Abstract
As human-dominated landscapes have expanded, the diversity of organic matter (OM) sources to rivers has increased, and knowledge of the composition and quality of these sources is lacking. It is important to characterize novel forms of OM that can affect retention, transport, and transformation of OM within watersheds. We collected fine particulate OM (FPOM) and dissolved OM (DOM) in four watersheds of Northeast Utah with a mixture of urban, forest, and agricultural land cover. We used the natural abundance of 13C, 15N, and 2H isotopes in mixing models to quantify the proportion of terrestrial, autochthonous, and anthropogenic OM. We also used spectroscopic properties of DOM to characterize OM composition. Percent land cover within a watershed did not account for the anthropogenic influence of wastewater treatment plants (WWTP) or grazing and recreation activities. OM was primarily derived from terrestrial sources, except at sites influenced by wastewater, and autochthonous sources at sites influenced by grazing and recreation activities. Sites influenced by WWTPs had more homogenous DOM that was consistently composed of 35% protein-like DOM, while all other sites had greater variability in DOM composition. Further study is needed to understand landscape drivers of DOM composition in watersheds with WWTPs, and such studies should incorporate samples directly from of WWTP effluent.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Organic matter (OM) in aquatic and terrestrial ecosystems is a complex mixture of thousands of organic molecules and exists in every compartment of the hydrologic cycle. The identification of OM sources is a challenge due to the multiple origins of OM in rivers such as inputs from terrestrial and anthropogenic sources, as well as OM produced in situ through primary production. OM produced in-stream, referred to as autochthonous OM, is considered more bioavailable (i.e., labile) to microbes compared to terrestrial sources. This is because microbial activity associated with photosynthesis produces exudates of simple, low molecular weight compounds that are easy for microbes to consume (Guillemette et al., 2013). Autochthonously derived OM has proportionally more nitrogen-rich compounds (e.g., amino acids, DNA) than terrestrial sources and therefore is considered high-quality OM that is preferentially consumed in nutrient limited conditions (Guenet et al., 2010; Knapik et al., 2015).
With increased urbanization and other land-use changes, the diversity of anthropogenic OM inputs to rivers has increased (Fork et al., 2018; Xenopoulos et al., 2021) adding to the complexity, and difficulty, of characterizing OM sources and composition. For example, wetland or forest conversion to agriculture was shown to destabilize fossil-aged soil OM, which contributed large OM loads to the Sacramento River (Sickman et al., 2007) and mobilization of a greater proportion of small, microbially available DOM in the Congo Basin (Drake et al., 2019). However, the same indicators of fossil soil OM (radiocarbon values) may also signify the contribution of anthropogenic sources of petroleum products from wastewater effluent, urban runoff, or pesticides (Sickman et al., 2010; Butman et al., 2015). Other anthropogenic sources of OM include detergents, pharmaceuticals (Kolpin et al., 2002), microplastics (McCormick et al., 2016), leaky sewage pipes and septic tanks (Kaushal and Belt 2012), grass clippings, and pet waste (Mallin et al., 2006). Many of these anthropogenic sources are discharged from wastewater treatment plants (WWTPs), which are not equipped to remove pharmaceuticals and petroleum products (Bridgeman et al., 2014). The ‘ecological footprint’ of wastewater effluent on downstream OM processing and production is not well understood and would help illuminate the ecological consequences of anthropogenic sources of OM to downstream lakes and estuaries (Wassenaar et al., 2010; Hansen et al., 2016).
The ability to link OM sources and composition to OM bioavailability is an exciting frontier in OM research. Traditional methods to assess bioavailability with bioassays that measure dissolved organic carbon (DOC) decay, biochemical oxygen demand (BOD), or bioavailable dissolved organic carbon (BDOC), are informative but labor intensive. Development of spectrofluorometers that can rapidly analyze DOM samples and produce 3-dimensional fluorescence excitation–emission matrices (EEMs) has driven advancements in characterizing DOM character and function (Shutova et al., 2014). EEMs produce peaks over a range of excitation and emission wavelengths, and depending on peak location, DOM can be characterized as humic-like or protein-like (Coble, 1996). The percent protein-like DOM in a sample has been directly correlated to DOC decay rate (Parr et al., 2015), BOD (Baker & Inverarity, 2004), and BDOC (Petrone et al., 2011; Hosen et al., 2014), making spectrofluorometry an inexpensive and efficient tool to describe DOM bioavailability.
Numerous studies have aimed to describe OM sources and bioavailability in watersheds with different land covers and gradients of human impact. Fine particulate OM (FPOM), OM between 0.45 and 1000 µm (Hutchens et al., 2017), was more autochthonously derived in agricultural and urban watersheds compared to watersheds with less human influence (Newcomer et al., 2012; Imberger et al., 2014; Lambert et al., 2017). Dissolved OM (< 0.45 µm) in urban watersheds was more bioavailable (Hosen et al., 2014), autochthonous (Petrone et al., 2011; Parr et al., 2015) and had greater proportions of hydrophobic OM (e.g., petroleum; McElmurry et al., 2013) than DOM in non-urban watersheds. Agricultural land use was associated with less complex, more microbially derived OM compared to forest land cover (Wilson & Xenopoulos, 2008; Lambert et al., 2017; Drake et al., 2019; Pisani et al., 2020). But, only one study has successfully distinguished DOM associated with urban versus agricultural land cover, and differences were dependent on the scale of comparison, and type of waterbody (e.g., lake vs. river, Williams et al., 2016).
Our first objective was to identify sources of FPOM and DOM in watersheds with varying land covers including urban, forest, shrub/scrub, and agriculture. We hypothesized that OM at sites with urban or agricultural influence would have a greater proportion of autochthonously derived DOM due to increased inorganic nutrient concentrations and primary production, compared to forest and shrub/scrub land covers. We used two types of stable isotope mixing models to characterize and quantify potential sources of FPOM and DOM among four watersheds, the Stable Isotope Mixing Model in R package (SIMMR) and a graphical gradient-based mixing model. We expected carbon, nitrogen, and hydrogen stable isotope values of autochthonous (algae, biofilms, macrophytes), terrestrial (soil and leaves), and anthropogenic-derived sources (e.g., wastewater effluent or agricultural runoff) to differ enough to use the SIMMR model to estimate the proportional contribution of each source to DOM and FPOM pools. A graphical gradient-based mixing model used stable isotope values of dissolved inorganic carbon (DIC-δ13C) and deuterium of river (water-δ2H) regressed against carbon and hydrogen stable isotopes of OM to characterize FPOM and DOM as primarily autochthonously derived or not autochthonously derived. Our second objective was to use water quality metrics of Chlorophyll a (Chla), dissolved organic carbon (DOC), and total dissolved nitrogen (TDN) as well as spectroscopic properties of DOM to infer DOM composition and relative lability at sites with varying land use.
Materials and methods
Study sites and land cover
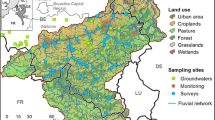
Four watersheds located in the Central Basin and Wasatch Mountain Ecoregions (Woods et al., 2001) were sampled in Northeastern Utah, USA (Fig. 1). The Logan River, Provo River, and Red Butte Creek watersheds all transition from forested, nationally protected areas (e.g., US Department of Agriculture National Forest and Wilderness Areas) to downstream reaches surrounded by either urban or suburban/rural land covers (Hall et al., 2015). The Jordan River flows north through the Salt Lake Valley metropolitan area which includes 2,125,000 people according to the 2018 USA Census Estimate (Census.gov). The source of the Jordan River is Utah Lake, a shallow, eutrophic lake that receives wastewater effluent from six WWTPs in the Orem, Provo, and Spanish Fork urban areas (Hogsett, 2015). The Jordan River also receives effluent from three WWTPs located 22, 37, and 50 km downstream of Utah Lake. It also receives runoff from six major tributaries with headwaters in the Wasatch Range (Epstein et al., 2016). There were eight to nine sampling sites within each of the four watersheds (Fig. 1). Nine sites were sampled on the Jordan River, two of which were above and below a WWTP on Mill Creek, a Jordan River tributary 37 km downstream of Utah Lake. Study site locations were chosen to sample river reaches that represented a range of natural and developed land covers.
Eight to nine sites were sampled in each of four watersheds in Northeast Utah (red box). The Provo River (green line) flows to Utah Lake and has two large reservoirs. The Jordan River (blue line) flows out of Utah Lake, and Red Butte creek is a tributary to the Jordan River (orange line). The Logan River (black line) flows into the Bear River (not shown) which flows into the Great Salt Lake
Watersheds and sub-watersheds were delineated for each site in ArcGIS (version 10.4.1; Environmental Systems Research Institute, Redlands, California) and the USGS StreamStats web application (https://water.usgs.gov/osw/streamstats). Land cover metrics were derived from the 2011 National Land Cover Dataset (NLCD; Homer et al., 2015); 18 land covers present in Northeast Utah were reduced to six types expected to explain OM concentrations and composition at a site (Petrone et al., 2011; Williams et al., 2016). Final land cover classes used in analysis were forest (deciduous + evergreen + mixed forest), scrub-grassland (shrub/scrub + grassland/herbaceous), wetland (woody + emergent herbaceous), pasture, cultivated crops, and urban development (open urban + low intensity + medium intensity + high intensity). Percent land cover was calculated for each of the seven land cover classes for the area above each site as a proportion of total watershed area above the site.
Study design and statistical approach
A principal components analysis (PCA) of percent land cover within a sub-watershed was run to identify sites along a gradient of land cover from low to high human influence from both agriculture (pasture or cropland) and urban development. The goal was to identify land cover types that could explain organic matter composition along a gradient of human influence, for example, through increased percent land cover of urban or agricultural development or through decreased forest or wetland land cover. The PCA was conducted with the FactoMineR package (Lê et al., 2008) and visualized with the factoextra package (Kassambara, 2015).
We used three isotope tracers (δ13C, δ15N, δ2H) in the SIMMR model to estimate the proportional source contributions to FPOM and DOM. SIMMR incorporates variability of endmembers into the model, and can estimate source contributions to a mixture regardless of the number of isotope tracers (Parnell & Inger, 2016). A total of eight potential sources were evaluated: macrophytes, biofilm, algae, tree leaves, soil, benthic OM, and DOM and FPOM collected in a stream directly below a wastewater effluent outfall (WWTP-DOM, WWTP-FPOM). The interquartile range (IQR) of δ13C, δ15N, and δ2H values was evaluated prior to conducting SIMMR models to evaluate whether different autochthonous and terrestrial forms should be averaged together or included separately in the model. If the IQR of all three isotope values overlapped among autochthonous or terrestrial endmembers, then these sources were averaged together in the SIMMR model (e.g., algae-biofilm isotope values were averaged together as once source). We also did not know if benthic OM would be a primarily terrestrial, autochthonous, or have a unique isotopic signature as it is presumably a mixture of terrestrial and senesced autochthonous sources so it was included as a distinct potential source of FPOM and DOM in the SIMMR model. A gradient of land use reflective of the human influence was not identified among sites (see results), so OM composition and quality at each site were analyzed and characterized by watershed and not land cover. Significant differences among source contributions for each watershed were assessed by comparing the 95% highest density intervals (HDI) of the posterior probability densities of each source. Parameter values within the HDI have higher probability density than values outside the HDI, and the total probability of values in the 95% HDI is 95% (Kruschke, 2018).
The graphical, gradient-based mixing model can partition OM as derived from either autochthonous or non-autochthonous sources (Mohamed & Taylor, 2009; Rasmussen, 2010; Wilkinson et al., 2013). Prevoius gradient-based mixing models assumed if OM was primarily derived from autochthonous sources, the δ13C and δ2H values would vary linearly with inorganic isotope values of aqueous DIC-δ13C or water-δ2H since autochthonously derived OM (e.g., algae) would have been created through carbon fixation with water (Wilkinson et al., 2013). Non-autochthonously derived OM was assumed to be terrestrial, and therefore, OM isotope values would yield a flat line with a y-intercept at average terrestrial OM isotope values. Similar to previous graphical, gradient-based models, we predicted if OM was primarily autochthonously derived there would be a linear relationship between inorganic and organic δ13C and δ2H values. Unlike previous models, we assumed non-autochthonously derived OM could be from either terrestrial or anthropogenic sources such as agricultural runoff or WWTP OM sources, and δ13C and δ2H values would yield a flat line with a y-intercept within the range of terrestrial or anthropogenic sources.
To better describe potential sources of DOM, spectroscopy was used to build a parallel factor analysis (PARAFAC) model and calculate spectroscopic indices of DOM that generally describe it as humic-like versus protein-like or terrestrially versus microbially derived. Spectroscopic indices and the percent of humic-like or protein-like fluorescing DOM were then correlated to water quality metrics including chlorophyll a (Chla), DOC, total dissolved N (TDN), and DOC:TDN.
FPOM and DOM isotope samples
FPOM and DOM were collected at 34 sites among four watersheds (Fig. 1). Samples were collected during baseflow in September and November of 2014, and November of 2015. FPOM for δ2H samples was collected instream with a one-liter, high-density polyethylene bottle from each site and transported back to the laboratory for filtering. FPOM was collected on 0.45 µm nylon filters (Whatman polyamide membrane filters, Maidstone, UK) then backwashed into deionized water, and dehydrated at 50 °C in a drying oven (Wilkinson et al., 2013). This material was packed in tin capsules. FPOM for δ13C and δ15N isotope analysis was collected instream with a drill-pump (Kelso & Baker, 2015) and filtered onto a 25-mm diameter glass fiber filter of 0.7 µm pore size (Whatman GF/F, Maidstone, UK). Filters were transported back to the laboratory in foil, dried at 50 °C, rewet with deionized water, and acidified by fumigation in a desiccator with 25% HCl for six hours (Brodie et al., 2011) before being packed into silver capsules.
DOM for isotope analysis was collected with two, one-liter grab samples at each site and filtered in the laboratory through 0.7 µm glass fiber filters (Whatman GF/F, Maidstone, UK). One liter was acidified to pH 2.5–3 with concentrated HCl to remove inorganic carbon. Acidified DOM was then evaporated in 8-inch diameter glass dishes at 50 °C, residue was scraped from plates (Wilkinson et al., 2013) and stored in coin envelopes or scintillation vials. DOM was then freeze dried, packed in silver capsules, and sent for δ13C and δ15N analysis. One liter of non-acidified DOM was also dehydrated in glass dishes, stored in coin envelopes or scintillation vials, and residue was sent to be packed and analyzed for δ 2H analysis. The 15N content of DOM samples was corrected for 15N of nitrate (Online Resource 1).
OM samples for carbon and nitrogen isotope analysis were sent to the Stable Isotope Facility (SIF) at University of California Davis and analyzed on a PDZ Europa ANCA-GSL elemental analyzer interfaced to a PDZ Europa 20–20 isotope ratio mass spectrometer (Sercon Ltd., Cheshire, UK). Deuterium analysis was conducted at the Colorado Plateau Stable Isotope Laboratory (CPSIL) at Northern Arizona University following Doucett et al. (2007).
Endmember isotope samples
OM endmembers were collected in all watersheds throughout the three sampling efforts. The goal of endmember sampling was to characterize autochthonous, terrestrial, and anthropogenic source of FPOM and DOM in all four watersheds. Primary producers within streams develop into complex physical structures and matrices that include bacteria and fungi (Lowe and LaLiberte 2007). Therefore, it was not realistic to sample autochthonous endmembers by taxonomy, or to separate algae from phytoplankton, or even algae from other heterotrophs. Autochthonous sources were therefore categorized by broad morphological characteristics that appeared to be dominated by photosynthesizing organisms and associated with common stream habitats present in all watersheds (e.g., riparian edge habitat, benthic habitat, and the water surface). Large submerged aquatic vegetation was classified as macrophytes, biofilm was scraped from benthic rocks, and algae were collected from green mats floating on the water surface. Live and senesced Elm tree leaves (Ulmus pumila) were sampled as a proxy for terrestrial vegetation because elm trees were a deciduous tree present in all four watersheds (Hall et al., 2015). Benthic OM was collected by sinking a stovepipe five to ten cm into river sediment, agitating with a meter stick, and then, a 100 ml sample of the sediment–water mixture was collected, transported back to the laboratory, and filtered through 0.7-µm glass fiber filters. Soil was collected by inserting a 10 × 1-inch soil auger into soil at the riparian zone of sites and removing the bottom 1 inch of soil from the auger. WWTP-FROM and WWTP-DOM were collected instream from the WWTP effluent outfall on the Mill Creek tributary of the Jordan River. WWTP OM was processed for isotopes as described for all FPOM and DOM isotope samples. In the laboratory, all isotope samples were dried for at least 48 h in a drying oven at 50 °C, ground in a coffee grinder, and packed for isotope analysis.
Water quality metrics
We collected samples for analysis of chlorophyll a (Chla) dissolved organic carbon (DOC) and total dissolved nitrogen (TDN) concurrently with organic matter sampling described above. DOC and TDN samples were filtered through 0.7-µm glass fiber filters into 40-mL amber vials and acidified with HCl to a pH of 2.5 for storage until carbon analysis. Acidified DOC and TDN samples were run on a Shimadzu TOC-L analyzer via catalytic oxidation combustion at 720 °C (DOC MDL 0.2 mg/l, TDN MDL 0.1 mg/l; Shimadzu Corp., Kyoto, Japan). Chla was collected on glass fiber filters, in-stream, with a drill-pump, wrapped in foil, frozen, and subsequently analyzed on a Turner handheld fluorometer (Turner Designs, Sunnyvale, CA) following Steinman et al. (2007).
DOM spectroscopic and water quality data analysis
Filtered water from each site and sampling effort were analyzed for DOM spectroscopic properties obtained from excitation–emission matrices (EEMs) collected on a Horiba Aqualog spectrofluorometer (Horiba Scientific, Edison, New Jersey; Online Resource 2). EEMs were used to create a PARAFAC model which decomposes a collection of EEMs into groups of organic compounds with similar fluorescence characteristics (Murphy et al., 2013). PARAFAC results are then used to identify humic- and protein-like fluorescent components of DOM to elucidate differences in DOM that varied by watershed and water quality. The drEEM toolbox was used to create a PARAFAC model in MATLAB™ (Murphy et al., 2013; Online Resource 2). In addition to PARAFAC components, EEMs were used to calculate six spectroscopic indices: the fluorescence index (FI), Yeomin fluorescence index (YFI), freshness index or biological index (BIX), humification index (HIX), peak T to peak C ratio (TC), and specific UV absorbance at 254 nm (SUVA). See Online Resource 2 for detailed description of spectroscopic index calculations.
Multi-way ANOVAs of water quality metrics, spectroscopic indices, and percent PARAFAC components were conducted in R to elucidate differences in DOM quality and character by watershed. Metrics included four water quality variables (DOC, TDN, DOC:TDN, and Chla), six indices (BIX, FI, YFI, HIX, SUVA, and TC), and four PARAFAC components (C1, C2, C3, and C4). Pearson’s correlations were conducted among water quality metrics, spectrofluorimetric indices, and PARAFAC components for DOM grouped by watershed to identify variables that revealed the source or character of DOM among watersheds. Correlations were conducted with the GGally package using all DOM samples and grouped by watershed (Schloerke et al., 2014). Correlations were considered significant when correlation coefficients were greater than 0.35 (Rohlf & Sokal, 1995). A PCA was also conducted with all spectroscopic indices, PARAFAC components, Chla, and DOC:TDN. DOC and TDN concentrations were excluded from the PCA to emphasize DOM composition and not concentration. Prior to conducting the PCA, all variables were Z-score standardized. The PCA was conducted with using the FactoMineR package (Lê et al., 2008) and visualized with the factoextra package (Kassambara, 2015).
Results
Watershed characteristics and land cover
The Jordan River had the maximum average watershed size (1578 km2) and maximum percent land cover associated with human development including urban development (mean 26.1%), crop (mean 2.1%), and pasture (mean 4.6) land covers (Table 1; Online Resource 3). The Provo and Logan Rivers had similar watershed area, 725 km2 and 893 km2, respectively, and Red Butte Creek was the smallest watershed (40 km2; Table 1). The Logan, Provo, and Red Butte Creek watersheds all had less than 5% mean urban development, crop, and pasture land cover within a sub-watershed and were dominated by forest and scrub-grassland land covers.
Correlations of percent land cover among the 34 sites sampled revealed land covers associated with human influence were positively correlated in all watersheds except in the Jordan River (Online Resource 4). Among all four watersheds, percent crops and agriculture were positively correlated (r = 0.97), and therefore, we summed pasture and crop land covers to create a total agricultural land cover for the PCA a percent land cover across all sites. Percent forest and agricultural land cover were negatively correlated in all watersheds (crops r = − 0.88; pasture r = − 0.92) as were percent forest and percent scrub-grassland (r = − 0.37).
The first three components of the land cover PCA explained 68%, 21%, and 7% of variation among percent urban development, agriculture (crop + pasture), forest, scrub-grassland, and wetland within the watershed for all 34 sites (Online Resource 5). Principle Component 1 (PC1) was negatively correlated with forest land cover (r = 0.98) and positively correlated with all other land covers (Online Resource 5). Percent scrub-grassland was positively correlated with Principle Component 2 (PC2; r = 0.86), and urban development was negatively correlated with PC2 (r = − 0.56). Ninety-five percent confidence interval ellipses of sites grouped by watershed indicated that difference among sites was greater by watershed than by gradients of any of the five land covers included in the PCA. Therefore, a gradient of land use reflective of the human influence within a sub-watershed was not identified among sites, and further analysis of OM composition and quality at each site were analyzed and characterized by watershed and not land cover.
Bayesian and gradient-based mixing models
Potential sources input into SIMMR mixing models
To determine if autochthonous or terrestrial sources should be averaged together for inclusion in the SIMMR mixing model, we assessed overlap in the IQR within potential autochthonous (macrophyte, biofilm, algae) and terrestrial (soil, tree leaves) sources. The IQR of δ13C, δ15N, and δ2H values of biofilm and algae overlapped (Fig. 2, Online Resource 6) so these sources were averaged together (algae-biofilm) when input into the SIMMR isotope mixing model. However, macrophyte δ2H values were more positive (IQR − 207.9 to − 190.5‰) than algae (IQR − 269.2 to − 240.3‰) and biofilm δ2H values (IQR − 262.9 to − 240.8‰) so macrophytes were evaluated as a distinct autochthonous source from algae-biofilm. Similarly, the IQR of tree leaves and soil δ13C and δ15N values overlapped, but soil δ2H values were more positive (IQR − 140.6 to − 125.4‰) than tree leave δ2H values (IQR − 181.8 to -− 170.3F‰) so soil and tree leaves were considered distinct terrestrial sources when input into SIMMR. We did not assume benthic OM was primarily an autochthonous or terrestrial source, so it was included as a distinct source in the SIMMR models. Wastewater-derived OM has nitrogen isotope values that are much more positive compared to other freshwater sources of OM (Cabana and Rasmussen, 1996; Archana et al., 2016). Therefore, we assumed WWTP-FPOM and WWTP-DOM were distinct potential sources of FPOM and DOM, respectively. Thus, there were six potential endmembers included in each of the DOM and FPOM SIMMR mixing models: algae-biofilm, macrophytes, tree leaves, soil, benthic OM, and WWTP-DOM or WWTP-FPOM.
Carbon, nitrogen, and deuterium isotope values of potential sources of organic matter. Boxes represent the interquartile range and whiskers represent the 1.5 times the interquartile range. WWTP FPOM and DOM were collected from a wastewater effluent outfall on the Jordan River. Tree leaves, soil, benthic organic matter (OM) macrophytes, biofilms, and algae were collected at multiple sites throughout the four watersheds. For raw endmember values, see Online Resource 6
As a result of sample loss related to issues associated with isotope preparation (e.g., insufficient material for FPOM analysis or DOM moisture reabsorption), DOM and FPOM SIMMR models included 50 and 75 OM samples, respectively. Isotope processing losses also resulted in a different number of FPOM and DOM samples analyzed for gradient mixing models with DIC-δ13C for DOM (n = 55) and FPOM (n = 49), and gradient mixing models with water-δ2H for DOM (n = 81) and FPOM (n = 59).
FPOM isotope mixing models
The FPOM SIMMR mixing model indicated FPOM in the Jordan River was primarily derived from WWTP sources, and to a lesser extent was sourced from a mixture of autochthonous sources and benthic OM (Fig. 3). FPOM in all other watersheds was primarily from terrestrial sources such as tree leaves, except the Logan River had greater variability in estimated contributes from algae-biofilm and benthic OM. Contributions of WWTP-FPOM for the Jordan River ranged from 19 to 61% (95% HDI) and ranged from 1 to 20% (95% HDI) for other watersheds. The Logan River had the highest estimate of autochthonous FPOM sources as algae-biofilm (95% HDI 2 to 28%) with similar estimates for the Jordan River (95% HDI 1 to 22%); the lowest estimates for autochthonous sources were in Red Butte Creek. Estimates of autochthonous sources from macrophytes were lower than algae-biofilm estimates in all watersheds except the Provo.

source was the average or algae and biofilm isotope values. WWTP-DOM was DOM collected directly below a wastewater treatment plant effluent discharge. Boxes represent 75% high density interval (HDI) and whiskers represent the 95% HDI
Percent feasible contributions of six sources to 75 FPOM samples collected in all watersheds. The algae-biofilm
Graphical gradient-based isotope mixing model results indicated FPOM was primarily terrestrially derived across watersheds except for the Jordan and Logan River. FPOM δ13C and δ13C-DIC isotope values were positively correlated for the Jordan (r = 0.76) and Logan River (r = 0.53) suggesting FPOM was autochthonously derived (Fig. 4a, Online Resource 7). Provo and Red Butte Creek FPOM δ13C values were not positively correlated, and were within the δ13C value range of tree leaves and soil suggesting primarily terrestrial FPOM sources. In contrast to the carbon graphical gradient-based model, there were no positive relationships between FPOM and water δ2H values in any watershed (Fig. 4b). Jordan, Provo, and Red Butte Creek FPOM δ2H values were within the range of tree leaves, soil, and WWTP FPOM δ2H values, suggesting a mixture of sources. While there was not a positive correlation among FPOM and water δ2H values within the Logan watershed, sample size was limited (n = 6) and some values were within the range of macrophyte δ2H values (− 190.5 to − 207.9 δ2H; Online Resource 6), and the range of tree leaf δ2H values, suggesting a mixture of tree leaves and autochthonous sources of FPOM in the Logan watershed.
FPOM-δ13C values compared to DIC-δ13C of river water (a), and FPOM-δ2H values compared to δ2H value of river water (b). The horizontal lines represent the interquartile range of δ13C and δ2H values of DOM from wastewater treatment plant effluent (WWTP; solid black), and DOM leached from tree leaves (gray dashed) and soil (gray dotted; see Online Resource 6 for endmember isotope values)
DOM isotope mixing models
According to the SIMMR model, DOM in the Jordan River was primarily WWTP-derived, with potentially significant terrestrial sources such as soil and tree leaves (Fig. 5). DOM in all other watersheds was primarily from terrestrial sources, and the Logan River had the highest variability in potential contributions from autochthonous sources. The 95% HDI of WWTP-derived DOM ranged from 3 to 87% in the Jordan River and ranged from 1 to 16% in all other watersheds. Tree leaves were the most likely source of DOM in the Logan watershed (95% HDI 6 to 74%). Tree leaves or soil were the most likely source of DOM in the Provo and Red Butte Creek watersheds. Maximum autochthonous contributions were estimated for the Logan River (95% HDI macrophytes 1 to 48%, 95% HDI algae-biofilm 2 to 30%) with lower estimates in all other watersheds for both macrophytes (95% HDI 1 to 24%) and algae-biofilm (95% HDI 0 to 14%). Estimated contributions of benthic OM were similar to estimates of autochthonous sources across watersheds likely due to similar ranges of δ13C, δ15N, and δ2H values among these sources (Fig. 2).

source was the average or algae and biofilm isotope values. WWTP-DOM was DOM collected directly below a wastewater treatment plant effluent discharge. Boxes represent 75% high density interval (HDI) and whiskers represent the 95% HDI
Percent feasible contributions of six sources to 50 DOM samples collected in four watersheds. The algae-biofilm
DOM-δ13C and DIC-δ13C values were not positively correlated within any watersheds (Fig. 6a, Online Resource 7), and DOM-δ13C values from all watersheds overlapped with the interquartile range of WWTP-derived DOM, tree leaves, and soil suggesting DOM was not autochthonously derived in any watershed. DOM-δ2H and water-δ2H were not positively correlated within any watersheds (Fig. 6b; Online Resource 7). Ten of the 17 Logan DOM samples were in the range of macrophyte δ2H values (− 190.5 to − 207.9 δ2H). Seven of the Logan δ2H samples, and most of the Provo and Red Butte Creek watersheds had DOM-δ2H values within the ranges of all non-autochthonous sources. The majority of Jordan River DOM δ2H values were more positive than all non-autochthonous source values.
DOM-δ13C values compared to DIC-δ13C of river water (a), and DOM-δ2H values compared to δ2H value of river water (b). The horizontal lines represent the interquartile range of δ13C and δ2H values of DOM from wastewater treatment plant effluent (WWTP; solid black), and DOM leached from tree leaves (gray dashed) and soil (gray dotted; see Online Resource 6 for endmember isotope values)
DOM spectroscopic and water quality analysis
DOM by watershed analysis
To better understand variability in DOM composition among watersheds, we assessed differences in water quality, spectroscopic indices, and PARAFAC components with multi-way ANOVAs and a PCA. A four component PARAFAC model was resolved and validated with split-half analysis (Online Resource 8). In this study, components 1 and 2 (C1, C2) were humic-like, and components 3 and 4 (C3, C4) were protein-like (see Online Resource 8 for previous studies that identified similar components). Percent protein-like fluorescing DOM was the sum of percent C3 and percent C4. DOC, TDN, and Chla were significantly higher for the Jordan River than all other watersheds (Online Resource 9). The FI, YFI, BIX, percent humic-like C1, and percent protein-like C3 were also higher for the Jordan River than all other watersheds. The Provo River had the highest HIX (7.4 ± 1.5 SD) and lowest percent protein (27.1 ± 3.8 SD) compared to all other watersheds, and tended to have high SUVA values (3.2 ± 1.0 SD) compared to all watersheds except for Red Butte Creek (Online Resource 9).
The first three components of the PCA explained 45%, 21%, and 10% of variation among DOM water quality metrics, spectroscopic indices, and PARAFAC components (Fig. 7, Online Resource 10). Indicators of humic DOM such as percent humic-like C2, HIX, DOC:TDN, and SUVA had significant negative correlations with PC1, and indicators of microbially derived DOM such as the YFI, BIX, percent protein-like C3, TC, and FI had significant positive correlations with PC1. The FI and percent humic-like C1, which were greatest in the Jordan, were positively correlated with PC2. The TC and percent protein-like C4 were negatively correlated with PC2. Chla and percent C3 were positively correlated with PC3, and FI values were negatively correlated with PC3 (Online Resource 10). PC3 appeared to distinguish Jordan River DOM collected below Utah Lake, with high percent C3, Chla and positive PC3 coordinate values from Jordan River DOM collected below WWTP influenced sites with high FI values and negative PC3 coordinate values.
Principle components 1(PC1) and 2 (PC2) of a PCA with 82 DOM samples from 34 sites (A). The larger symbol at the center of the 95% confidence ellipses represents the mean of PC1 and PC2 values within a watershed. If 95% confidence ellipses do not overlap, DOM composition between watersheds was different. Covariates inlcuded two humic-like flourescent components (percent C1 and C2), two protein-like components (percent to C3 and C4), Chla concetrations, the ratio of DOC:TDN concentrations, and spectroscopic indices of microbial derived DOM (FI, BIX), protein-like peak T to humic-like peak C ratio (TC), and humic DOM (SUVA, HIX)
Microbial versus autochthonous DOM sources
We expected microbial-like DOM to be derived from autochthonous DOM, and therefore expected a positive relationship between Chla and microbial indices of DOM (e.g., “FI, YFI”), Chla, and percent protein-like DOM. In contrast to this expectation, Chla and “FI, YFI” microbial indices were negatively correlated for the Jordan River (r = − 0.69, r = − 0.62), and there were no strong relationships between Chla and “FI, YFI” in other watersheds (Fig. 8; Online Resource 11). Also counter to our expectation, there was a positive relationship between Chla and DOC:TDN for the Jordan River, but DOC:TDN was extremely low for the Jordan River (mean 0.9, SD 0.6) compared to all other watersheds (mean 6.1, SD 4.9). Chla and percent protein-like DOM were positively correlated in the Jordan River (r = 0.52), but this relationship was driven by one sample with a very low Chla concentration (0.45 µg/l) collected at the site directly below WWTP effluent discharge. We reasoned autochthonous sources were present at WWTP sites, but a different microbial source also contributed to a significant portion of DOM at WWTP sites.
Correlations between chlorophyll a (Chla) and fluorescence indices of microbially derived DOM (FI, YFI), dissolved organic carbon to totoal dissolved nitrogen ratio (DOC:TDN), and percent protein-like DOM. Chla concentrations were log transformed because they were not normally distributed. Percent protein was the sum of percent C3 and C4 PARAFAC components. For pearson’s correlation significance results, see Online Resrouce 11
Discussion
NLCD land-use metrics, such as percent developed land cover within a watershed, did not explain differences in FPOM and DOM across watersheds. OM composition was significantly different in the Jordan River due to the influence of WWTP effluent. FPOM in the Jordan River was primarily derived from WWTP FPOM or benthic organic matter, and Jordan River DOM was characterized as a consistent source of microbial derived, highly labile, protein-like DOM likely derived from WWTP effluent. OM sources in the Provo, Red Butte Creek, and Logan watersheds were primarily terrestrial; however, OM sources in the Logan were more variable with some influence from autochthonous or other microbially derived sources. Greater variability in OM composition in forested sub-watersheds of the Logan River may have been associated with cattle grazing or recreational activities that were not accounted for by NLCD land cover.
Land cover metrics did not capture variability in OM composition
We did not identify a human land cover metric that could adequately predict OM composition or bioavailability. Despite numerous studies, it remains difficult to detect differences in OM composition in response to gradients of urban development, and across multiple land covers. Studies of DOM quality among multiple land covers (e.g., agriculture, wetland, urban, forest) (Petrone et al., 2011; McElmurry et al., 2013; Parr et al., 2015; Williams et al., 2016; Lambert et al., 2017), and watersheds that included WWTP effluent, or leaky sewage infrastructure, have found human impact increased DOM lability (Newcomer et al., 2012; Hosen et al., 2014; Lambert et al., 2017). But, only one study has successfully distinguished the effect of agricultural versus urban land cover on DOM composition, and results were dependent on landscape scale of analysis (Williams et al., 2016). Several studies have anecdotally mentioned WWTP OM as a possible influence on OM composition and lability, but WWTP effluent was not considered in the initial study design (Newcomer et al., 2012; Hosen et al., 2014). Many studies have identified WWTP effluent as a major source of inorganic nutrients and labile OM within urban and agricultural land cover matrices (Sickman et al., 2007; Petrone et al., 2011; Hossler and Bauer 2012; Lambert et al., 2017; Yates et al., 2019a), but WWTP effluent was not directly sampled and considered as a distinct endmember. We acknowledge that many studies have collected and characterized the composition and bioavailability of WWTP effluent (Westerhoff & Anning 2000; Griffith et al., 2009; Yates et al., 2019b), but few studies have directly sampled DOM from WWTP effluent to compare to OM collected in catchments with mixed land covers. Duan et al. (2014) compared wastewater OM stable isotopes to surrounding urban, non-urban, and storm water DOM, and found terrestrial derived OM was replaced by wastewater and autochthonous sources of DOM. We had similar results to Duan et al. (2014), but ours is the first study to report proportional contributions of autochthonous, terrestrial, and WWTP sources in watersheds with multiple land covers. Proportional estimates of OM sources can inform OM reduction strategies that focus on WWTP effluent versus autochthonous sources, non-point sources from agriculture, or terrestrial sources within a watershed.
DOM composition of freshwaters in wet, temperate ecoregions is controlled by different landscape-scale drivers than in the arid west U.S. For example, wetland land cover is a strong driver of DOM composition in the Eastern U.S. and is also influenced by reservoirs, legacy land cover, and differences in urban growth patterns (Grimm et al., 2000). Wetland land cover is less extensive in the west (4% of sites sampled by EPA in 2011) compared to eastern ecoregions (EPA 2016). In this western U.S. study, percent and total area of wetland land cover was near zero, and therefore had little influence on DOM composition. Regardless of the reason for low wetland cover in west, wetlands appear to play a smaller role in DOM composition in the arid west U.S. compared to regions such as the Canadian Great Lakes (Wilson & Xenopoulos, 2008; Williams et al., 2016), the boreal forest (Kothawala et al., 2014), eastern U.S. (Newcomer et al., 2012; Parr et al., 2015), and coastal ecosystems (Sickman et al., 2007; Fellman et al., 2011).
We also did not consider how storms or seasonal changes in hydrology could influence DOM source and bioavailability. The effect of storms in urban watersheds may supersede the influence of urban land cover as storms may replace autochthonous derived OM with terrestrial OM (Imberger et al., 2014; Smith & Kaushal, 2015). In general, lower flows in summer result in more autochthonous OM compared to terrestrial OM in other seasons (Kendall et al., 2001; Hudson et al., 2007; Hosen et al., 2020). In contrast, terrestrial sources are greater in spring runoff in snowmelt-driven ecosystems with little deciduous riparian cover (Hornberger et al., 1994; Hood et al., 2005) and during high flow stages in temperate watersheds (Hosen et al., 2020). The influence of WWTP effluent can increase during low flows in summer due to decreased dilution from other sources (e.g., tributaries and groundwater), and results in greater autochthonous OM production (Wassenaar et al., 2010; Duan et al., 2014). While there has been extensive research on the effect of seasonal hydrology or land cover metrics on OM sources and composition, studies of the interacting effects of season, hydrology, and land cover at large spatial scales that include multiple aquatic ecosystem types (e.g., streams, reservoirs, lakes) will help elucidate anthropogenic controls on OM composition (Spencer et al., 2012; Williams et al., 2016).
FPOM characterization and source composition by watershed
We concluded WWTP-FPOM was the dominant source of FPOM in the Jordan River, followed by benthic OM. In contrast, FPOM in the Provo and Red Butte Creek watersheds was dominated by terrestrial sources and was a mixture of multiple sources in the Logan watershed. Greater variability in FPOM source estimates for the Logan River could have been due to cattle grazing or recreation activity in publicly accessible forested reaches of the watershed. Forested sites along the Logan River were historically grazed by cattle from 1935 to 2005, and currently have cattle trailed up tributaries of the river each fall (Hough-Snee et al., 2013). In addition, the U.S. Department of Agriculture National Forest property composes the majority of the middle and upper watershed and includes networks of trails and dirt roads used for recreation throughout the year. Cow manure, sediment from dirt roads or exhaust from vehicles could contribute to inorganic nutrient runoff at forested sites along the Logan River. Inorganic nutrients and sediment disturbance associated with these activities could stimulate microbial activity and autochthonous production explaining increased variability in sources of FPOM in the Logan watershed.
DOM characterization and composition by watershed
The composition of DOM at sites influenced by WWTP effluent was of high quality for microbial assimilation and mineralization, and therefore characterized as extremely labile. DOM in the Jordan River was characterized by high values for indices of microbially derived DOM including FI, YFI, BIX, and had very low DOC:TDN values indicating it was more bioavailable than DOM of other watersheds. It is important to note that FI values are based on the assumption that OM concentration varies proportionally with fluorescence intensity, which is generally true except at high DOC concentrations (e.g., > 5 mg/l), and FI values above two are rarely, if ever, reported for non-human impacted water bodies. Several studies have reported FI values greater than two from samples of algal leachate and WWTP effluent (Dong & Rosario-Ortiz, 2012; Hansen et al., 2016; Ateia et al., 2017), and one study described DOM with FI values above 1.8 as entirely microbially derived DOM. Similar to other studies that investigated DOM in urban watersheds influenced by WWTP effluent, we concluded DOM from the Jordan River was dominated by microbially derived DOM, some from Utah Lake and some from wastewater effluent, but unlike previous studies, we were able to estimate the proportional contributions of autochthonous versus wastewater derived microbial DOM.
While some extremely bioavailable DOM in the Jordan River was likely derived from autochthonous sources, such as primary production in Utah Lake, several lines of evidence suggest significant sources of microbially derived DOM from WWTP effluent influenced sites was not from autochthonous sources. First, Chla was negatively correlated to the FI and WWTP effluent sites along PC3. Second, in the Jordan River, Chla was negatively correlated with FI, and YFI, and positively correlated with the DOC:TDN ratio. Percent protein was significantly correlated to Chla for the Jordan River, but percent protein never exceeded 35% in that watershed. Thus, we characterized DOM at WWTP sites as a more consistently produced, protein-like, homogenous source of microbial DOM compared to other watersheds that had a wider range of DOM composition (e.g., greater range in percent protein; Inamdar et al., 2012). We posit DOM at WWTP sites was derived from a consistent, homogenous, microbial derived source, such as lysed microbes or microbial exudates from a “microbe factory” like a WWTP.
Management implications
It is important to characterize organic matter sources and bioavailability so watershed managers can understand the origins of OM loads and infer its relative bioavailability. The quality of OM in rivers regulates functions such as decomposition and nutrient assimilation that affect material retention and transport within watersheds. Excessive labile OM and inorganic nutrient loads to rivers can saturate microbial demand for high-quality OM, thereby reducing transformation and retention of semi-labile OM (Wollheim et al., 2018). Consequently, OM and associated nutrients and pathogens are transported downstream to lakes and estuaries causing pollution and eutrophication which pose significant public health risks and increase the cost of drinking water treatment (Chow et al., 2005; Shutova et al., 2014). Therefore, describing the sources and composition of OM in rivers can inform management strategies aimed at improving water quality in both rivers and downstream water bodies.
Identifying relative proportions of OM sources can also help to characterize the quality of OM, or bioavailability to microbes. Constituent removal, a common goal of watershed management is tightly linked with OM supply and demand (Wollheim et al., 2018) which also depends on OM quality. For example, the ratio of dissolved iron and copper to DOM increases with increased aromatic DOM content (Kikuchi et al., 2017). Therefore, management practices aimed at reducing labile OM inputs (e.g., WWTP effluent or autochthonous sources) would not sufficiently address iron and copper constituent transport and accumulation. Similarly, if reduced algal and macrophyte growth is the primary management goal, targeted reduction of labile OM is likely necessary, and identification of primary labile OM sources, e.g., point sources versus instream production, would be crucial. Thus, management aimed at constituents of concern and reduced autochthonous production within a watershed can be improved through knowledge of OM source, composition, and quality.
Conclusions
As human-dominated landscapes increase, it will become more important to understand the sources and quality of OM in rivers. Depending on source and composition, OM can increase or decrease river ecosystem functions such as constituent removal and retention. With increased human development, impervious surfaces, dams, and diversions, sources of OM to rivers will increase and become more variable (Kaushal & Belt, 2012). Scientists and watershed managers need access to information that describes OM composition in rivers with varying land cover to predict how OM composition will influence ecosystem functions such primary production, decomposition, pollutant transport, and nutrient retention and transformation. Land cover in this study did not account for stark differences in DOM quality due to the influence of WWTPs. Therefore, we recommend all OM studies in watersheds with WWTP effluent obtain a representative WWTP sample and incorporate wastewater OM into study designs that compare land cover across watersheds.
Data availability
Kelso and Baker (2017). Dissolved Organic Matter spectrofluorometric properties along a mountain to urban gradient in North East Utah, HydroShare, http://www.hydroshare.org/resource/7722d73be4b348a9ad874fe29086c1fb. Kelso and Baker (2017). FPOM and DOM isotope values, HydroShare, http://www.hydroshare.org/resource/4eb5c9c871e34aa4ae6951ce6d15020d.
Code availability
Not applicable.
References
Archana, A., L. Li, K. Shuh-Ji, B. Thibodeau & D. M. Baker, 2016. Variations in nitrate isotope composition of wastewater effluents by treatment type in Hong Kong. Marine Pollution Bulletin 111: 143–152.
Ateia, M., O. G. Apul, Y. Shimizu, A. Muflihah, C. Yoshimura & T. Karanfil, 2017. Elucidating adsorptive fractions of natural organic matter on carbon nanotubes. Environmental Science & Technology 51: 7101–7110.
Baker, A. & R. Inverarity, 2004. Protein-like fluorescence intensity as a possible tool for determining river water quality. Hydrological Processes 18: 2927–2945.
Bridgeman, J., P. Gulliver, J. Roe & A. Baker, 2014. Carbon isotopic characterisation of dissolved organic matter during water treatment. Water Research 48: 119–125.
Brodie, C. R., M. J. Leng, J. S. Casford, C. P. Kendrick, J. M. Lloyd, Z. Yongqiang & M. I. Bird, 2011. Evidence for bias in C and N concentrations and δ13C composition of terrestrial and aquatic organic materials due to pre-analysis acid preparation methods. Chemical Geology 282: 67–83.
Butman, D. E., H. F. Wilson, R. T. Barnes, M. A. Xenopoulos & P. A. Raymond, 2015. Increased mobilization of aged carbon to rivers by human disturbance. Nature Geoscience 8: 112–116.
Cabana, G. & J. B. Rasmussen, 1996. Comparison of aquatic food chains using nitrogen isotopes. Proceedings of the National Academy of Sciences 93: 10844–10847.
Chow, A. T., S. Gao & R. A. Dahlgren, 2005. Physical and chemical fractionation of dissolved organic matter and trihalomethane precursors: a review. Journal of Water Supply: Research and Technology-AQUA 54: 475–507.
Coble, P. G., 1996. Characterization of marine and terrestrial DOM in seawater using excitation-emission matrix spectroscopy. Marine Chemistry 51: 325–346.
Dong, M. M. & F. L. Rosario-Ortiz, 2012. Photochemical formation of hydroxyl radical from effluent organic matter. Environmental Science & Technology 46: 3788–3794.
Doucett, R. R., J. C. Marks, D. W. Blinn, M. Caron & B. A. Hungate, 2007. Measuring terrestrial subsidies to aquatic food webs using stable isotopes of hydrogen. Ecology 88: 1587–1592.
Drake, T. W., K. Van Oost, M. Barthel, M. Bauters, A. M. Hoyt, D. C. Podgoski, J. Six, P. Boeckx, S. E. Trumbore, N. L. C. Ntaboba & R. G. M. Spencer, 2019. Mobilization of aged and biolabile soil carbon by tropical deforestation. Nature Geosciences 12: 541–546.
Duan, S., R. M. Amon & R. L. Brinkmeyer, 2014. Tracing sources of organic matter in adjacent urban streams having different degrees of channel modification. Science of the Total Environment 485: 252–262.
Epstein, D. M., J. E. Kelso & M. A. Baker, 2016. Beyond the urban stream syndrome: organic matter budget for diagnostics and restoration of an impaired urban river. Urban Ecosystems 19: 1041–1061.
Fellman, J. B., K. C. Petrone & P. F. Grierson, 2011. Source, biogeochemical cycling, and fluorescence characteristics of dissolved organic matter in an agro-urban estuary. Limnology and Oceanography 56: 243–256.
Fork, M. L., J. R. Blaszczak, J. M. Delesantro & J. B. Heffernan, 2018. Engineered headwaters can act as sources of dissolved organic matter and nitrogen to urban stream networks. Limnology and Oceanography Letters 3: 215–224.
Griffith, D. R., R. T. Barnes & P. A. Raymond, 2009. Inputs of fossil carbon from wastewater treatment plants to US rivers and oceans. Environmental Science & Technology 43: 5647–5651.
Grimm, N. B., J. G. Grove, S. T. Pickett & C. L. Redman, 2000. Integrated approaches to long-term studies of urban ecological systems: Urban ecological systems present multiple challenges to ecologists—Pervasive human impact and extreme heterogeneity of cities, and the need to integrate social and ecological approaches, concepts, and theory. AIBS Bulletin 50: 571–584.
Guenet, B., M. Danger, L. Abbadie & G. Lacroix, 2010. Priming effect: bridging the gap between terrestrial and aquatic ecology. Ecology 91: 2850–2861.
Guillemette, F., S. L. McCallister, P. A. del Giorgio, 2013. Differentiating the degradation dynamics of algal and terrestrial carbon within complex natural dissolved organic carbon in temperate lakes. Journal of Geophysical Research: Biogeosciences 118: 963–973.
Hall, S. J., R. L. Hale, M. A. Baker, D. R. Bowling & J. R. Ehleringer, 2015. Riparian plant isotopes reflect anthropogenic nitrogen perturbations: robust patterns across land use gradients. Ecosphere 6: 1–16.
Hansen, A. M., T. E. C. Kraus, B. A. Pellerin, J. A. Fleck, B. D. Downing & B. A. Bergamaschi, 2016. Optical properties of dissolved organic matter (DOM): effects of biological and photolytic degradation. Limnology and Oceanography 61: 1015–1032.
Hogsett, M.C. Water quality and sediment biogeochemistry in the urban Jordan River, UT, 2015. Dissertation University of Utah, Salt Lake City, UT.
Homer, C. G., J. A. Dewitz, L. Yang, S. Jin, P. Danielson, G. Xian, J. Coulston, N. D. Herold, J. D. Wickham & K. Megown, 2015. Completion of the 2011 national land cover database for the conterminous United States-Representing a decade of land cover change information. Photogrammetric Engineering and Remote Sensing 81: 345–354.
Hood, E., M. W. Williams & D. M. McKnight, 2005. Sources of dissolved organic matter (DOM) in a Rocky Mountain stream using chemical fractionation and stable isotopes. Biogeochemistry 74: 231–255.
Hornberger, G., K. Bencala & D. McKnight, 1994. Hydrological controls on dissolved organic carbon during snowmelt in the Snake River near Montezuma, Colorado. Biogeochemistry 25: 147–165.
Hosen, J. D., O. T. McDonough, C. M. Febria & M. A. Palmer, 2014. Dissolved organic matter quality and bioavailability changes across an urbanization gradient in headwater streams. Environmental Science & Technology 48: 7817–7824.
Hosen, J. D., K. S. Aho, J. H. Fair, E. D. Kyzivat, S. Matt, J. Morrison, A. Stubbins, L. C. Weber, B. Yoon & P. A. Raymond, 2020. Source switching maintains dissolved organic matter chemostasis across discharge levels in a large temperate river network. Ecosystems 24: 227–247.
Hossler, K. & J. E. Bauer, 2012. Estimation of riverine carbon and organic matter source contributions using time-based isotope mixing models. Journal of Geophysical Research 117: G03035.
Hough-Snee, N., B. B. Roper, J. M. Wheaton, P. Budy & R. L. Lokteff, 2013. Riparian vegetation communities change rapidly following passive restoration at a northern Utah stream. Ecological Engineering 58: 371–377.
Hudson, N., A. Baker & D. Reynolds, 2007. Fluorescence analysis of dissolved organic matter in natural, waste and polluted waters—a review. River Research and Applications 23: 631–649.
Hutchens, J. J., J. B. Wallace & J. W. Grubaugh, 2017. Transport and storage of fine particulate organic matter. In Hauer, F. R. & G. A. Lamberti (eds), Methods in Stream Ecology 3rd ed. Elsevier, Amsterdam: 37–53.
Imberger, S. J., P. L. Cook, M. R. Grace & R. M. Thompson, 2014. Tracing carbon sources in small urbanising streams: catchment-scale stormwater drainage overwhelms the effects of reach-scale riparian vegetation. Freshwater Biology 59: 168–186.
Inamdar, S., N. Finger, S. Singh, M. Mitchell, D. Levia, H. Bais, D. Scott & P. McHale, 2012. Dissolved organic matter (DOM) concentration and quality in a forested mid-Atlantic watershed, USA. Biogeochemistry 108: 55–76.
Kassambara, A., 2015. Factoextra: Extract and visualize the results of PCA, CA and MCA. R package version 1.0.3. R project for statistical computing, Vienna. (Available from: https://cran.r-project.org/web/packages/factoextra/index.html)
Kaushal, S. S. & K. T. Belt, 2012. The urban watershed continuum: evolving spatial and temporal dimensions. Urban Ecosystems 15: 409–435.
Kelso, J. E. & M. A. Baker, 2015. Filtering with a drill pump: an efficient method to collect suspended sediment. Journal of the American Water Resources Association 52: 262–268.
Kendall, C., S. R. Silva & V. J. Kelly, 2001. Carbon and nitrogen isotopic compositions of particulate organic matter in four large river systems across the United States. Hydrological Processes 15: 1301–1346.
Kikuchi, T., M. Fujii, K. Terao, R. Jiwei, Y. P. Lee & C. Yoshimura, 2017. Correlations between aromaticity of dissolved organic matter and trace metal concentrations in natural and effluent waters: a case study in the Sagami River Basin, Japan. Science of the Total Environment 576: 36–45.
Knapik, H. G., C. V. Fernandes, J. C. R. de Azevedo, M. M. dos Santos, P. Dall’Agnol, & D. G. Fontane, 2015. Biodegradability of anthropogenic organic matter in polluted rivers using fluorescence, UV, and BDOC measurements. Environmental Monitoring and Assessment 187: 1–15.
Kolpin, D. W., E. T. Furlong, M. T. Meyer, E. M. Thurman, S. D. Zaugg, L. B. Barber & H. T. Buxton, 2002. Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999–2000: a national reconnaissance. Environmental Science & Technology 36: 1202–1211.
Korak, J. A., E. C. Wert & F. L. Rosario-Ortiz, 2015. Evaluating fluorescence spectroscopy as a tool to characterize cyanobacteria intracellular organic matter upon simulated release and oxidation in natural water. Water Research 68: 432–443.
Kothawala, D. N., C. A. Stedmon, R. A. Müller, G. A. Weyhenmeyer, S. J. Köhler & L. J. Tranvik, 2014. Controls of dissolved organic matter quality: evidence from a large-scale boreal lake survey. Global Change Biology 20: 1101–1114.
Kruschke, J. K., 2018. Rejecting or accepting parameter values in Bayesian estimation. Advances in Methods and Practices in Psychological Science 1: 270–280.
Lowe, R. L. & G. D. LsLiberte, 2007. Dissolved organic matter. In Hauer, F. R. & G. A. Lamberti (eds), Methods in Stream Ecology Elsevier, Amsterdam: 327–356.
Lambert, T., S. Bouillon, F. Darchambeau, C. Morana, F. A. Roland, J.-P. Descy & A. V. Borges, 2017. Effects of human land use on the terrestrial and aquatic sources of fluvial organic matter in a temperate river basin (The Meuse River, Belgium). Biogeochemistry 136: 191–211.
Lê, S., J. Josse & F. Husson, 2008. FactoMineR: an R package for multivariate analysis. Journal of Statistical Software 25: 1–18.
Mallin, M. A., V. L. Johnson, S. H. Ensign & T. A. MacPherson, 2006. Factors contributing to hypoxia in rivers, lakes, and streams. Limnology and Oceanography 51: 690–701.
McCormick, A. R., T. J. Hoellein, M. G. London, J. Hittie, J. W. Scott & J. J. Kelly, 2016. Microplastic in surface waters of urban rivers: concentration, sources, and associated bacterial assemblages. Ecosphere 7: e01556.
McElmurry, S. P., D. T. Long & T. C. Voice, 2013. Stormwater dissolved organic matter: influence of land cover and environmental factors. Environmental Science & Technology 48: 45–53.
Mohamed, N. M. & W. D. Taylor, 2009. Relative contribution of autochthonous and allochthonous carbon to limnetic zooplankton: a new cross-system approach. Archiv Für Hydrobiologie 175: 113–124.
Murphy, K. R., C. A. Stedmon, D. Graeber & R. Bro, 2013. Fluorescence spectroscopy and multi-way techniques. PARAFAC. Analytical Methods 5: 6557–6566.
Newcomer, T. A., S. S. Kaushal, P. M. Mayer, A. R. Shields, E. A. Canuel, P. M. Groffman & A. J. Gold, 2012. Influence of natural and novel organic carbon sources on denitrification in forest, degraded urban, and restored streams. Ecological Monographs 82: 449–466.
Parnell, A., & R. Inger, 2016. Stable isotope mixing models in R with SIMMR. R package version 0.3. R Project for Statistical Computing, Vienna. (Available from: https://cran.r.-project.org/web/packages/simmr/vignettes/simmr.html)
Parr, T. B., C. S. Cronan, T. Ohno, S. E. G. Findlay, S. M. C. Smith & K. S. Simon, 2015. Urbanization changes the composition and bioavailability of dissolved organic matter in headwater streams. Limnology and Oceanography 60: 885–900.
Petrone, K. C., J. B. Fellman, E. Hood, M. J. Donn & P. F. Grierson, 2011. The origin and function of dissolved organic matter in agro-urban coastal streams. Journal of Geophysical Research 116: G01028.
Pisani, O., D. D. Bosch, A. W. Coffin, D. M. Endale, D. Liebert & T. C. Strickland, 2020. Riparian land cover and hydrology influence stream dissolved organic matter composition in an agricultural watershed. Science of the Total Environment 717: 137165.
Rasmussen, J. B., 2010. Estimating terrestrial contribution to stream invertebrates and periphyton using a gradient-based mixing model for δ13C. Journal of Animal Ecology 79: 393–402.
Schloerke, B., J. Crowley, D. Cook, H. Hofmann, H. Wickham, F. Briatte, M. Marbach, & E. Thoen, 2014. GGally: Extension to ggplot2. R package version 1.4. R Project for Statistical Computing, Vienna, Austria. (Available from: https://cran.r-project.org/web/packages/GGally/index.html)
Shutova, Y., A. Baker, J. Bridgeman & R. K. Henderson, 2014. Spectroscopic characterisation of dissolved organic matter changes in drinking water treatment: from PARAFAC analysis to online monitoring wavelengths. Water Research 54: 159–169.
Sickman, J., M. Zanoli & H. Mann, 2007. Effects of urbanization on organic carbon loads in the Sacramento River California. Water Resources Research 43: W11422.
Sickman, J. O., C. L. DiGiorgio, M. L. Davisson, D. M. Lucero & B. Bergamaschi, 2010. Identifying sources of dissolved organic carbon in agriculturally dominated rivers using radiocarbon age dating: Sacramento-San Joaquin River Basin, California. Biogeochemistry 99: 79–96.
Spencer, R. G., K. D. Butler & G. R. Aiken, 2012. Dissolved organic carbon and chromophoric dissolved organic matter properties of rivers in the USA. Journal of Geophysical Research 117: G03001.
Steinman, A. D., G. A. Lamberti & P. R. Leavitt, 2007. Biomass and pigments of benthic algae. In Hauer, F. R. & G. A. Lamberti (eds), Methods in Stream Ecology Elsevier, Amsterdam: 357–379.
US Environmental Protection Agency, 2016. National Wetland Condition Assessment: 2011 Technical Report. EPA-843-R-15-006. US Environmental Protection Agency, Washington, DC.
Wassenaar, L., J. Venkiteswaran, S. Schiff & G. Koehler, 2010. Aquatic community metabolism response to municipal effluent inputs in rivers quantified using diel δ18O values of dissolved oxygen. Canadian Journal of Fisheries and Aquatic Sciences 67: 1232–1246.
Westerhoff, P. & D. Anning, 2000. Concentrations and characteristics of organic carbon in surface water in Arizona: influence of urbanization. Journal of Hydrology 236: 202–222.
Wilkinson, G. M., M. L. Pace & J. J. Cole, 2013. Terrestrial dominance of organic matter in north temperate lakes. Global Biogeochemical Cycles 27: 43–51.
Williams, C. J., P. C. Frost, A. M. Morales-Williams, J. H. Larson, W. B. Richardson, A. S. Chiandet & M. A. Xenopoulos, 2016. Human activities cause distinct dissolved organic matter composition across freshwater ecosystems. Global Change Biology 22: 613–626.
Wilson, H. F. & M. A. Xenopoulos, 2008. Effects of agricultural land use on the composition of fluvial dissolved organic matter. Nature Geoscience 2: 37–41.
Wollheim, W., S. Bernal, D. Burns, J. Czuba, C. Driscoll, A. Hansen, R. Hensley, J. Hosen, S. Inamdar, S. S. Kaushal, L. E. Koenig, Y. H. Lu, A. Marzadri, P. A. Raymond, D. Scott, R. J. Stewart, P. G. Vidon & E. Wohl, 2018. River network saturation concept: factors influencing the balance of biogeochemical supply and demand of river networks. Biogeochemistry 141: 503–521.
Woods, A. J., D. A. Lammers, S. A. Bryce, J. M. Omernik, R. L. Denton, M. Domeier, & J.A. Comstock, 2001. Ecoregions of Utah. U.S. Geological Survey, Reston, VA.
Xenopoulos, M. A., R. T. Barnes, K. S. Boodoo, D. Butman, N. Catalan, S. D’amario, C. Fasching, D. Kothawala, O. Pisani, C. Solomon, R. Spencer, C. Williams & H. Wilson, 2021. How humans alter dissolved organic matter composition in freshwater: relevance for the Earth’s biogeochemistry. Biogeochemistry 154: 323–348.
Yates, C. A., P. J. Johnes, A. T. Owen, F. L. Brailsford, H. C. Glanville, C. D. Evans, M. R. Marshall, D. L. Jones, C. E. M. Lloyd, T. Jickells & R. P. Evershed, 2019. Variation in dissolved organic matter (DOM) stoichiometry in UK freshwaters: assessing the influence of land cover and soil C: N ratio on DOM composition. Limnology and Oceanography 64: 2328–3234.
Yates, C. A., P. J. Johnes & R. G. M. Spencer, 2019. Characterization of treated effluent from four commonly employed wastewater treatment facilities: a UK case study. Journal of Environmental Management 232: 919–927.
Acknowledgements
We would like to thank Dave Ericksson, Dave Epstein, Joe Crawford, Chris Cox, Richard Walker, Kavoka Jackson, and Erin Jones for their help in the field. We thank Lindsay Capito, Hayden Campbell, and Lisa Ward for help in the lab. This manuscript was improved by comments from Zach Aanderud, Matt Miller, Beth Neilson, and John Stark. We thank Melanie Caron (CPSIL), Dennis Newell (USUSIL), and Joy Matthews (SIF) for their help with stable isotope samples and results. We thank our funders, Utah State University Office of Graduate and Research Studies, the Ecology Center at Utah State University and the National Science Foundation (IIA-1208732).
Funding
This research was supported in part by NSF EPSCoR cooperative agreement IIA- 1208732 awarded to Utah State University, as part of the State of Utah EPSCoR Research Infrastructure Improvement Award. Additional support was provided by the South Valley Water Reclamation Facility and Utah Department of Environmental Quality. Any opinions, findings, and conclusions or recommendations expressed are those of the authors and do not reflect the views of the National Science Foundation, South Valley Water Reclamation Facility, or the State of Utah.
Author information
Authors and Affiliations
Contributions
JK and MA developed the study design and wrote the manuscript. JK collected and analyzed samples and analyzed the data.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Ethical approval
Not applicable.
Additional information
Handling editor: Maria de los Angeles Gonzalez Sagrario
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kelso, J.E., Baker, M.A. Organic matter sources and composition in four watersheds with mixed land cover. Hydrobiologia 849, 2663–2682 (2022). https://doi.org/10.1007/s10750-022-04884-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-022-04884-y