Abstract
Decomposition of allochthonous organic matter is an essential process in headwater streams. Damming of streams alters decomposition rates in the benthic zone downstream, but little is known about the effects on hyporheic decomposition. We examined the effects of dams on hyporheic and benthic organic matter decomposition, using the cotton-strip assay over five seasons, in two forest mountain streams in western Japan. The decomposition rates in the hyporheic zone were lower downstream of the dams than at the unregulated reach in spring, rainy season and fall, but they did not differ in winter and summer. Hyporheic decomposition rates were comparable to the benthic rates in one river and were lower in the other river. Decomposition rates did not differ between coarse- and fine-mesh bags in many seasons at all sites, and the densities of macroinvertebrates were low, suggesting that the contribution of macroinvertebrate to decomposition in the hyporheic zone was small. These results showed that the hyporheic zone is an important zone for decomposition and that presence of dams altered the hyporheic decomposition in some seasons. Thus, it is crucial to examine both the benthic and hyporheic zones when addressing the effects of dams and reservoirs on stream ecosystem processes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Forest mountain streams are often heterotrophic due to limited in-stream photosynthesis, and stream organisms are dependent on organic matter inputs from the riparian zone (Meyer et al., 2000). Leaf litter decomposition are thus pivotal ecosystem functions (Gessner & Chauvet, 2002). Leaf litter decomposition is a complex process, influenced by various biotic and abiotic factors. Microbes and invertebrates play essential roles on leaf litter decomposition in streams (Piscart et al., 2011; Flores et al., 2013), and elevated water temperature and nutrient concentrations affect their activities and stimulate leaf litter decomposition rates (Ferreira et al., 2015). Stream flow affects decomposition rates directly through physical abrasion and indirectly by stimulating microbial activities (Ferreira & Graça, 2006; Arroita et al., 2015). Leaf litter decomposition controlled by various environmental factors is considered a useful indicator of stream ecosystem health (Young et al., 2008).
Damming of streams changes water and sediment fluxes and alters many of those environmental factors influencing leaf litter decomposition (Lessard & Hayes, 2003; Tieman et al., 2004; Poff et al., 2007; Harrison et al., 2009). Previous studies found lower leaf litter decomposition rates in the benthic zone in reaches downstream of dams compared to the upstream reaches (Martínez et al., 2013, 2016). The lower leaf litter decomposition rates were often explained by differences in biotic factors, such as lower abundance and biomass of shredders (González et al., 2013; Mollá et al., 2017) and lower microbial biomass and invertebrate richness (Salomão et al., 2019). Other studies showed no effects or even higher benthic leaf litter decomposition rates downstream of dams because hypolimnetic release tends to increase nutrient concentrations downstream (Casas et al., 2000; Menéndez et al., 2012).
The hyporheic zone is a major site of organic matter storage. A substantial part of leaf litter inputs can be buried in the streambed sediments (Metzler & Smock, 1990; Storey et al., 1999), and the amount of coarse particulate organic matter may be much greater in the streambed sediment than in the benthic zone in headwater streams (Cornut et al., 2012; Wohl et al., 2012). Hyporheic exchange stimulates hyporheic respiration (González-Pinzón et al., 2014), which can account for a large portion of stream ecosystem respiration (Naegeli & Uehlinger, 1997; Corson-Rikert et al., 2016). Despite the potential high rates of organic matter processing in the hyporheic zone, most studies focus only on the benthic zone, and decomposition processes in the hyporheic zone are not well understood.
Still, several studies have compared leaf litter decomposition between the hyporheic zone and the benthic zone. Some studies reported lower decomposition rates in the hyporheic zone (Metzler & Smock, 1990; Cornut et al., 2010; Peralta-Maraver et al., 2019), whereas others reported similar or higher rates in the hyporheic zone (Solagaistua et al., 2015; Burrows et al., 2017). In the benthic zone, macroinvertebrates play a significant role in leaf litter decomposition. In the hyporheic zone, due to the size of pore space, access of macroinvertebrates may be limited, and microbes may become the primarily decomposers (Cornut et al., 2010; Flores et al., 2013). Microbial-driven leaf litter decomposition may also be lower in the hyporheic zone than the benthic zone (Cornut et al., 2010), although some studies reported comparable decomposition rates (Risse-Buhl et al., 2017). Thus, further studies on organic matter decomposition in the hyporheic zone are needed. Furthermore, the effects of dams on subsurface processes are poorly understood (Graham et al., 2019). Flow regulation that alters frequency, duration and magnitude of flow fluctuation impacts hyporheic exchange flow (Graham et al., 2019), and altered stream water temperature and nutrient concentration downstream are also expected to affect hyporheic processes, especially in the downwelling zone (Franken et al., 2001).
Leaf litter decomposition show seasonality due to the seasonal changes in environmental factors such as water temperature (Ferreira & Canhoto, 2014; Mora-Gómez et al. 2015). The effects of dams on environmental factors may differ depending on season. For example, presence of a dam generally dampens seasonal variation in water temperature downstream, and the effects on water temperature depend on season (Maheu et al., 2016). The existing studies on the effects of dams are often carried out in a single season (González et al., 2013; Mollá et al., 2017; Salomão et al., 2019), and seasonal variability in the effects of dams on leaf litter decomposition remains understudied.
The present study investigated the effects of dams and reservoirs on organic matter decomposition in the hyporheic and benthic zones by comparing decomposition of cotton-strips, enclosed in coarse-mesh bags that allow macroinvertebrates to enter and in fine-mesh bags that exclude macroinvertebrates, in the reaches downstream of dams and in the unregulated reaches that were located upstream and in a tributary in two forest mountain streams, over five seasons. Cotton-strips were used to keep a consistent initial organic matter quality across sites and seasons (Tiegs et al., 2013; Griffiths & Tiegs, 2016; Peralta-Maraver et al., 2019). We examined (1) whether hyporheic decomposition rates downstream of the dams differed from those at the unregulated reaches in all seasons, (2) whether contribution of macroinvertebrates to decomposition was significant in the hyporheic zone and (3) the relative differences in decomposition rates between the hyporheic zone and the benthic zone.
Materials and methods
Study sites
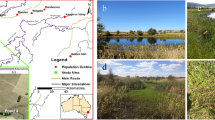
This study was carried out in the headwater area of two neighboring rivers, the Inunaki (INU) River and the Ino (INO) River, which are both tributaries of the Tatara River that flows into the Hakata Bay in Fukuoka, Japan (Fig. 1). The dams and reservoirs in both rivers were similar in dam height, catchment area, storage capacity, and water retention time (Table 1). Water retention time was calculated as the average volume of water stored in the reservoir divided by the mean annual outflow from the dam. The data of outflow from the dams were provided by Fukuoka Prefecture. Both catchments were covered by forest, and there were no agricultural fields or industrial facilities in the watershed. The two dams were multi-purpose dams with epilimnetic-release, where water from surface layer of the reservoirs was released downstream.
The study sites were located in a mountain area, where the riparian canopy covered a large portion of the narrow wetted channel, and where the channel gradient was steeper than 0.03 m/m (Table 2). The substrates were dominated by pebbles and cobbles with presence of boulders at all sites. In each river, the downstream site was located about 200 m downstream of the dam. The unregulated sites were located about 200 m upstream from the area flooded by the reservoir in both rivers; for the INU River, an additional unregulated site was set up in a tributary that had similar catchment size and channel size to the downstream site (Fig. 1, Table 2). The downstream sites in the INU River and the INO River were called INU_D and INO_D, respectively, the sites upstream of the reservoirs were called INU_U and INO_U, respectively, and the site in the INU River tributary was called INU_T. All the study sites were set up in the zone of downwelling to examine the effect of altered flow, stream water temperature, and nutrient concentration on hyporheic decomposition rates. Downwelling trend was confirmed by installing piezometers.
The study was carried out in 2016, and annual rainfall that year was 2836 mm, which was higher than the average annual precipitation (2008–2017) of 2099 mm, based on the rainfall data obtained from the nearby Haruda weather station (33°28’ N, 130°33ʹ E; 35 m a.s.l.), maintained by the Ministry of Land, Infrastructure, Transport, and Tourism. Rainy season is from June to July, and over 30% of the total annual precipitation fell in these two months in 2016.
Measurement of environmental factors
Several factors that affect organic matter decomposition were measured. A piezometer was installed at each site in November 2015, and water level and vertical head gradient (VHG, m/m) were measured about once a month from February to November 2016. Piezometers were made from PVC pipes by making numerous holes at the bottom 10 cm. The porous portion of the piezometer was inserted to the depth of 20 to 30 cm. The piezometer was also used to sample hyporheic water for analysis of chemical components.
Dissolved oxygen (DO, mg/l), specific conductance (μS/cm) and water temperature (°C) of stream and hyporheic water were measured in-situ at each study site about eight times during February to November 2016, using a portable sensor in the piezometers (YSI Pro2030, OH, USA). Stream water temperature during each incubation was measured at 10 min intervals using the pendant data loggers in stream channel (HOBO, MA, USA). Hyporheic water and stream water were also sampled about six times during February to November 2016, and the samples were brought back to the laboratory on ice and filtered within 24 h using 0.7-μm glass-fiber filters. The filtered samples were stored in the refrigerator until analysis. We run the samples through ion chromatography (DX-120, Dionex, Osaka, Japan) to determine the concentrations of inorganic nitrogen (NO3–, NH4 +) after passing the samples through 0.45-μm membrane filter immediately prior to the analyses.
Particle size distribution of streambed sediment was estimated by measuring > 100 particles at each site, covering the area of two riffle-pool sequences. In addition, in the INU River, freeze cores were taken to measure particle size distribution and organic matter content of hyporheic sediment. Freeze cores were taken from the depth of 15 to 30 cm, and three samples were taken from each INU River site. The cores were taken back to the laboratory, dried at 60˚C oven for 72 h and sieved to measure particle size distribution. The fraction of organic matter in the sediment that was < 1 mm in size was measured from the mass loss after putting the samples of sediment in 550°C furnace for 6 h. Because we did not find visible coarse organic matter in the samples, only the sediment samples < 1 mm in size were analyzed.
Measurements of cotton strip decomposition
The cotton-strip assay was used to measure organic matter decomposition rates in this study. To evaluate the seasonal variation in decomposition rates, the cotton-strip assay was carried out five times in 2016: in winter (starting on February 2nd), spring (May 2nd), rainy season (June 27th), summer (August 5th), and autumn (November 4th). The cotton strip assay was carried out following the protocol described in Tiegs et al. (2013). Cotton-strips used were approximately 8-cm long and 2.5-cm wide and made from a roll of Fredrix-brand unprimed 12-oz. heavy-weight cotton fabric, Style#548 (Tiegs et al., 2013). Each cotton-strip was inserted to a plastic mesh bag during incubation in the hyporheic zone and the benthic zone. Two different mesh sizes, 5 and 0.1 mm, were used to make the bags for cotton-strips. The 5-mm mesh bags allowed macroinvertebrate access to cotton-strips, whereas the 0.1-mm mesh bags prevented macroinvertebrate access (Gonçalves et al., 2006; Braatne et al., 2007).
Two cotton-strips, one in a 5-mm mesh bag and another in a 0.1-mm mesh bag, were treated as a set, and five sets each were placed in the hyporheic zone and the benthic zone at each site. Thus, there were total of 20 cotton-strips incubated at each site in each season. The mesh bags, which housed the cotton-strips, were tied to a nylon cord (4 mm in diameter) using banding bands, and the nylon cord was fixed to a rebar hammered into the streambed so that the cotton-strips in the hyporheic zone could be found easily at the time of collection, and the cotton-strips in the benthic zone would not be washed downstream. In the hyporheic zone, cotton-strips were buried in the sediments to a depth of 10–15 cm in the downwelling zone. The effects of stream water in the downwelling zone decrease with depth, and thus relatively shallow depth was selected. Cotton-strips were placed parallel to the streambed, and the sediment was gently placed back to minimize the damage by installation. In the benthic zone, cobbles were placed on the nylon cord and the edges of mesh bags, not on the cotton-strips, so that the cotton-strips remained in the benthic zone.
To determine the length of incubation period, a preliminary trial was carried out to estimate the time to reach 50% tensile strength loss (TSL) in autumn 2015. Twelve cotton-strips in 5-mm mesh bags were placed in the hyporheic zone and in the benthic zone, and three cotton-strips were removed every week for a month. The results suggested that 20 days of incubation was sufficient to reach 50% TSL in both the hyporheic zone and the benthic zone.
The cotton-strips removed from the hyporheic zone and the benthic zone were gently handled to minimize the loss of tensile strength due to handling. Mesh bags that housed cotton-strips were cut open, and the cotton-strips were placed in 80% ethanol for approximately 30 s. Attached sediments were then removed using distilled water and a soft brush. The cotton-strips were oven-dried at 40 °C and stored in a desiccator until tensile strength measurements.
A tensile tester (Shimazu AG 100kNE, Kyoto, Japan) was used to measure tensile strength of the cotton-strips. The grips were placed at approximately 1 cm from the end of the strips and tighten so that grips would not slip during the measurement, or the strip would not tear at where the grips were placed. The rate the tension on the strip increases was set to 2 cm/min. The maximum tensile strength was recorded, and TSL per day was calculated using the following equation (Griffiths & Tiegs, 2016):
where tensile strengthincubated is the maximum tensile strength of the cotton-strips removed from the hyporheic zone or the benthic zone after incubation, and tensile strengthreference is the mean maximum tensile strength of five strips that were not placed in the stream, but were cleaned with 80% ethanol, oven-dried at 40 °C, and stored in the desiccator.
Water temperature strongly influence TSL (Griffiths & Tiegs, 2016), and to normalize the effects of water temperature, we also calculated TSL per degree-days by substituting the incubation days in the Eq. 1 by degree-days. Degree-days were calculated by summing up the daily mean stream water temperature for the incubation period.
Contribution of macroinvertebrates to cotton strip decomposition
Contribution of macroinvertebrate to decomposition was assessed using cotton-strips in bags with two different mesh sizes. The cotton-strips in 5-mm mesh bags were accessible to macroinvertebrates. In contrast, the cotton-strips in 0.1-mm mesh bags excluded macroinvertebrates. Tiegs et al. (2013) suggested that cotton-strips were not a palatable food resource for invertebrates, and thus the use of cotton-strips might not be suitable to examine decomposition by invertebrates. However, in this study, the cotton-strips after incubation in the benthic zone showed apparent differences in size and shape between the two mesh bags (Fig. 2). All cotton-strips in 0.1-mm mesh bags maintained rectangular shapes, whereas the sizes of the several cotton-strips in 5-mm mesh bags became smaller and jagged after 20 days of incubation, suggesting a contribution of macroinvertebrates to decomposition. The cotton-strips are basically cellulose, and it differs in quality from leaf litter. Thus, contribution of macroinvertebrate to organic matter decomposition estimated from this experiment may be lower than the contribution estimated from experiments using leaf litter. However, we considered that this approach could still be used to compare relative contribution of macroinvertebrates among sites.
The differences in TSL of the strips between the two types of bags were used to calculate the effects of macroinvertebrates on decomposition rates following Eq. 2 (Mora-Gomez et al., 2016):
To support the estimated contribution of macroinvertebrates to decomposition calculated from TSL, sampling of hyporheic invertebrate was carried out at INU_D and INU_T sites in March 2016. Abundances of hyporheic invertebrates and its community composition were compared between the two sites. Three replicate samples were collected at each sampling site from a depth of 15 to 30 cm using the freeze core method (Stocker & Williams, 1972). The collected freeze cores were gently removed from the pipe, brought back to the laboratory while keeping the temperature low, and stored temporarily in a quick freezer (− 30°C). Each core taken out from the freezer was returned to room temperature, and the sample volume was measured by Archimedes’ principle. The samples were separated into invertebrates, organic matter and sediment by the floating sorting method. The invertebrates and organic matter were transferred to sample bottles and fixed with 70% ethanol. The invertebrates sorted by sieves (0.063 and 0.125 mm) were examined under a stereomicroscope with a magnification of 10–80 × (Nikon, SMZ800N). Testacean amoebae (Protozoa: Sarcomastigophora) were identified and counted at a stereomicroscope, and used for analysis together with hyporheic invertebrates. Hyporheic invertebrates were identified to the most precise taxonomic level using keys provided in Kawai & Tanida, (2005) and Mizuno & Takahashi (1991). Each taxon was assigned to a functional feeding group (FFG; collector-filterers, collector-gatherers, grasping feeders, predators, scrapers, shredders), according to Takemon (2005), Kobayashi (2019), Thorp & Covich (2010), and Merritt et al. (2008). Some taxa are known to have different FFG among the subordinate taxa, and all applicable FFG were assigned to those taxa.
Data analysis
All values were reported as mean ± standard deviation. The differences among sites in mean TSL, daily mean temperature, and nitrate concentration was tested using a one-way analysis of variance (ANOVA). ANOVA was also used to compare TSL between 5-mm and 0.1-mm mesh bags, and between hyporheic zone and benthic zone. Effects of water temperature on TSL was tested using analysis of co-variance. The effect of study site and seasons, and their interaction was tested using the two-way ANOVA. The density of hyporheic invertebrate assemblage between INU_T and INU_D were compared using Kruskal–Wallis test.
Results
Environmental factors
Inflow into the reservoir and release from the dam showed that the flow fluctuation patterns were similar because both dams had epilimnetic release (Fig. 3). The exceptions were during the low flow period over the winter, when inflow peaks did not propagate to the dam release, and in August, when the release was artificially increased to prepare for the typhoon season in September and October. During incubation experiments in spring and rainy season, the study sites experienced several high peak flows, whereas during winter, summer and fall incubation, the flow was relatively stable (Fig. 3). Vertical head gradients (VHG) showed that all the incubation sites maintained downwelling trend throughout the year (Fig. 4ab). In the INU River, the downwelling trend was weaker compared to the INO River, and during the rainy season, the site shifted slightly to upwelling trend (Fig. 4b). In the INO river, downwelling trend was often stronger at INO_D than INO_U (Fig. 4a).
Vertical head gradients measured at a the INO River and b the INU River. The negative values indicate downwelling of stream water into the hyporheic zone. Dissolved oxygen concentrations of hyporheic water in c the INO River and d the INU River. Nitrate-N concentration of hyporheic water in e the INO River and f the INU River. Open symbols indicate downstream sites, black symbols upstream sites, and grey symbols tributary site
The lowest mean daily stream water temperature (less than 10 °C) was observed in winter, and it gradually increased in spring and rainy season (Fig. 5ad, Table 4). The highest mean water temperature was observed in summer (exceeding 20°C at all sites) and then it decreased in fall. The differences between the two rivers were small when water temperature at upstream sites was compared (Table 4). Sites downstream of the dams had significantly warmer water temperature than upstream sites in both rivers, except for spring in the INO River and winter in the INU River (Table 4). INU_T showed significantly lower water temperature than INU_D in all seasons (Table 4).
Tensile strength loss per day calculated from the cotton-strip in 5 mm mesh in the benthic zone in a the INO River and d the INU River, and in the hyporheic zone in b the INO River and e the INU River. The error bars show one standard deviation. The black asterisk (*) indicate significant difference between the downstream and upstream sites, and gray asterisk (*) between the downstream and tributary sites (ANOVA, n = 5, P < 0.05). Dashed lines indicate water temperature variation at downstream sites, and solid and grey lines at upstream and tributary sites, respectively. Tensile strength loss per degree day in c the INO River and f the INU River. Open symbols indicate downstream sites, black symbols upstream sites, and grey symbols the tributary site
Concentrations of dissolved oxygen in the hyporheic zone showed seasonal trend at all sites, except for INU_D site (Fig. 4cd). Concentrations were high during spring, gradually decreased to a minimum in summer and increased again during autumn. Concentrations of dissolved oxygen in the hyporheic zone were lower at downstream sites than at unregulated sites in both rivers throughout the year (Fig. 4cd).
Concentrations of nitrate–N in the hyporheic zone fluctuated and did not show apparent seasonality, except for INU_D where slightly higher values were observed from July to October (Fig. 4ef). In the INO River, the mean concentrations of nitrate–N did not significantly differ between INO_U and INO_D (Fig. 4e, Table 2), but INO_D had lower nitrate-N concentration than INO_U at all sampling date, except for June. In the INU River, the mean concentrations were significantly lower at INU_D compared to INU_U and INU_T (Fig. 4f, Table 2).
Streambed substrate was dominated by pebbles and cobbles at all sites, where D50 ranged from 13 to 94 mm across sites (Table 2). D50 of the hyporheic sediment, measured from freeze cores, was 14 mm at INU_U and INU_T, and 26 mm at INU_D. Fractions of organic matter in < 1 mm sediment size were similar among sites, 5.5 (± 0.4) % at INU_U, 3.4 (± 1.0) % at INU_T, and 5.5 (± 0.7) % at INU_D.
Tensile strength loss (TSL) in the hyporheic zone
For cotton-strips in 5-mm mesh bags, TSL in the hyporheic zone (TSLhypo) was significantly lower by 0.7 to 1.7%/d downstream of the dams than at unregulated sites in all seasons, except on summer in both rivers and on winter in the INU River (Fig. 5b, e, Table 3). For cotton-strips in 0.1-mm mesh bags, TSLhypo was significantly lower by 0.7 to 1.5%/d downstream of the dams than at unregulated sites in spring and fall in both rivers, and in winter in the INU River (Fig. 6b, e). Thus, the trend of lower TSLhypo at the downstream sites was observed in both rivers but it was not maintained in all seasons.
Tensile strength loss per day calculated from the cotton-strip in 0.1 mm mesh in the benthic zone in a the INO River and d the INU River, and in the hyporheic zone in b the INO River and e the INU River. The error bars show one standard deviation. The black asterisk (*) indicate significant difference between the downstream and upstream sites, and gray asterisk (*) between the downstream and tributary sites (ANOVA, n = 5, P < 0.05). Dashed lines indicate water temperature variation at downstream sites, and solid and grey lines at upstream and tributary sites, respectively. Tensile strength loss per degree day in c the INO River and f the INU River. Open symbols indicate downstream sites, black symbols upstream sites, and grey symbols the tributary site
TSLhypo showed seasonality in both rivers, where the lowest values were found in winter and the highest values in the rainy season and summer (Figs. 5be, 6be). This seasonal variation in TSLhypo was similar to the seasonal variation in stream water temperature. When degree-days were used in TSLhypo calculation to remove the effects of water temperature, seasonality of TSLhypo disappeared at all sites (Figs. 5cf, 6cf), suggesting that water temperature was the primary factor resulting in seasonal variation in TSLhypo.
Comparison of tensile strength loss between the hyporheic and benthic zones
To compare the differences in organic matter decomposition rates between the hyporheic and benthic zones, the TSL from the cotton-strips in 5-mm mesh bags, that allow access of macroinvertebrate, was used. TSLhypo was significantly lower than TSLstream by 0.5 to 1.7%/d in the INO River from spring to fall, and by 0.1 to 1.1%/d in the INU River from spring to summer (Table 3). In winter, mean TSLhypo was higher than TSLstream by 0.5 to 0.8%/d in the INO River and 0.3 to 1.0%/d in the INU River, although the difference was significant only at INU_D.
Contribution of macroinvertebrate to tensile strength loss in the hyporheic zone
Contribution of macroinvertebrates to the decomposition rates were estimated using the differences in TSLhypo between cotton-strips in the two mesh sizes. The mean TSLhypo from 5-mm mesh bags showed higher values compared to TSLhypo from 0.1-mm mesh bags, but the differences were not significant in many seasons. In the INO River, the significant differences were only observed in winter at INO_U and in rainy season at INO_D (Table 4), and the estimated contribution of macroinvertebrate to decomposition was 50 (± 17) % and 12 (± 7)%, respectively. In the INU River, the significant differences were observed at INU_U in Spring, and at INU_D in winter (Table 4); the estimated contribution of macroinvertebrate was 9 (± 6) % at INU_U in spring and 53 (± 36) % at INU_D in winter. At INU_T, the differences between TSLhypo of 5-mm and 0.1-mm mesh bag were not significant in all seasons. Thus, the seasons with significant contribution by macroinvertebrate was limited and was not consistent among sites.
Hyporheic samples at INU_D and INU_T retrieved 72 invertebrate taxa from six phyla. The dominant taxa in hyporheic invertebrate assemblages 0.063 to 0.125 mm in size were Centropyxis, Difflugia and other Testacealobosia at INU_T, and Difflugia and Centropyxis at INU_D (Table 5). The dominant taxa of hyporheic invertebrate assemblages > 0.125 mm in size were Nematoda, Harpacticoida, Centropyxis, and Difflugia in INU_D, and Harpacticoida, Centropyxis, and Nematoda at INU_T. These dominant taxa constituted more than 70% of the average total population density in each size and site (Table 5). The mean density of hyporheic invertebrates, including testate amoeba, 0.063 to 0.125 mm in size was significantly higher at INU_D than at INU_T (Kruskal–Wallis; P = 0.0495), whereas the mean density of hyporheic invertebrates > 0.125 mm in size was not significantly different between the two sites (Table 6, Fig. 7).
Considering hyporheic invertebrates 0.063 to 0.125 mm in size by FFG, the densities of PR and CG/PR were significantly higher at INU_D than at INU_T (Kruskall-Wallis test; respectively P = 0.0495 and P = 0.0369, Table 6). Considering hyporheic invertebrates > 0.125 mm in size, the density of SC was significantly higher at INU_T than at INU_D (Kruskall–Wallis test; P = 0.0369, Table 6, Fig. 7b). When comparing the total density of SC and CF/SC, there was no significant difference between sites (Kruskall–Wallis test; P = 0.1266). The SC contained temporary interstitial organisms such as some Coleoptera (i.e. Elmidae, Elodes) and some Ephemeroptera (i.e. Ameletus, Ecdyonurus), while the CF/SC contained permanent interstitial organisms such as some Crustacea (i.e. Alona, Harpacticoida) and Bdelloidea.
a Density of hyporheic invertebrate (individuals/1000 cm3 of core) in two size groups (0.063 ~ 0.125 mm and > 0.125 mm), b density of scraper (SC) and shredder (SH) at INO_D and INO_N sites. The error bars indicate 1 standard deviation. The asterisk (*) indicates significant difference between INO_D and INO_N (P < 0.05)
Discussion
Effects of dams and reservoirs on hyporheic decomposition rates
Decomposition rates in the hyporheic zone in two rivers were lower downstream of the dams, compared to the unregulated reach located upstream and in a tributary, in spring, rainy season and fall. In winter and summer, when decomposition rates were lowest and highest respectively, the differences between downstream and unregulated sites were not significant. There are no studies that examined the effects of dams on hyporheic decomposition rates as far as we know, but there are several studies that examined the effects of dams on benthic decomposition rates. The studies in the benthic zone showed lower decomposition rates at downstream of epilimnetic-release dams, and the lower rates were explained by altered water quality and microbial and invertebrate activities (Mendoza-Lera et al., 2012; Menéndez et al., 2012; González et al., 2013). Many of those studies were carried out only in one or two seasons, but Li et al. (2020) studied over five seasons and found lower decomposition rates downstream of the dam in all seasons. Thus, the results of this study and findings from previous studies suggest that the presence of dams and reservoirs results in lower decomposition rates downstream not only in the benthic zone but also in the hyporheic zone. The effects of dam may be consistent throughout the year in the benthic zone (Li et al., 2020), but in the hyporheic zone, the effects may be significant only in some seasons.
Various environmental factors, such as flow, nutrient concentration and water temperature, and biological activities of invertebrates and microbes influence decomposition rates (Young et al., 2008). Inflow to and outflow from the reservoir showed that peak flow magnitudes were dampened at downstream sites in both rivers. Flow fluctuations affect decomposition rates in the benthic zone through abrasion, but the effects in the hyporheic zone is considered limited (Boulton & Quinn, 2000). Water temperature influenced decomposition rates in the benthic zone (Li et al., 2020). Increase in water temperature stimulates microbial and invertebrate activities, and results in higher decomposition rates (Ferreira & Canhoto, 2014). In this study, when the TSLhypo was normalized by water temperature, seasonality of TSLhypo disappeared (Figs. 5cf, 6cf), and mean TSLhypo showed a positive correlation with surface water temperature in both rivers (Fig. 8). These results suggest that in the shallow hyporheic zone of downwelling, where strong influence of stream water is expected, stream water temperature plays a primary role in the seasonal variations of hyporheic decomposition rates.
Relationship between average water temperature during incubation period and tensile strength loss in a INO River and b INU River. Open symbols indicate downstream sites, black symbols indicate upstream sites and grey indicate tributary site. The lines indicate linear regression relationship (P < 0.05)
When water temperature was compared among sites, the downstream sites were warmer than unregulated sites in almost all seasons, because both dams released water from surface. Temperature differences between the downstream and unregulated sites were more than 3˚C from spring to summer in the INU River and in fall in the INO River, but the difference was smaller during winter. Ferreira & Canhoto (2015) showed that warming by about 3°C stimulated litter decomposition but only in cold months. The temperature differences of less than 3˚C in winter may be a factor, resulting in no significant differences in hyporheic decomposition rates between downstream and unregulated sites. Besides, the temperature differences between downstream and the unregulated sites were relatively small, compared to seasonal variation. The differences between summer and winter water temperature were larger than 15°C. Therefore, downstream sites had higher water temperature, but the differences were not large enough to affect decomposition processes.
Nutrient concentration is another factor positively influencing decomposition rates (Ferreira et al., 2015). For example, enrichment of nitrate resulted in increased microbial biomass and higher decomposition rates of leaf litter (Ferreira et al., 2006). In this study, nitrate-N and DO concentrations on hyporheic water downstream of the dams were often lower than the unregulated sites. Pooling of water in reservoirs increases opportunities for denitrification and results in lower nitrate-N concentrations downstream of dams (David et al., 2006; Harrison et al., 2009). The same trend was observed in this study, where the nitrate concentrations at the unregulated site was about 0.2 mg/l higher than at the downstream sites (Li et al., 2020). All the study sites had downwelling trend, and nitrate-N concentrations in the hyporheic zone were influenced by the concentrations of surface water. In addition, DO concentrations in the hyporheic zone downstream of the dams were lower than at the unregulated sites, and sometimes even below 2.0 mg/l, favoring denitrification. Thus, lower nitrate-N and DO concentrations in the hyporheic zone downstream of the dams might have contributed to the lower decomposition rates.
Macroinvertebrates are often considered as primary players in leaf litter decomposition in the benthic zone. Shredders, especially, play an important role, and differences in decomposition rates among sites have been explained by differences in the abundance of shredders in several studies (Mendoza-Lera et al., 2012; González et al., 2013; Mbaka & Schafer, 2015). In the hyporheic zone too, shredders were important players (Navel et al., 2009), and grazing activity by hyporheic invertebrates also enhanced microbial activity (Boulton, 2007). In this study, contribution of macroinvertebrates to decomposition was estimated using two mesh sizes, and found that their contribution was often not significant in the hyporheic zone. Each site showed significant contribution of macroinvertebrates in just one season. The benthic zone, on the other hand, showed a significant contribution of macroinvertebrate in many seasons at all sites (Li et al., 2020). In the INU River, densities of hyporheic invertebrate > 0.125 mm in size was much lower than those of ≦ 0.125 mm in size, and limited access of macroinvertebrate to the hyporheic zone due to small pore sizes may have resulted in lower contribution of macroinvertebrates to decomposition (Cornut et al., 2010; Flores et al., 2013). In addition, densities of shredders and scraper were low at both INU_D and INU_T. Thus, both the results of TSL and sampling of hyporheic invertebrates suggested lower contribution of macroinvertebrates in the hyporheic zone, and macroinvertebrates did not explain the seasonality in the downstream effects in this study.
We need to bear in mind that cotton-strips and their tensile strength loss were used in this study. The observed changes in shape of many cotton strips in 0.5 mm mesh bags in the benthic zone after just 20 days of incubation were considered as the signs of shredder activities. However, the cotton-strips are basically cellulose, and the quality is different from leaf litter. Tensile strength loss is sensitive for fiber cutting by macroinvertebrate activities and fiber maceration by microbial activity, and their relative effects are not known. Thus, the results of this study may underestimate the actual contribution of macroinvertebrate to leaf litter decomposition. To examine the effects of macroinvertebrates on leaf litter decomposition at each site, other approach, preferably use of leaf litter, should also be used.
Comparison of organic matter decomposition rates between the hyporheic zone and the benthic zone
Previous studies that examined the decomposition rates both on the benthic zone and in the hyporheic zone showed contradicting results. For example, Boulton & Quinn (2000) and Peralta-Maraver et al. (2019) that used both cotton-strips and tea bags, and Piscart et al. (2011) and Danger et al. (2012) that used leaf litter, found lower decomposition rates in the hyporheic zone due to lower effects of abrasion from bedload transport and invertebrate and microbial activities. Solagaistua et al. (2015) and Risse-Buhl et al. (2017) that used leaf litter found similar decomposition rates between the benthic zone and the hyporheic zone due to comparable contribution by the microbial community. In intermittent streams, Burrows et al. (2017) used cotton-strips and leaf litter and found higher decomposition rates in the hyporheic zone compared to benthic zone because of consistently saturated conditions. The present study also showed mixing results. The INO River sites showed lower decomposition rates in the hyporheic zone than in the benthic zone from spring to fall, whereas the INU River sites showed similar decomposition rates in the hyporheic zone and the benthic zone in many seasons. The studied rivers were perennial, so the effects of dryness were not expected. Microbial community was not examined in this study, but microbial decomposition rates between benthic zone and hyporheic zone may be similar despite the differences in the physical environment (Risse-Buhl et al., 2017). Macroinvertebrate decomposition rates, in contrast, differ between the hyporheic zone and the benthic zone, where the effects of macroinvertebrate are greater in the benthic zone (Cornut et al., 2010). The contribution of macroinvertebrate examined in this study showed that the effects were small in the hyporheic zone, whereas it was significant in the benthic zone at the same sites (Li et al., 2020). The contribution of macroinvertebrates to decomposition in the benthic zone was larger in the INO River sites, ranging from 23 to 29%, than the INU River sites, ranging from 11 to 20% (Li et al., 2020). Therefore, the difference in the contribution of macroinvertebrates to decomposition between the benthic zone and the hyporheic zone might have resulted in the lower decomposition rates in the hyporheic zone in the INO River, and comparable decomposition rates in the INU River.
In winter at INU_D, the decomposition rate was significantly higher in the hyporheic zone than in the benthic zone, and a similar trend was also found in other sites, although the differences were not significant. Menéndez et al. (2003) found that the density of macroinvertebrates was more than twofold higher and so was the decomposition rate in the benthic zone in spring–summer, compared to fall-winter. The effects of macroinvertebrate in the benthic zone may become small in winter. The contribution of macroinvertebrate to decomposition in the benthic zone was lowest in winter in the INU River (Li et al., 2020). Due to these seasonal variation in the effects of macroinvertebrate, hyporheic decomposition rates may become comparable to the rates in the benthic zone in winter.
This study demonstrated that the presence of a dam influences decomposition rates not only in the benthic zone but also in the hyporheic zone downstream, that the effects of dams on hyporheic decomposition may depend on season, and that the hyporheic decomposition rates and benthic decomposition rates may be comparable in some seasons in some rivers. These results suggest that the hyporheic zone can be an important zone of decomposition, and it is crucial to examine both benthic and hyporheic zones when addressing the effects of dams and reservoirs on stream ecosystem processes.
Data availability
The datasets obtained during the present study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Arroita, M., I. Aristi, J. Díez, M. Martinez, G. Oyarzun & A. Elosegi, 2015. Impact of water abstraction on storage and breakdown of coarse organic matter in mountain streams. Science of the Total Environment 503–504: 233–240.
Boulton, A. J., 2007. Hyporheic rehabilitation in rivers: restoring vertical connectivity. Freshwater Biology 52: 632–650.
Boulton, A. J. & J. M. Quinn, 2000. A simple and versatile technique for assessing cellulose decomposition potential in floodplain and riverine sediments. Archiv Fur Hydrobiologie 150: 133–151.
Braatne, J. H., S. M. P. Sullivan & E. Chamberlain, 2007. Leaf decomposition and stream macroinvertebrate colonisation of Japanese knotweed, an invasive plant species. International Review of Hydrobiology 92: 656–665.
Burrows, R., H. Rutlidge, N. Bond, S. Eberhard, A. Auhl, M. Andersen, D. Valdez & M. Kennard, 2017. High rates of organic carbon processing in the hyporheic zone of intermittent streams. Scientific Reports 7: 13198.
Casas, J. J., C. Zamora-Muñoz, F. Archila & J. Alba-Tercedor, 2000. The effect of a headwater dam on the use of leaf bags by invertebrate communities. Regulated Rivers-Research & Management 16: 577–591.
Cornut, J., A. Elger, D. Lambrigot, P. Marmonier & E. Chauvet, 2010. Early stages of leaf decomposition are mediated by aquatic fungi in the hyporheic zone of woodland streams. Freshwater Biology 55: 2541–2556.
Cornut, J., A. Elger, A. Greugny, M. Bonnet & E. Chauvet, 2012. Coarse particulate organic matter in the interstitial zone of three French headwater streams. International Journal of Limnology 48: 303–313.
Corson-Rikert, H. A., S. M. Wondzell, R. Haggerty & M. V. Santelmann, 2016. Carbon dynamics in the hyporheic zone of a headwater mountain stream in the Cascade Mountains, Oregon. Water Resources Research 52: 7556–7576.
Danger, M., J. Cornut, L. Elger & E. Chauvet, 2012. Effects of burial on leaf litter quality, microbial conditioning and palatability to three shredder taxa. Freshwater Biology 57: 1017–1030.
David, M. B., L. G. Wall, T. V. Royer & J. L. Tank, 2006. Denitrification and the nitrogen budget of a reservoir in an agricultural landscape. Ecological Applications 16: 2177–2190.
Ferreira, V. & M. A. S. Graça, 2006. Do invertebrate activity and current velocity affect fungal assemblage structure in leaves? International Review of Hydrobiology 91: 1–14.
Ferreira, V. & C. Canhoto, 2014. Effect of experimental and seasonal warming on litter decomposition in a temperate stream. Aquatic Sciences 76: 155–163.
Ferreira, V. & C. Canhoto, 2015. Future increase in temperature may stimulate litter decomposition in temperate mountain streams: evidence from a stream manipulation experiment. Freshwater Biology 60: 881–892.
Ferreira, V., V. Gulis & M. A. S. Graça, 2006. Whole-stream nitrate addition affects litter decomposition and associated fungi but not invertebrates. Oecologia 149: 718–729.
Ferreira, V., B. Castagneyrol, J. Koricheva, V. Gulis, E. Chauvet & M. A. S. Graça, 2015. A meta-analysis of the effects of nutrient enrichment on litter decomposition in streams. Biological Reviews 90: 669–688.
Flores, L., D. J. Ramón, L. Aitor, C. Pascoal & E. Arturo, 2013. Effects of retention site on breakdown of organic matter in a mountain stream. Freshwater Biology 58: 1267–1278.
Franken, R. J. M., R. G. Storey & D. D. Williams, 2001. Biological, chemical and physical characteristics of downwelling and upwelling zones in the hyporheic zone of a north-temperate stream. Hydrobiologia 44: 183–195.
Gessner, M. O. & E. Chauvet, 2002. A case for using litter breakdown to assess functional stream integrity. Ecological Applications 12: 498–510.
Gonçalves, J. F., M. A. S. Graça & M. Callisto, 2006. Leaf-litter breakdown in 3 streams in temperate, Mediterranean, and tropical Cerrado climates. Journal of the North American Benthological Society 25: 344–355.
González, J. M., S. Mollá, N. Roblas, E. Descals, O. Moya & C. Casado, 2013. Small dams decrease leaf litter breakdown rates in Mediterranean mountain streams. Hydrobiologia 712: 117–128.
González-Pinzón, R., R. Haggerty & A. Argerich, 2014. Quantifying spatial differences in metabolism in headwater streams. Freshwater Science 33: 798–811.
Graham, E. B., J. C. Stegen, M. Y. Huang, X. Y. Chen & T. D. Scheibe, 2019. Subsurface biogeochemistry is a missing link between ecology and hydrology in dam-impacted river corridors. Science of the Total Environment 657: 435–445.
Griffiths, N. A. & S. D. Tiegs, 2016. Organic-matter decomposition along a temperature gradient in a forested headwater stream. Freshwater Science 35: 518–533.
Harrison, J. A., R. J. Maranger, R. B. Alexander, A. E. Giblin, P. A. Jacinthe, E. Mayorga, S. P. Seitzinger, D. J. Sobota & W. M. Wollheim, 2009. The regional and global significance of nitrogen removal in lakes and reservoirs. Biogeochemistry 93: 143–157.
Kawai, T. & K. Tanida, 2005. Aquatic insects of Japan: manual with keys and illustrations, Tokai University Press Division, Tokai:
Kobayashi, S., 2019. 4.1 Benthic Invertebrates. In Inoue, M. & F. Nakamura (eds), Field and analytical methods in stream ecology Kodansha Scientific Ltd., Tokyo: 232–292.
Lessard, J. L. & D. B. Hayes, 2003. Effects of elevated water temperature on fish and macroinvertebrate communities below small dams. River Research and Applications 19: 721–732.
Li, Y., T. Kasahara, M. Chiwa & N. Fujimoto, 2020. Effects of dams and reservoirs on organic matter decomposition in forested mountain streams in western Japan. River Research and Applications 36: 1257–1266.
Maheu, A., A. St-Hilaire, D. Caissie, N. El-Jabi, G. Bourque & D. Boisclair, 2016. A regional analysis of the impact of dams on water temperature in medium-size rivers in eastern Canada. Canadian Journal of Fisheries and Aquatic Sciences 73: 1885–1897.
Martínez, A., A. Larrañaga, A. Basaguren, J. Pérez, C. Mendoza-Lera & J. Pozo, 2013. Stream regulation by small dams affects benthic macroinvertebrate communities: from structural changes to functional implications. Hydrobiologia 711: 31–42.
Martínez, A., A. Larrañaga, J. Pérez, C. Casado, J. J. Casas, J. M. González, M. Menéndez, S. Mollá & J. Pozo, 2016. Climate modulates the magnitude of the effects of flow regulation on leaf-litter decomposition. Aquatic Sciences 79: 507–514.
Mbaka, J. G. & R. B. Schafer, 2015. Effect of Small Impoundments on Leaf Litter Decomposition in Streams. River Research and Applications 32: 907–913.
Mendoza-Lera, C., A. Larrañaga, J. Pérez, E. Descals, A. Martínez, O. Moya, I. Arostegui & J. Pozo, 2012. Headwater reservoirs weaken terrestrial-aquatic linkage by slowing leaf-litter processing in downstream regulated reaches. River Research and Applications 28: 13–22.
Menéndez, M., O. Hernandez & F. A. Comin, 2003. Seasonal comparisons of leaf processing rates in two Mediterranean rivers with different nutrient availability. Hydrobiologia 495: 159–169.
Menéndez, M., E. Descals, T. Riera & O. Moya, 2012. Effect of small reservoirs on leaf litter decomposition in Mediterranean headwater streams. Hydrobiologia 691: 135–146.
Merritt, R. W., K. W. Cummins & M. B. Berg, 2008. An Introduction to the Aquatic Insects of North America, 4th ed. Kendall/Hunt publishing company, United States of America:
Metzler, G. M. & L. A. Smock, 1990. Storage and dynamics of subsurface detritus in a sand-bottomed stream. Canadian Journal of Fisheries and Aquatic Sciences 47: 588–594.
Meyer, J. L., C. Hax, J. B. Wallace, S. L. Eggert & J. R. Webster, 2000. Terrestrial litter inputs as determinants of food quality of organic matter in a forest stream. Verhandlungen Der Internationalen Vereinigung Fur Theoretische Und Angewandte Limnologie 27: 1346–1350.
Mizuno, T. & E. Takahashi, 1991. An illustrated guide to freshwater zooplankton in Japan, Tokai University Press, Tokai:
Mollá, S., J. J. Casas, M. Menéndez, A. Basaguren, C. Casado, E. Descals, J. M. González, A. Larrañaga, M. Lusi, A. Martínez, C. Mendoza-Lera, O. Moya, J. Pérez, T. Riera, N. Roblas & J. Pozo, 2017. Leaf-litter breakdown as an indicator of the impacts by flow regulation in headwater streams: Responses across climatic regions. Ecological Indicators 73: 11–22.
Mora-Gómez, J., A. Elosegi, E. Mas-Martí & A. M. Romaní, 2015. Factors controlling seasonality in leaf-litter breakdown in a Mediterranean stream. Freshwater Science 34: 1245–1258.
Mora-Goméz, J., A. Elosegi, S. Duarte, F. Cássio, C. Pascoal & A. M. Romani, 2016. Differences in the sensitivity of fungi and bacteria to season and invertebrates affect leaf litter decomposition in a Mediterranean stream. Fems Microbiology Ecology 92: fiw121.
Naegeli, M. W. & U. Uehlinger, 1997. Contribution of the hyporheic zone to ecosystem metabolism in a prealpine gravel-bed river. Journal of the North American Benthological Society 16: 794–804.
Navel, S., F. Mermillod-Blondin, B. Montuelle, E. Chauvet, L. Simon, C. Piscart & P. Marmonier, 2009. Interactions between fauna and sediment control the breakdown of plant matter in river sediments. Freshwater Biology 55: 753–766.
Peralta-Maraver, I., D. M. Perkins, M. S. A. Thompson, K. Fussmann, J. Reiss & A. L. Robertson, 2019. Comparing biotic drivers of litter breakdown across stream compartments. Journal of Animal Ecology 88: 1146–1157.
Piscart, C., S. Navel, C. Maazouzi, B. Montuelle, J. Cornut, F. Mermillod-Blondin & M.C. des Chatelliers, L. Simon & P. Marmonier, 2011. Leaf litter recycling in benthic and hyporheic layers in agricultural streams with different types of land use. Science of the Total Environment 409: 4373–4380.
Poff, N. L., J. D. Olden, D. M. Merritt & D. M. Pepin, 2007. Homogenization of regional river dynamics by dams and global biodiversity implications. PNAS 104: 5732–5737.
Risse-Buhl, U., C. Mendoza-Lera, H. Norf, J. Pérez, J. Pozo & J. Schlief, 2017. Contrasting habitats but comparable microbial decomposition in the benthic and hyporheic zone. Science of the Total Environment 605–606: 683–691.
Salomão, V. P., A. M. Tonin, R. S. Rezende, G. F. M. Leite, E. A. C. C. Alvim, J. M. B. Quintão & J. F. Gonçalves, 2019. Small dam impairs invertebrate and microbial assemblages as well as leaf breakdown: a study case from a tropical savanna stream. Limnologica 77: 125685.
Solagaistua, L., M. Arroita, I. Aristi, A. Larrañaga & A. Elosegi, 2015. Changes in discharge affect more surface than subsurface breakdown of organic matter in a mountain stream. Marine and Freshwater Research 67(12): 1826–1834.
Stocker, Z. S. J. & D. D. Williams, 1972. A freezing core method for describing the vertical distribution of sediments in streambed. Limnology and Oceanography 17: 136–138.
Storey, R. G., R. R. Fulthorpe & D. D. Williams, 1999. Perspectives and predictions on the microbial ecology of the hyporheic zone. Freshwater Biology 41: 119–130.
Takemon, Y., 2005. Life-type concept and functional feeding groups of benthos communities as indicators of lotic ecosystem condition. Japanese Journal of Ecology 55: 189–197.
Thorp, J. H. & A. P. Covich, 2010. Ecology and Classification of North American Freshwater Invertebrates, 3rd ed. Academic Press, Burlington:
Tiegs, S. D., J. E. Clapcott, N. A. Griffiths & A. J. Boulton, 2013. A standardized cotton-strip assay for measuring organic-matter decomposition in streams. Ecological Indicators 32: 131–139.
Tiemann, J. S., D. P. Gillette, M. L. Wildhaber & D. R. Edds, 2004. Effects of lowhead dams on riffle-dwelling fishes and macroinvertebrates in a midwestern river. Transactions of the American Fisheries Society 133: 705–717.
Wohl, E., K. Dwire, N. Sutfin, L. Polvi & R. Bazan, 2012. Mechanisms of carbon storage in mountainous headwater rivers. Nature Communications 3: 1263.
Young, R. G., C. D. Matthaei & C. R. Townsend, 2008. Organic matter breakdown and ecosystem metabolism: functional indicators for assessing river ecosystem health. Journal of the North American Benthological Society 27: 605–625.
Acknowledgements
This work was supported by funding from the Water Resources Environment Center (WEC) in Japan under Grant Agreement No 2015-02 and JSPS KAKENHI 19K12298. We are grateful to Tomoya Kubo and Mizuki Koga for their help with field data collection, and Dr. Noboru Fujimoto for support in tensile strength measurement. We thank Mr. Gyo Yoshinari for his contribution in identifying Hydrachnella, Dr. Nao Yamamoto for identification of Chironomidae, and Dr. Taira Akikazu for identification of other common aquatic insects. We also thank the three anonymous reviewers for their constructive comments, which helped us to much improve the manuscript.
Funding
This work was supported by funding from the Water Resources Environment Center (WEC) in Japan under Grant Agreement No 2015-02 and by JSPS KAKENHI Grant number 19K12298.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by TK and YL. Analysis of hyporheic invertebrates were carried out by AT. The first draft of the manuscript was written by TK and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethical approval
This manuscript has not been submitted to any other journals. This manuscript does not contain any studies with human participants or animals under the Japanese Act.
Additional information
Handling Editor: Verónica Ferreira
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kasahara, T., Li, Y. & Tanaka, A. Effects of dams and reservoirs on organic matter decomposition in the hyporheic zone in forest mountain streams. Hydrobiologia 849, 2949–2965 (2022). https://doi.org/10.1007/s10750-022-04905-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-022-04905-w