Abstract
Cryptotanshinone (CTS), a major constituent extracted from the medicinal herb Salvia miltiorrhiza Bunge, has well-documented antioxidative and anti-inflammatory effects. In the present study, the pharmacological effects and underlying molecular mechanisms of CTS on lipopolysaccharide (LPS)-induced inflammatory responses were investigated. By enzyme-linked immunosorbent assay, we observed that CTS reduced significantly the production of proinflammatory mediators (tumor necrosis factor-α and interleukin-6) induced by LPS in murine macrophage-like RAW264.7 cells. Mechanistically, CTS inhibited markedly the phosphorylation of mitogen-activated protein kinases (MAPKs), including ERK1/2, p38MAPK, and JNK, which are crucially involved in regulation of proinflammatory mediator secretion. Moreover, immunofluorescence and western blot analysis indicated that CTS abolished completely LPS-triggered nuclear factor-κB (NF-κB) activation. Taken together, these data implied that NF-κB and MAPKs might be the potential molecular targets for clarifying the protective effects of CTS on LPS-induced inflammatory cytokine production in macrophages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Proinflammatory cytokines, including tumor necrosis factor (TNF), interleukin-1 (IL-1), IL-6, and IL-8, are important inflammatory mediators that are rapidly induced in the early stage of inflammatory diseases or injury process and modulate a myriad of healing processes, but if over-produced, these cytokines may exacerbate the severity of multiple inflammatory diseases such as in rheumatoid arthritis, atherosclerosis, Alzheimer's disease, acute ischemic stroke, especially in sepsis. Among the inflammatory cytokines, TNF-α plays a key role in regulating inflammation, mostly through the induction of other inflammatory cytokines including IL-1 (IL-1α and IL-1β), IL-6, IL-8, macrophage inflammatory protein 2 (MIP-2), granulocyte-macrophage colony-stimulating factor (GM-CSF), and adhesion molecules. Therefore, anti-inflammation is an important therapeutic strategy for various inflammatory diseases.
Salvia miltiorrhiza (SM), a well-known traditional Chinese herbal medicine, has been widely used in the clinical treatment of different diseases such as cardiovascular disease and neurodegenerative diseases. Cryptotanshinone (CTS), as the major active component of SM, was reported to have multiple pharmacological activities, such as anti-inflammatory, antioxidative, antiapoptosis, and antiplatelet aggregation activities [1–4]. It also benefited patients with stroke and ischemic diseases [5], with few or no side effects.
As nuclear factor-Kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) pathways are crucially implicated in inflammatory responses, the effects of CTS on NF-κB activation and phosphorylation of ERK1/2, p38MAPK, and JNK were explored in activated RAW264.7 macrophages induced by lipopolysaccharide (LPS) in order to illuminate the molecular mechanisms of the anti-inflammatory effect of CTS.
Materials and Methods
Chemicals and Reagents
Cryptotanshinone (over 98% purity) was kindly provided by Professor Gu Lianquan (Institute of Pharmacy Synthesis, Sun Yat-sen University). The structure of the compound was established based on nuclear magnetic resonance and mass spectral data and by comparison with those of authentic sample. LPS (Escherichia coli 055:B5), dimethyl sulfoxide (DMSO), and 3-(4,5-dimethylthiazol-2-y1)-2,5-dipheny-ltetrazolium bromide (MTT) were obtained from Sigma Aldrich (St. Louis, MO, USA). Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum (FBS) were obtained from Invitrogen-Gibco (Grand Island, NY). Primary antibodies of p65 and IκB-α were purchased from Santa Cruz (Santa Cruz, CA, USA). Primary antibodies of ERK, p38, JNK, p-ERK, p-p38, and p-JNK were obtained from Cell Signaling Technology Inc. (San Francisco, CA, USA). Flourescein isothiocyanate (FITC)-conjugated secondary antibody was from Invitrogen.
Cell Culture
RAW264.7 cells, a murine macrophage-like cell line, were purchased from American Type Culture Collection (ATCC TIB71, Manassas, VA) and maintained in DMEM supplemented with 10% heat-inactivated FBS, 100 IU/ml of penicillin G, and 100 µg/ml streptomycin under 5% CO2 at 37°C in humidified atmosphere. In brief, macrophages were plated at a density of 2 × 105 cells/ml and serum-starved for 24 h, then the cells were pretreated with indicated compounds for 2 h prior to stimulation with LPS (100 ng/ml) for another 24 h. Test compounds were freshly dissolved in DMSO on the day of the experiment and diluted with serum-free DMEM at appropriate concentrations. The final concentration of DMSO was adjusted to 0.1% (v/v). Control groups received the same amount of DMSO.
MTT Assay for Cell Viability
To measure cell viability, MTT assay was performed as described previously. RAW264.7 cells were seeded in 96-well plates at 2 × 105 cells/ml and incubated in a 37°C, 5% CO2 incubator. After 24 h, the cells were pretreated with different concentrations of CTS (0–10 µM) for 2 h, followed by stimulation with LPS for 24 h. Subsequently, MTT at 5 mg/ml was added to each well and incubated for an additional 4 h. The MTT/medium in each well was carefully removed, and 150 μl DMSO was added into each well, followed by incubation at 37°C for 10 min with horizontal shaking. The absorbance at 570 nm was measured with an automated microplate reader (Bio-Tek, Winooski, VT, USA).
Quantitative Analysis of Cytokine Production
RAW264.7 cells (2 × 105 cells/ml) in 96-well plates were treated with LPS (100 ng/ml) in the presence or absence of CTS for 24 h, and then culture supernatants were harvested. TNF-α and IL-6 in the cell supernatants was measured with commercial enzyme-linked immunosorbent assay (ELISA) kits according to the instructions of the manufacturer.
Western Blot Analysis
RAW264.7 cells (2 × 105 cells/ml) were cultured in six-well plates for 24 h, then pretreated with 2.5, 5, or 10 µM of CTS for 2 h prior to treatment with 100 ng/ml of LPS for 30 min. Whole-cell lysates were prepared using ice-cold cell lysis buffer (Cell Signaling, Danvers, MA, USA). Protein concentration was determined using BCA Protein Assay Kit (Pierce, Rockford, IL, USA). Samples of whole-cell lysates were separated by 10% SDS-PAGE and then transferred onto polyvinylidene difluoride membranes (Bio-Rad, Hercules, CA, USA). The membranes were incubated in blocking solution (5% skimmed milk) for 1 h at room temperature and immunoblotted with primary antibodies that recognize α-tubulin, IκB-α, p65, MAPKs (ERK, JNK, p38, p-ERK, p-JNK, and p-p38), and Histone-1 (Cell Signaling, Danvers, MA, USA). After washing with Tris-buffered saline Tween-20 (TBST), horseradish peroxidase-conjugated secondary antibodies (Cell Signaling; 1:2,000 dilution in TBST) were applied. Blots then were developed by an enhanced chemiluminescence detection system (Pierce, Rockford, IL, USA). The intensities of the protein bands were analyzed by Labworks software. α-Tubulin protein was used as the internal control to normalize for protein loading. Western blot analysis of nuclear protein (for NF-κB and Histone H1) isolated from RAW264.7 cells was performed as previously described [6].
P65 Subunit Translocation by Immunofluorescent Staining
RAW264.7 cells (2 × 105 cells/ml) cultured on glass cover slips were plated into six-well plates for 24 h, pretreated with 2.5, 5, or 10 µM of CTS for 2 h prior to treatment with 100 ng/ml of LPS for 30 min. Cells were washed with 0.01 M phosphate-buffered saline (PBS) and fixed in 4% formaldehyde for 30 min. After being permeabilized with 1% Triton X-100 for 10 min, cells were then blocked with PBS containing 5% bovine serum albumin for 30 min and processed for immunofluorescent staining with mouse anti-NF-κB/p65 polyclonal antibody followed by FITC-conjugated goat anti-mouse IgG. Finally, cover slips were mounted on slides, and fluorescence signals were analyzed by microscopy (Olympus, Tokyo, Japan).
Statistical Analysis
All values were expressed as means ± the standard error of the mean (SEM). Differences between mean values of normally distributed data were assessed by one-way ANOVA (Dunnett's t test) and Student's t test. The analysis was performed using GraphPad Prism Software version 4.0 (GraphPad Software Inc. La Jolla, CA). P values less than 0.05 were considered significant.
Results
Effect of CTS on Macrophage Cell Viability
As determined by the MTT assay (Fig. 1), CTS (0–10 μM) did not display any cellular toxicity against RAW264.7 cells over 24 h, thus excluding a nonspecific cytotoxicity as a possible explanation for the decreased cytokine output.
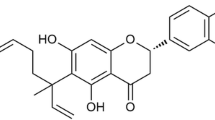
a Chemical structure of cryptotanshinone. b Effect of CTS on the viability of RAW264.7 cells. Cells were cultured with CTS (0–10 μM) in the absence or presence of 100 ng/ml LPS for 24 h, then cell viability was measured by MTT assay. Data are presented as means ± SEM of three independent experiments.
CTS Inhibited Efficiently LPS-Induced Production of TNF-α and IL-6 in Macrophages
To investigate the anti-inflammatory effects of CTS, we first quantified TNF-α and IL-6 production in the culture supernatants of RAW264.7 cells. As shown by sandwich ELISA results, both cytokines were relatively low in resting cells but markedly increased upon exposure to LPS alone. Treatment with CTS inhibited LPS-induced TNF-α and IL-6 production in a dose-dependent manner (Fig. 2, P < 0.01).
Effect of different concentrations of CTS on the secretion of TNF-α and IL-6 from RAW264.7 macrophages. The cells were treated with LPS alone or LPS plus different concentrations (2.5, 5, or 10 μM) of CTS for 24 h and then subjected to ELISA for the determination of TNF-α and IL-6 secretion. The values represent means ± SEM of three independent experiments. *P < 0.05, **P < 0.01.
CTS Suppressed the Phosphorylation of ERK 1/2, JNK, and p38 MAPK
MAPK signaling pathways are well-recognized to control the synthesis and release of proinflammatory mediators by activated macrophages during the inflammatory response. To determine whether MAPK signaling pathways are implicated in the anti-inflammatory effects of CTS, RAW264.7 cells were treated with various concentrations of CTS for 2 h and then stimulated with LPS for 30 min. The phosphorylation of three MAPK signaling molecules including ERK1/2, JNK, and p38 MAPK were analyzed by western blot. The phosphorylation levels of the MAPK isoforms were dramatically decreased in CTS-treated cells compared with the only LPS-treated cells. However, total levels of the MAPK isoforms did not differ significantly among these groups. These results indicated that signal transduction by MAPK molecules might be effectively blocked by CTS in activated macrophages (Fig. 3).
Effect of CTS on LPS-induced phosphorylation of MAPKs in RAW264.7 macrophages. RAW264.7 macrophages were treated with 2.5, 5, or 10 μM CTS for 2 h before the addition of 100 ng/ml LPS for 30 min. Cell extracts were subjected to western blot analysis with phospho-specific antibodies. The total MAPK levels were used as internal controls. Shown in the right panel are means ± SEM of three independent experiments. A representative western blot is shown in the left panel. *P < 0.05, **P < 0.01.
CTS Prevented NF-κB Activation in LPS-Stimulated RAW264.7 Cells
NF-κB is an important transcription factor orchestrating proinflammatory mediators' production in activated macrophages. Therefore, we next investigated whether CTS has an inhibitory effect on the NF-κB signaling pathway, which is implicated in the transcriptional regulation of inflammatory mediators in LPS-stimulated RAW264.7 cells.
As shown in Fig. 4, LPS stimulation induced the IκB-α degradation in cytosol and the translocation of NF-κB p65 subunit into the nucleus of RAW264.7 cell; however, CTS pretreatment significantly attenuated the cytosolic levels of IκB-α and the nuclear levels of NF-κB p65 subunit in LPS-stimulated RAW264.7 cells. By immunofluorescent staining of p65, we observed that p65 was exclusively distributed in the cytoplasmic compartment before LPS stimulation. Treatment with LPS resulted in the enrichment of p65 in the nucleus (Fig. 5). The nuclear translocation of p65 was markedly attenuated in a dose-dependent manner by CTS treatment. These results indicated the potential role of NF-κB in the suppression of inflammatory mediators-TNF-α and IL-6 production by CTS.
CTS suppressed LPS-induced IκB degradation and NF-κB activation in RAW264.7 cells. RAW264.7 cells were pretreated with CTS (2.5, 5, and 10 μM) for 2 h and then stimulated with LPS (100 ng/ml) for 30 min. The cells were harvested, and then cytosolic extract was prepared for the detection of total forms of IκB and nuclear extracts for the detection of the NF-κB p65 subunit by western blot. Shown in the right panel are means ± SEM of three independent experiments. A representative western blot is shown in the left panel. *P < 0.05, **P < 0.01.
CTS prevented LPS-induced nuclear translocation of NF-κB p65 subunit by immunofluorescent studies. Cells were treated with or without CTS for 2 h and stimulated with LPS (100 ng/ml) for 30 min, fixed, permeabilized, and incubated with mouse anti-p65 antibody followed by FITC-conjugated anti-mouse IgG (green). The nuclei of the corresponding cells were visualized by Hoechst 33342 staining (blue). Magnification ×400. a In untreated cells, NF-κB p65 is limited to the cytoplasm. b LPS-stimulated cells show NF-κB p65 (green) translocation into the nucleus. c–e CTS inhibited p65 nuclear translocation in a dose-dependent manner.
Discussion
Activated monocytes/macrophages liberate cytokines at the site of inflammation and are involved in the progression of disease states resulting from chronic inflammation. LPS activates monocytes/macrophages by binding to its receptor, Toll-like receptor-4 (TLR4), and then TLR4 activates the intracellular signaling cascade by recruiting myeloid differentiation factor 88 (MyD88), IL-1 receptor-associated kinase (IRAK)-1, and IRAK-4 to the membrane. IRAKs associate with the receptor complex transiently. Once released, IRAKs can associate with and activate TNF receptor-activated factor 6 (TRAF6), causing activation of the IκB kinase (IKK) complex and MAPK [7–9] which are known to be involved in the regulation proinflammatory cytokine secretion [10–12]. The activated IKK complex induces phosphorylation of IκB, causing degradation of IκB and liberation of the transcription factor, NF-κB, which promotes the transcription of inflammatory cytokines such as IL-1, IL-6, TNF-α, and IFN-γ [13, 14].
Several proinflammatory cytokines such as TNF-α, IL-6, IL-1β, and cyclooxygenase-2 are instrumental in inflammatory responses; therefore, the inhibitory effect on these proinflammatory cytokines and mediator production is a key factor to evaluate the efficacy of anti-inflammatory drugs. In the present study, we showed that CTS inhibited significantly the production of proinflammatory mediators (TNF-α and IL-6) in RAW264.7 cells after stimulating with LPS, suggesting that CTS had an anti-inflammatory function.
Despite several reports documenting the anti-inflammatory properties of CTS [15–17], the precise molecular mechanisms remain largely unexplored. NF-κB, as the main regulator for most of proinflammatory genes, mediates a variety of important cellular functions by regulating immune and inflammatory responses [18, 19]. In unstimulated cells, NF-κB forms a heterodimer of p65/p50 binding to the inhibitor proteins IkB. After stimulation, p65/p50 is released from the IκB resulting in p65 translocation into the nucleus to regulate gene transcription. Our studies indicated that CTS could inhibit IκB-α degradation and p65 translocation into the nucleus upon LPS stimulation in RAW264.7 cells, indicating that NF-κB pathways might be involved in the suppressive effects of CTS on the release of proinflammatory cytokines in LPS-treated RAW264.7 cells.
MAPKs are a family of serine/threonine protein kinases responsible for most cellular responses to cytokines and external stress signals and crucial for regulation of the production of inflammation mediators; thus, this pathway may be an important therapeutic target in the treatment of inflammatory diseases [20]. A growing body of evidence indicated that many natural products have been shown to inhibit the expression of proinflammatory genes by modulating the phosphorylation of MAPKs pathways [21–24]. In our experiments, rapid phosphorylation of ERK1/2, JNK, and p38 MAPK followed by LPS stimulation in RAW264.7 cells were inhibited by CTS in a dose-dependent manner, implying that CTS may inhibit MAPK signaling cascade.
To summarize, our data provide the first line of evidence, suggesting that CTS suppressed LPS-induced production of TNF-α and IL-6 via inhibiting the activation of NF-κB and MAPKs. However, the molecular basis that contributes to these inhibitory effects remains unknown, and future work is underway in our lab to determine upstream events of these signaling pathways such as Toll-like receptor-4. In addition, future studies should also address the clinical relevance of our studies.
References
Kim, S.Y., T.C. Moon, H.W. Chang, K.H. Son, S.S. Kang, and H.P. Kim. 2002. Effects of tanshinone I isolated from Salvia miltiorrhiza Bunge on arachidonic acid metabolism and in vivo inflammatory responses. Phytotherapy Research 16: 616–620.
Ng, T.B., F. Liu, and Z.T. Wang. 2000. Antioxidative activity of natural products from plants. Life Sciences 66: 709–723.
Park, E.J., Y.Z. Zhao, Y.C. Kim, and D.H. Sohn. 2007. PF2401-SF, standardized fraction of Salvia miltiorrhiza and its constituents, tanshinone I, tanshinone IIA, and cryptotanshinone, protect primary cultured rat hepatocytes from bile acid-induced apoptosis by inhibiting JNK phosphorylation. Food and Chemical Toxicology 45: 1891–1898.
Zhou, L., Z. Zuo, and M.S. Chow. 2005. Danshen: An overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. Journal of Clinical Pharmacology 45: 1345–1359.
Adams, J.D., R. Wang, J. Yang, and E.J. Lien. 2006. Preclinical and clinical examinations of Salvia miltiorrhiza and its tanshinones in ischemic conditions. Chinese Medicine 1: 3.
Jung, H.W., U.K. Seo, J.H. Kim, K.H. Leem, and Y.K. Park. 2009. Flower extract of Panax notoginseng attenuates lipopolysaccharide-induced inflammatory response via blocking of NF-kappaB signaling pathway in murine macrophages. Journal of Ethnopharmacology 122: 313–319.
Choi, H.S., D.I. Cho, H.K. Choi, S.Y. Im, S.Y. Ryu, and K.M. Kim. 2004. Molecular mechanisms of inhibitory activities of tanshinones on lipopolysaccharide-induced nitric oxide generation in RAW 264.7 cells. Archives of Pharmacal Research 27: 1233–1237.
van der Bruggen, T., S. Nijenhuis, E. van Raaij, J. Verhoef, and B.S. van Asbeck. 1999. Lipopolysaccharide-induced tumor necrosis factor alpha production by human monocytes involves the raf-1/MEK1-MEK2/ERK1-ERK2 pathway. Infection and Immunity 67: 3824–3829.
Andersson, K., and R. Sundler. 2000. Signalling to translational activation of tumour necrosis factor-alpha expression in human THP-1 cells. Cytokine 12: 1784–1787.
Fitzgerald, K.A., D.C. Rowe, and D.T. Golenbock. 2004. Endotoxin recognition and signal transduction by the TLR4/MD2-complex. Microbes and Infection 6: 1361–1367.
Kerfoot, S.M., and P. Kubes. 2005. Local coordination verses systemic disregulation: Complexities in leukocyte recruitment revealed by local and systemic activation of TLR4 in vivo. Journal of Leukocyte Biology 77: 862–867.
Beutler, B. 2000. Tlr4: Central component of the sole mammalian LPS sensor. Current Opinion in Immunology 12: 20–26.
Ghosh, S., M.J. May, and E.B. Kopp. 1998. NF-kappa B and Rel proteins: Evolutionarily conserved mediators of immune responses. Annual Review of Immunology 16: 225–260.
Dobrovolskaia, M.A., and S.N. Vogel. 2002. Toll receptors, CD14, and macrophage activation and deactivation by LPS. Microbes and Infection 4: 903–914.
Jeon, S.J., K.H. Son, Y.S. Kim, Y.H. Choi, and H.P. Kim. 2008. Inhibition of prostaglandin and nitric oxide production in lipopolysaccharide-treated RAW 264.7 cells by tanshinones from the roots of Salvia miltiorrhiza Bunge. Archives of Pharmacal Research 31: 758–763.
Lee, P., J. Hur, J. Lee, J. Kim, J. Jeong, I. Kang, S.Y. Kim, and H. Kim. 2006. 15, 16-dihydrotanshinone I suppresses the activation of BV-2 cell, a murine microglia cell line, by lipopolysaccharide. Neurochemistry International 48: 60–66.
Jin, D.Z., L.L. Yin, X.Q. Ji, and X.Z. Zhu. 2006. Cryptotanshinone inhibits cyclooxygenase-2 enzyme activity but not its expression. European Journal of Pharmacology 549: 166–172.
Baldwin, A.S., Jr. 1996. The NF-kappa B and I kappa B proteins: New discoveries and insights. Annual Review of Immunology 14: 649–683.
Suh, S.J., U.H. Jin, H.J. Choi, H.W. Chang, J.K. Son, S.H. Lee, S.J. Jeon, K.H. Son, Y.C. Chang, Y.C. Lee, and C.H. Kim. 2006. Cryptotanshinone from Salvia miltiorrhiza BUNGE has an inhibitory effect on TNF-alpha-induced matrix metalloproteinase-9 production and HASMC migration via down-regulated NF-kappaB and AP-1. Biochemical Pharmacology 72: 1680–1689.
Cao, W., C. Bao, E. Padalko, and C.J. Lowenstein. 2008. Acetylation of mitogen-activated protein kinase phosphatase-1 inhibits Toll-like receptor signaling. The Journal of Experimental Medicine 205: 1491–1503.
Wu, M.J., L. Wang, H.Y. Ding, C.Y. Weng, and J.H. Yen. 2004. Glossogyne tenuifolia acts to inhibit inflammatory mediator production in a macrophage cell line by downregulating LPS-induced NF-kappa B. Journal of Biomedical Science 11: 186–199.
Cheng, Y.W., C.Y. Chang, K.L. Lin, C.M. Hu, C.H. Lin, and J.J. Kang. 2008. Shikonin derivatives inhibited LPS-induced iNOS in RAW 264.7 cells via downregulation of MAPK/NF-kappaB signaling. Journal of Ethnopharmacology 120: 264–271.
Chen, C.C., P.C. Tsai, B.L. Wei, and W.F. Chiou. 2008. 8-Prenylkaempferol suppresses inducible nitric oxide synthase expression through interfering with JNK-mediated AP-1 pathway in murine macrophages. European Journal of Pharmacology 590: 430–436.
Jung, H.W., C.H. Yoon, Y.H. Kim, Y.C. Boo, K.M. Park, and Y.K. Park. 2007. Wen-Pi-Tang-Hab-Wu-Ling-San extract inhibits the release of inflammatory mediators from LPS-stimulated mouse macrophages. Journal of Ethnopharmacology 114: 439–445.
Acknowledgments
This study was supported by research grants from National Natural Science Foundation of China (no. 30811120434, no. 30772576), the National Science and Technology Major Project of China “Key New Drug Creation and Manufacturing Program” [Grant 2009ZX09102-152, 2009ZX09303-007], Major Program in Key Field of People's Government of Guangdong Province (P. R. of China, no. 2003A30904), and the Key Natural Science Foundation of Guangdong Province, People's Republic of China (no. 7117380).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tang, S., Shen, XY., Huang, HQ. et al. Cryptotanshinone Suppressed Inflammatory Cytokines Secretion in RAW264.7 Macrophages through Inhibition of the NF-κB and MAPK Signaling Pathways. Inflammation 34, 111–118 (2011). https://doi.org/10.1007/s10753-010-9214-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-010-9214-3