai, H. Murayama, K. Tanaka, and M. Fujita. 2003. New ELISA system for myeloid-related protein complex (MRP8/14) and its clinical significance as a sensitive marker for inflammatory response associated with transplant rejection. Clinical Chemistry 49: 594–600.
Ikemoto, M., H. Murayama, H. Itoh, M. Totani, and M. Fujita. 2007. Intrinsic function of S100A8/A9 complex as an anti-inflammatory protein in liver injury induced by lipopolysaccharide in rats. Clinica Chimica Acta 376: 197–204.
Ryckman, C., K. Vandal, P. Rouleau, M. Talbot, and P.A. Tessier. 2003. Proinflammatory activities of S100: proteins S100A8, S100A9, and S100A8/A9 induce neutrophil chemotaxis and adhesion. Journal of Immunology 170: 3233–3242.
Ryckman, C., C. Gilbert, R. de Médicis, A. Lussier, K. Vandal, and P.A. Tessier. 2004. Monosodium urate monohydrate crystals induce the release of the proinflammatory protein S100A8/A9 from neutrophils. Journal of Leukocyte Biology 76: 433–440.
Qing, G., L.C. Ma, A. Khorchid, G.V. Swapna, T.K. Mal, M. Takayama, B. Xia, S. Phadtare, H. ke, T. Acton, G.T. Montelione, M. Ikura, and M. Lnouyeet. 2004. Cold-shock induced high-yield protein production in Escherichia coli. Nature Biotechnology 22: 877–882.
Namura, T., S. Arai, K. Okawa, A. Koike, S. Yamada, N. Saita, A. Nagae, H. Itoh, M. Totani, S. Uemoto, and M. Ikemoto. 2010. Identification of serum proteins that bind with S100A8, S100A9 and S100A8/A9: clinical significance of using proteins for monitoring the postoperative condition of liver recipients. Clinica Chimica Acta 411: 1766–1773.
Imamichi, T., I. Uchida, S.M. Wahl, and N. MaCartney-Fransis. 1993. Expression and cloning of migration inhibitory factor-related protein (MRP)8 and MRP14 in arthritis-susceptible rats. Biochemical and Biophysical Research Communications 194: 819–825.
Lowry, O.H., N.J. Rosebrough, A.L. Faee, and R.J. Randall. 1951. Protein measurement with the Folin phenol reagent. The Journal of Biological Chemistry 193: 265–275.
Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proceedings of the National Academy of Sciences of the United States of America 76: 4350–4354.
Zanetti, M., L.V. d'Uscio, T.E. Peterson, Z.A. Katusic, and T. O'Brien. 2005. Analysis of superoxide anion production in tissue. Methods in Molecular Medicine 108: 65–72.
Itou, H., M. Yao, I. Fujita, N. Watanabe, M. Suzuki, J. Nishihira, and I. Tanaka. 2002. The crystal structure of human MRP14 (S100A9), a Ca(2+)-dependent regulator protein in inflammatory process. Journal of Molecular Biology 316: 265–276.
Viemann, D., A. Strey, A. Janning, K. Jurk, K. Klimmek, T. Vogl, K. Hirono, F. Ichida, D. Foell, B. Kehrel, V. Gerke, C. Sorg, and J. Roth. 2005. Myeloid-related proteins 8 and 14 induce a specific inflammatory response in human microvascular endothelial cells. Blood 105: 2955–2962.
Vandal, K., P. Rouleau, A. Boivin, C. Rickman, M. Talbot, and P.A. Tessier. 2003. Blockade of S100A8 and S100A9 suppresses neutrophil migration in response to lipopolysaccharide. Journal of Immunology 171: 2602–2609.
Vogl, T., K. Tenbrock, S. Ludwig, N. Leukert, C. Ehrhardt, M.A. van Zoelen, W. Nacken, D. Foell, T. van der Poll, C. Sorg, and J. Roth. 2007. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nature Medicine 13: 1042–1049.
ACKNOWLEDGEMENTS
We thank Dr Hiroshi Murayama (Yamasa Shoyu Co., Choshi, Chiba, Japan) for the gift of ELISA plates, and Dr Kyoichi Matsumoto (Mikuri Immunological Laboratory Co., Osaka, Japan) for his technical teaching in the preparation of monoclonal antibody. This work was supported by a Grant-in-Aid for Scientific Research (C: 20590567) from the Ministry of Education, Science, Sports and Culture of Japan (to M.I.)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koike, A., Arai, S., Yamada, S. et al. Dynamic Mobility of Immunological Cells Expressing S100A8 and S100A9 in vivo: A Variety of Functional Roles of the two Proteins as Regulators in Acute Inflammatory Reaction. Inflammation 35, 409–419 (2012). https://doi.org/10.1007/s10753-011-9330-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-011-9330-8